Abstract
In Saccharomyces cerevisiae, Cdc13, Stn1, and Ten1 are essential for both chromosome capping and telomere length homeostasis. These three proteins have been proposed to perform their roles at chromosome termini as a telomere-dedicated t-RPA complex, on the basis of several parallels with the conventional RPA complex. In this study, we have used several approaches to test whether a predicted α-helix in the N-terminal domain of the S. cerevisiae Stn1 protein is required for formation of the proposed t-RPA complex, in a manner analogous to the comparable helix in Rpa2. Analysis of a panel of Rpa2–OBStn1 chimeras indicates that whether a chimeric protein contains the Rpa2 or Stn1 version of this α-helix dictates its ability to function in place of Rpa2 or Stn1, respectively. In addition, mutations introduced into a hydrophobic surface of the predicted Stn1 α-helix eliminated association with Ten1. Strikingly, allele-specific suppression of a stn1 mutation in this helix (stn1–L164D) by a ten1 mutation (ten1–D138Y) resulted in a restored Stn1–Ten1 interaction, supporting the identification of a Stn1–Ten1 interface. We conclude that Stn1 interacts with Ten1 through an α-helix, in a manner analogous to the interaction between the comparable subunits of the RPA complex.
IN the budding yeast Saccharomyces cerevisiae, a trio of essential proteins—Cdc13, Stn1, and Ten1—orchestrate a number of interactions to ensure chromosome end protection and telomere length homeostasis. In cells depleted for any of these three proteins, telomeres become subject to unregulated 5′ → 3′ resection of the C strand of telomeres, resulting in extensive single-stranded regions that signal cell cycle arrest and lethality if left unrepaired (Weinert and Hartwell 1993; Garvik et al. 1995; Lydall and Weinert 1995; Grandin et al. 1997, 2001; Vodenicharov and Wellinger 2006). The exact mechanism by which these three proteins protect chromosome termini has not been elucidated. However, there are several notable parallels between the DNA degradation that occurs at both unprotected telomeres and newly generated double-strand breaks (Ira et al. 2004; Frank et al. 2006; Vodenicharov and Wellinger 2006; Mimitou and Symington 2008; Zhu et al. 2008; Bonetti et al. 2009). In addition to an essential role in telomere capping, this complex regulates telomere length through both positive and negative mechanisms. Cdc13 interacts with the telomerase Est1 subunit, to recruit the telomerase enzyme to its site of action, thereby ensuring that telomeres do not become critically short (Nugent et al. 1996; Pennock et al. 2001; Bianchi et al. 2004). All three proteins also contribute to negative regulation of telomere length (Grandin et al. 1997, 2001; Chandra et al. 2001; Gelinas et al. 2009), although the mechanism by which this second regulatory step occurs remains unclear.
Increasing evidence supports the hypothesis that Cdc13, Stn1, and Ten1 form a telomere-dedicated version of the RPA complex (Gao et al. 2007; Martin et al. 2007; Gelinas et al. 2009; Sun et al. 2009), dubbed the t-RPA complex, which acts at chromosome ends in a manner that is potentially analogous to how the canonical replication protein A (RPA) complex performs its genome-wide roles. The heterotrimeric RPA complex is the major single-strand DNA binding activity in eukaryotic cells, participating in multiple DNA transactions throughout the genome through its ability to bind single-stranded DNA with high affinity but low specificity (Wold 1997). In contrast, the proposed t-RPA complex is exclusively localized to chromosome ends as a consequence of the high specificity and affinity that the Cdc13 protein exhibits for single-stranded telomeric DNA (Nugent et al. 1996; Anderson et al. 2002). The t-RPA complex is further distinguished from the conventional RPA complex by the acquisition of an additional domain at the C terminus of Stn1, which confers a telomere-specific function (Gelinas et al. 2009), as well as the telomerase recruitment domain of Cdc13 (Pennock et al. 2001).
The t-RPA hypothesis was initiated with the observation that the N-terminal domains of the Stn1 and RPA32 protein families exhibit notable protein similarities, suggesting that Stn1 contains an N-terminal oligosaccharide/oligonucleotide binding (OB)-fold domain (Gao et al. 2007). Consistent with this, a chimeric Rpa2–OBStn1 protein, in which the OB-fold domain of the yeast Rpa2 protein was replaced by the comparable region of Stn1, retains Rpa2 function in vivo. Furthermore, the Stn1–Ten1 and Rpa2–Rpa3 subcomplexes share several biochemical properties (Gao et al. 2007). More direct support for an evolutionary relationship has come from two recent structural studies, which have demonstrated that Stn1 and Ten1 proteins are composed of several domains with striking structural similarities to comparable domains found in RPA32 and RPA14 (Gelinas et al. 2009; Sun et al. 2009). (The middle and small subunits of the RPA complex are called RPA32 and RPA14 in humans and Rpa2 and Rpa3 in budding yeast).
In the conventional RPA complex, interaction between the middle and small subunits is mediated through two α-helices located on the C-terminal side of the OB-fold present in each protein (Bochkarev et al. 1999; Bochkareva et al. 2002). As an additional experimental test of the t-RPA hypothesis, this current study asked whether the S. cerevisiae Stn1 and Ten1 proteins interact through a similar interface. The starting point for this analysis employed a statistically robust prediction for the structure of the N terminus of the S. cerevisiae Stn1 protein. However, a comparable prediction for the structure of the S. cerevisiae Ten1 protein has so far remained elusive (even with the recent report of the crystal structures for the Schizosaccharomyces pombe and Candida tropicalis Ten1 proteins; Gelinas et al. 2009; Sun et al. 2009). This study therefore tests the idea that a proposed structure for one partner of a complex might be sufficient to define a protein–protein interaction domain, employing in vivo methods. Specifically, mutagenesis was used to demonstrate that an α-helix in the predicted OB-fold domain of the S. cerevisiae Stn1 protein was required for interaction with Ten1. Mutations in residues on one surface of this helix eliminated association with Ten1, as monitored by two different biochemical assays. These stn1− mutations were subsequently used to identify mutations in a potential interaction surface in Ten1. This analysis ultimately led to a pair of stn1− and ten1− mutations (stn1–L164D and ten1–D138Y), which exhibited allele-specific reciprocal cosuppression, as the result of a restored interaction between these mutant Stn1 and Ten1 proteins. Therefore, these in vivo data have identified amino acids that are required for the S. cerevisiae Stn1–Ten1 interface and presumably are in close proximity in the Stn1–Ten1 complex. These observations indicate that Stn1 interacts with Ten1 through an α-helix, analogous to the interaction between the comparable subunits of the RPA complex.
MATERIALS AND METHODS
Strains and plasmids:
A list of the yeast strains, as well as details of construction, is shown in supporting information, Table S1; all yeast strains used for genetic analysis were isogenic. A list of the plasmids, as well as the starting vectors used for each set of plasmid constructions, is shown in Table S2. All plasmids containing missense mutations were generated by QuickChange mutagenesis, and either a sequenced restriction fragment was subcloned back into an unmutagenized backbone or the relevant gene (STN1 or TEN1) was completely sequenced. The Stn1–(G)9–(myc)7 and Ten1–(G)8–(FLAG)3 constructs used for coimmunoprecipitation experiments were expressed by the respective STN1 and TEN1 native promoters on CEN plasmids; these tagged proteins were functional, as determined by telomere length analysis in stn1-Δ or ten1-Δ strains, respectively (data not shown).
Genetic methods:
Diploid strains with integrated stn1− and ten1− mutations were dissected and haploid spores allowed to germinate on rich media. For spores that generated visible colonies, TEN1 genotype was determined by Kan resistance, whereas the STN1 genotype was determined by sequence analysis of PCR products of the relevant region of the STN1 gene. Spores that did not give rise to a visible colony were examined under the microscope, which revealed two classes of inviable spores: spores that failed to germinate and spores that underwent ≥1 division following germination. Tetrads with spores that failed to germinate were excluded from subsequent analysis. For each dissection, a minimum of a dozen tetrads were analyzed for genotype and growth phenotype, thereby ensuring that the inferred genotype of the inviable spores was correctly identified. Other standard genetic methods (telomere length analysis, plasmid shuffle, viability assays, and two-hybrid tests) were performed as previously described (Lendvay et al. 1996; Bertuch and Lundblad 2003; Gao et al. 2007).
Biochemical methods:
The biochemical association between Stn1 and Ten1, and various mutant versions, was determined by coimmunoprecipitation of proteins expressed either in reticulocyte extracts or in yeast. In the first method, FLAG–Stn1 and Ten1 were translated in a coupled transcription/translation reaction with 35S-methionine, subjected to immunoprecipitation with anti-FLAG M2 beads (Sigma), and radio-labeled proteins in the immunoprecipitates were resolved on a 15% SDS–PAGE gel, as described previously (Gao et al. 2007). For experiments in yeast, myc-tagged Stn1 and FLAG-tagged Ten1 proteins were expressed from single-copy plasmids by native promoters in the protease-deficient strain JB811 (with intact genomic copies of STN1 and TEN1). Extract preparation and immunoprecipitations with anti-FLAG M2 beads (Sigma) were performed as described previously (Evans and Lundblad 2002); proteins were detected on 15% SDS–PAGE gels with anti-myc 2272 (Cell Signaling Technology) or anti-FLAG F7425 (Sigma) antibodies.
Screening for mutations in TEN1 that suppressed stn1–L164D:
The Gal4DBD–Ten1 plasmid (pVL2678) was mutagenized by passage through an Escherichia coli mutator strain (XL1Red). Plasmid DNA was recovered from ∼60,000 transformants grown as single colonies for ∼36 hr at 37°. Two variants of the S. cerevisiae strain pJ69–4A (James et al. 1996), containing either pVL3670 (Gal4AD–Stn1–L164D) or pVL3674 (Gal4AD–Stn1–I168D), were transformed with the mutagenized pVL2678 library and plated on media that selected for expression of either the ADE2 or HIS3 reporter genes; an estimated 300,000 transformants were screened for each. Candidate plasmids were rescued, retransformed to confirm the phenotype, and subsequently sequenced to identify potential missense mutations. A total of 15 plasmids, corresponding to mutations in six different amino acid residues in TEN1, were recovered from the screen with pVL3670 (Gal4AD–Stn1–L164D); ten1–D138A and ten1–D138Y were recovered twice and once, respectively. From the screen with pVL3674 (Gal4AD–Stn1–I168D), 13 plasmids were recovered, corresponding to missense mutations in 11 different amino acids (which did not include mutations in residue D138). The in vivo analysis of these additional mutations in TEN1 will be described elsewhere.
RESULTS
Domain swaps between Rpa2 and Stn1 implicate a helical region common to both proteins:
As the starting point for this study, a predicted structure for the N-terminal domain of the S. cerevisiae Stn1 protein was constructed. Structure predictions were performed by comparing the results from HHpred, SAM-T08, and I-TASSER servers (Karplus et al. 2005; Söding et al. 2005; Zhang 2008), as described previously (Gao et al. 2007). Structural alignment between the HHpred model and models from SAM and I-TASSER using the TM-Align server gave an average TM score of 0.7 ± 0.1 and a root mean square deviation of 2.2 ± 0.7 Å, representing highly similar predicted structures (Zhang and Skolnick 2005). These were used to construct a three-dimensional structural model of the S. cerevisiae Stn1 protein, based on the HHpred profile–profile comparison (Figure S1).
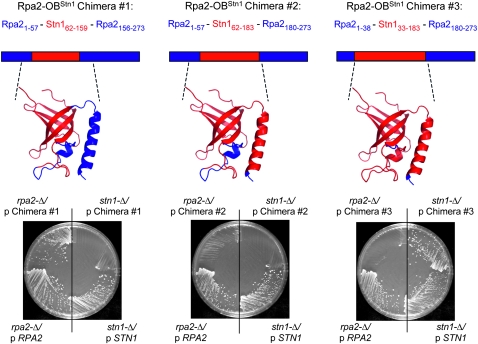
In a previous test of this structural prediction, we constructed a chimeric Rpa2 protein, in which the N-terminal OB-fold of the yeast Rpa2 protein, which is required for viability, was replaced by the predicted OB-fold of Stn1 (Gao et al. 2007). This Rpa2–OBStn1 chimera (chimera 1, Figure 1) was still capable of performing the essential role(s) of Rpa2, indicating that the OB-folds of Rpa2 and Stn1 are functionally related, thereby supporting an evolutionary relationship between these two proteins. The design of this Rpa2 chimera was based on the structural prediction for Stn1, described above, and also incorporated a particular observation about the RPA complex. Structural analysis of the human RPA complex has revealed that a trimerization interface is formed through contacts between three α-helices, contributed by each of the three RPA subunits (Bochkareva et al. 2002; Figure S1). The α-helix of the middle RPA subunit (called RPA32 in Homo sapiens and Rpa2 in S. cerevisiae) contains two hydrophobic surfaces that interact with the other two subunits of the complex. Therefore, chimera 1 was designed to retain the α-helix of Rpa2, on the assumption that this helix would be essential for RPA complex formation. Consistent with this, chimera 1 rescued the inviability of a rpa2-Δ null strain, but not that of a stn1-Δ strain (Gao et al. 2007 and Figure 1), as expected if this chimera associated with Rpa1 and Rpa3.
Figure 1.—
In vivo assessment of Rpa2–OBStn1 chimeric proteins. The predicted structure of the N-terminal domain of the three chimeras are shown as ribbon representations, with the position of the domain relative to each full-length Rpa2–OBStn1 protein; Rpa2 and Stn1 sequences are depicted in blue or red, respectively. Viability of a rpa2-Δ strain (YVL2924) or a stn1-Δ strain (YVL2394), following eviction of a RPA2 or STN1 plasmid by passage on 5-FOA-containing media, is shown on the left and right half of each plate, as indicated; the ability of each chimera to rescue the lethality of rpa2-Δ or stn1-Δ is compared to control plasmids expressing the intact RPA2 or STN1 genes.
This further predicts that an Rpa2–OBStn1 chimera, which includes the C-terminal α-helix of Stn1, would be unable to rescue rpa2-Δ, but instead should rescue the lethality of a stn1-Δ strain. To test this, we constructed chimera 2, which extended the boundary of chimera 1 to include this α-helix. Unexpectedly, this second chimera was incapable of rescuing either strain (Figure 1). This could be due to nonspecific reasons, such as protein instability. However, an alternative possibility was that chimera 2 did not contain all of the elements necessary for complex formation with the other subunits of the proposed t-RPA complex. In particular, we considered the possibility that a second smaller α-helix present on the N-terminal side of the OB-fold of Rpa2 (as well as the predicted OB-fold of Stn1) might also contribute, given the location of this second helix in the RPA32–RPA14 interface (Bochkarev et al. 1999; Bochkareva et al. 2002). To test this, we constructed a third chimera, which replaced the Rpa2 OB-fold, as well as the flanking N- and C-terminal α-helices, with the comparable region of Stn1 (chimera 3). Strikingly, this Rpa2 chimera exhibited an in vivo behavior that was the reciprocal of that of chimera 1, in that chimera 3 restored viability to the stn1-Δ strain, but did not rescue an rpa2-Δ strain (Figure 1).
The behavior of this set of three chimeric proteins argues that the ability of a given Rpa2–OBStn1 protein to functionally replace either Rpa2 or Stn1 is determined by the two α-helices that flank either side of the β barrel of the OB-fold. Thus, chimera 2, which contained the N-terminal α-helix of Rpa2 but the C-terminal α-helix of Stn1, was nonfunctional. In contrast, Rpa2–OBStn1 chimeras that possessed both helices, from either Rpa2 or Stn1, dictated whether the resulting chimeric protein could function as a subunit of the RPA or t-RPA complexes, respectively.
One surface of the predicted α-helix of Stn1 is required for interaction with Ten1:
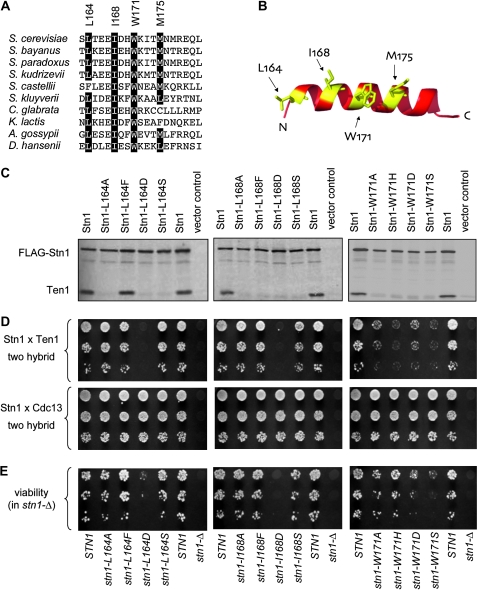
In the RPA complex, the interface between RPA32 and RPA14 (the equivalent of Rpa2 and Rpa3, in S. cerevisiae) is mediated by hydrophobic interactions between the α-helices of these two proteins. To determine whether Stn1 might similarly employ such an interface, the surface of the predicted structure of the α-helix of Stn1 was examined, as well as a primary sequence alignment of this same region of Stn1. We focused our attention on the side of the Stn1 helix which, by analogy with RPA32, should contact Ten1. As shown in Figure 2, A and B, four residues—L164, I168, W171, and M175—define a hydrophobic surface of the proposed α-helix; furthermore, three of these four hydrophobic residues are highly conserved among an alignment of 10 Stn1 proteins.
Figure 2.—
Mutagenesis of the predicted Stn1 α-helix disrupts interaction with Ten1. (A) Alignment of the predicted Stn1 α-helix, for 10 Stn1 proteins; amino acids 163–181 for the S. cerevisiae protein are shown. (B) Predicted structure of the α-helix of the S. cerevisiae protein, with the hydrophobic face indicated in yellow; see Figure S1 for a comparison with the comparable surface of the RPA32 α-helix, as well as an alignment of this region of RPA32. (C) FLAG–Stn1 (pVL2848, or the indicated missense mutations introduced into pVL2848) and Ten1 (pVL3115) were translated in a coupled transcription/translation reaction with 35S-methionine, aliquots were subjected to FLAG-immunoprecipitation, and immunoprecipitates were resolved on a 15% SDS–PAGE gel, as described previously (Gao et al. 2007). (D) Yeast two-hybrid analysis of the interaction between Stn1 (wild-type or mutant versions of pVL859) and either Ten1 (upper; pVL2678) or Cdc13 (lower; pVL3125); dilutions of the S. cerevisiae strain pJ69–4A, coexpressing Gal4AD–Stn1 and either Gal4DBD–Ten1 or Gal4DBD–Cdc13, were plated on appropriate selective plates to monitor viability (not shown) and the ability to activate the GAL2–ADE2 reporter gene. (E) Viability of stn1− missense mutations, expressed by the STN1 native promoter and on a CEN plasmid (wild-type or mutant versions of pVL1492), which were transformed into a stn1-Δ/p STN1 URA3 shuffle strain (YVL2394); serial dilutions were plated on selective plates to monitor total viable cells (not shown) and on 5-FOA-containing plates, to monitor viability following loss of the covering STN1 plasmid.
To determine whether this proposed surface of Stn1 is required for interaction with Ten1, three conserved hydrophobic residues (L164, I168, and W171) were mutated, such that each was changed to either alanine (A), a bulky residue (F or H), a charged residue (D), or a polar residue (S). This panel of 12 mutations was initially examined for effects on interaction with Ten1 using an in vitro coimmunoprecipitation assay and a two-hybrid test. We have previously shown that Stn1 and Ten1 form a stable complex in vitro (Gao et al. 2007) using an assay that monitors radio-labeled Stn1 and Ten1 proteins expressed in reticulocyte extracts, followed by immunoprecipitation of Stn1 (which bears an N-terminal FLAG tag). Notably, 11 of the 12 mutations introduced into the hydrophobic surface of the Stn1 α-helix substantially destabilized the Stn1–Ten1 interaction, as assessed in this in vitro assay (Figure 2C). The only Stn1 mutant protein (Stn1–L164F) that retained a wild-type interaction with Ten1 contained a amino acid change that appears to be well tolerated at this position, on the basis of the sequence of the wild-type S. castellii Stn1 protein at this same position (Figure 2A).
Surprisingly, however, the in vitro binding defects were not recapitulated in the two-hybrid assay (Figure 2D). For residues L164 and I168, three of the four mutant changes still exhibited an interaction with Ten1, as assessed by this two-hybrid test. Thus, only Stn1–L164D and Stn1–I168D failed to interact with Ten1 by both the in vitro and two-hybrid assays. For residue W171, all four mutational changes exhibited a weak two-hybrid interaction (even though each of these Stn1–W171 mutant constructs still interacted with Cdc13 by two hybrid).
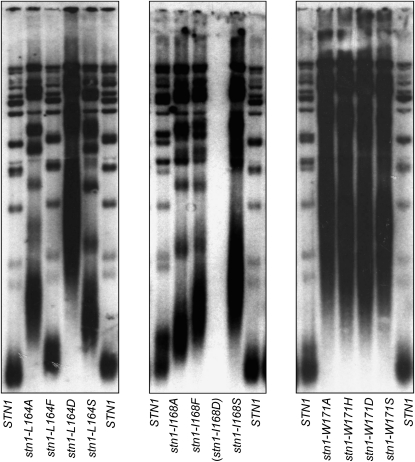
These 12 mutations were also examined for their effects on telomere function in vivo. Each mutation was subcloned into the STN1 gene, expressed by its native promoter, and on a single-copy CEN plasmid, and examined in a stn1-Δ strain for effects on viability (Figure 2E) and telomere length (Figure 3). Notably, the two mutations in residues L164 and I168, which were defective for Ten1 interaction by both assays, also displayed a severe growth defect: stn1–L164D was barely viable, and stn1–I168D was inviable, whereas the remaining mutations in these two residues did not display a noticeable growth defect (Figure 2E). The only mutant strain that was completely wild type for telomere length regulation was stn1–L164F (Figure 3). In contrast, the stn1–L164A, stn1–L164S, stn1–I168A, stn1–I168F, and stn1–I168S strains all exhibited substantially elongated telomeres in vivo (Figure 3), consistent with the inability of these mutant Stn1 proteins to form a stable complex with Ten1 as assessed by in vitro coimmunoprecipitation (Figure 2C). These results underscore the fact that the ability to detect an interaction between two proteins in a two-hybrid assay provides an extremely minimal assessment of protein function.
Figure 3.—
Mutations in the proposed Ten1-interacting domain of Stn1 result in telomere elongation. Telomere length analysis of a panel of stn1− missense mutations introduced into pVL1492, and transformed into the stn1-Δ/p STN1 URA3 shuffle strain (YVL2394); strains were propagated for ∼25 generations following evicting of the covering STN1 plasmid, prior to preparing genomic DNA for analysis of telomere length.
Strains expressing any of the four mutations introduced into residue W171 exhibited extreme telomere elongation phenotypes (Figure 3), as well as a range of growth phenotypes (Figure 2E). Because all four amino acid changes introduced at this location conferred what appeared to be nonspecific effects on Stn1 function, mutations at amino acid W171 were not included in further experiments.
Increased levels of Ten1 suppresses mutations in residues L164 and I168 of Stn1:
As a further test of whether these two residues define an interaction surface between Stn1 and Ten1, mutant strains were examined for whether viability or telomere length could be influenced by overexpression of Ten1. As shown in Figure 4A, the growth defects exhibited by the stn1–L164D and stn1–I168D strains were suppressed by increased expression of Ten1. Furthermore, the severe telomere elongation phenotype of the stn1–L164D strain was restored to almost wild type in the presence of increased levels of Ten1 (Figure 4B). In contrast, the stn1–II68D + ADH–TEN1 strain, although viable, still exhibited elongated telomeres, indicating that this mutation was not fully suppressed by Ten1 overexpression (Figure S2). The telomere elongation phenotypes of the remaining mutations in L164 and I168 were also suppressed by Ten1 overexpression (Figure S2).
Figure 4.—
Ten1 overexpression suppresses stn1–L164D and stn1–I168D. (A) The stn1-Δ shuffle strain (YVL2394), with a control vector (pVL248) or a vector expressing Ten1 by the strong constitutive ADH promoter (pVL3541), as well as pVL1492 (STN1), pVL3573 (stn1–L164D), pVL3577 (stn1–L164D), or YCplac111 (control vector); cultures of single colony isolates of strains expressing the indicated mutations were plated on selective media to monitor total viable cells and on 5-FOA-containing media to monitor viability following loss of the covering STN1 plasmid. (B) Telomere length analysis of the selected strains from A, analyzed after ∼25 generations following loss of the covering STN1 plasmid.
Reciprocal cosuppression identifies a site on Ten1 that interacts with Stn1:
The overexpression suppression data supported the premise that a surface of Stn1, minimally defined by amino acids L164 and I168, mediated interaction with Ten1. To test this directly, we screened for mutations in Ten1 that could restore interaction with Stn1 proteins bearing mutations at either of these two residues, using two hybrid as a screening tool. The details of this screen, which are described in materials and methods, are briefly summarized here. The Gal4DBD–Ten1 plasmid was mutagenized by passage through an E. coli mutator strain and transformed into a two-hybrid strain, to identify mutant Gal4DBD–Ten1 plasmids that interacted with either Gal4AD–Stn1–L164D or Gal4AD–Stn1–I168D. A large number of candidates bearing missense mutations in multiple residues of Ten1 were recovered; the high number of hits in this screen is presumably a reflection of the fact that this two-hybrid assay is sensitive to even minimal levels of interaction between Stn1 and Ten1. We therefore performed a secondary screen to identify Gal4DBD–Ten1 plasmids that were positive in this assay with only one of the two mutant Gal4AD–Stn1 constructs. These criteria led us to two Gal4DBD–Ten1 plasmids, bearing two different missense mutations in the same amino acid (ten1–D138A and ten1–D138Y), which interacted with Gal4AD–Stn1–L164D, but not with Gal4AD–Stn1–LI168D (Figure S3). Unexpectedly, Ten1–D138A and Ten1–D138Y also interacted with wild-type Stn1 in this two-hybrid test (Figure S3), which again may reflect the sensitivity of this assay. However, the allele-specific behavior of the stn1–L164D and stn1–I168D mutations in this two-hybrid test prompted a further analysis of the genetic and biochemical behavior of mutations in residue D138 of Ten1.
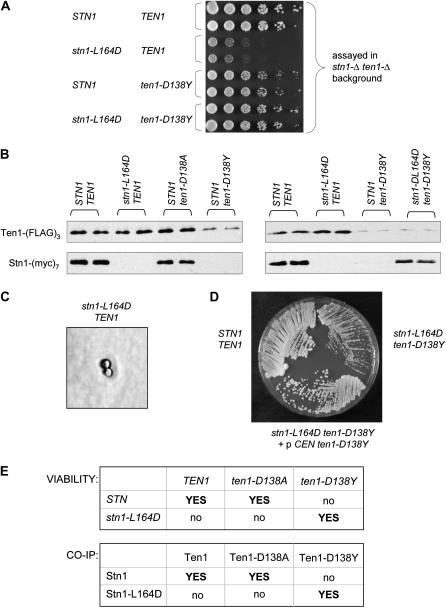
To examine whether ten1–D138A or ten1–D138Y exhibited allele-specific suppression of either stn1–L164D or stn1–I168D, a double shuffle strain was constructed with genomic ten1-Δ and stn1-Δ mutations, which was kept alive by a URA3 plasmid that contained both STN1 and TEN1. Additional plasmids, expressing mutant versions of STN1 or TEN1 (both expressed by their native promoters and on CEN plasmids), were transformed into this strain, and the covering URA3 plasmid was shuffled out by propagation on 5-FOA. Examination of the growth defect of various mutant combinations, using this double-mutant shuffle strain, demonstrated that the ten1–D138A and ten1–D138Y mutations were capable of suppressing the near lethal phenotype of a stn1–L164D strain, but not the inviability of a stn1–I168D strain (Figure 5A, Figure S4, and data not shown). An additional allele of TEN1, ten1–D138L, was also tested for its ability to suppress stn1–L164D; as shown in Figure S4, the stn1–L164D ten1–D138L strain (constructed in the stn1-Δ ten1-Δ shuffle strain) exhibited the same growth defect as the stn1–L164D TEN1 strain.
Figure 5.—
ten1–D138Y and stn1–L164D exhibit reciprocal cosuppression. (A) Viability of stn1-Δ ten1-Δ strains, containing pVL1492 (STN1) or pVL3573 (stn1–L164D) and pVL3779 (TEN1) and pVL3858 (ten1–D138Y), following loss of the covering STN1 TEN1 plasmid. In this experiment, single colony isolates of each strain were identified following propagation on 5-FOA-containing media, grown in liquid culture, and serial dilutions plated on rich media. (B) Western analysis of mutant or wild-type Stn1–(myc)7 and Ten1–(FLAG)3 proteins, following anti-FLAG immunoprecipitation. The Ten1–D138Y–(FLAG)3 protein consistently displayed lower protein levels in immunoprecipitates, suggesting that this protein might be unstable; a longer exposure of the panel on the right is shown in Figure S4. (C) Photo micrograph of a stn1–L164D strain, following dissection of YVL3421, photographed after 3 days growth with a Zeiss Axioskop 50 with a Nikon Digital Sight DS-5M camera. (D) Single colony streak-outs of STN1 TEN1, stn1–L164D ten1–D138Y and stn1–L164D ten1–D138Y/p CEN ten1–D138Y strains, generated by dissection of YVL3422 transformed with pVL3858 (CEN TRP1 ten1–D138Y), propagated on rich media at 30° for 3 days; Figure S5 shows a comparison of these strains at 2 vs. 3 days. (E) A summary of (i) the viability of strains with mutations integrated at their corresponding genomic loci and (ii) co-immunoprecipitation data for the indicated wild-type and mutant proteins.
The in vivo cosuppression data predicts that the Ten1–D138A and Ten1–D138Y proteins should be capable of forming a complex with the Stn1–L164D protein. To test this, extracts were prepared from strains expressing mutant or wild-type Stn1–(myc)7 and Ten1–(FLAG)3 proteins (expressed from a single copy CEN plasmid and by the native STN1 and TEN1 promoters, respectively). These extracts were subjected to immunoprecipitation with anti-FLAG antibodies, and immunoprecipitates (IPs) were examined on anti-myc and anti-FLAG westerns. An interaction between the wild-type Stn1 and Ten1 proteins could be readily detected in IPs (Figure 5B), consistent with the results shown in Figure 2C with reticulocyte-expressed proteins. As expected, no interaction between Stn1–L164D and wild-type Ten1 was observed. The mutant Ten1–D138Y protein was also defective for the ability to associate with the wild-type Stn1 protein (Figure 5B), although a longer exposure suggested a very weak interaction (Figure S4). This is consistent with the observation that a ten1-Δ/p ten1–D138Y strain was viable, although not completely healthy (Figure 5A and data not shown). Strikingly, Stn1–L164D and Ten1–D138Y could be coimmunoprecipitated, indicating that these two mutant proteins could physically interact, even though neither mutant protein was capable of forming a complex with its wild-type partner (Figure 5B). Thus, these genetic and biochemical observations demonstrate that stn1–L164D and ten1–D138Y are reciprocal cosuppressing mutations, indicating that amino acid L164 of Stn1 and D138 of Ten1 are in close proximity in the S. cerevisiae Stn1–Ten1 complex.
In contrast, the Ten1–D138A mutant protein did not behave as expected in these biochemical experiments, as Ten1–D138A could be coimmunoprecipitated with the wild-type Stn1 protein, but not with Stn1–L164D (Figure 5B and data not shown). This was inconsistent with the apparent ability of the ten1–D138A mutation to suppress the stn1–L164D mutation, when assayed in the stn1-Δ ten1-Δ shuffle strain, with the ten1− and stn1− missense mutations introduced as CEN plasmids. However, at several points during this study, certain observations suggested to us that even slight alterations in Ten1 expression levels, presumably due to fluctuations in plasmid copy number, were influencing the ability to suppress stn1− mutations. Therefore, as a more rigorous assessment of potential cosuppression between alleles of TEN1 and STN1, two diploid strains were constructed, as described in materials and methods, with the relevant stn1− and ten1− mutations integrated into the genome. These two strains—stn1–L164D/STN1 ten1–D138A/TEN1 and stn1–L164D/STN1 ten1–D138Y/TEN1—were sporulated, and haploid strains following dissection were analyzed for viability and telomere length regulation. Examination of the phenotypes of stn1− and ten1− strains revealed several differences, when compared with strains in which the comparable missense mutations were present on plasmids over the relevant null mutations. As previously noted, the stn1-Δ/p stn1–L164D strain was viable (although just barely). In contrast, the stn1–L164D strain (with the integrated allele) was inviable: stn1–L164D spores underwent a single cell division following spore germination, arresting as a single large budded cell (Figure 5C). Similarly, the haploid ten1–D138Y mutant strain generated from dissection of the relevant diploid was also inviable, in sharp contrast to the viable (although slightly sick) ten1-Δ/p ten1–D138Y strain. We assume that the differing behavior, when comparing plasmid-borne vs. integrated alleles for both of these mutations, was due to selective pressure driving up the copy number of plasmids expressing the stn1–L164D and ten1–D138Y mutations. In contrast to the inviability of the ten1–D138Y strain, the ten1–D138A strain generated from a diploid strain was viable, although it exhibited a notable growth defect and extremely elongated telomeres (Figure S5). Thus, the in vivo phenotypes of the three haploid mutant strains (generated from these dissections) correlated well with the in vitro coimmunoprecipitation data: the only missense mutation which was viable (ten1–D138A) was also the only mutant protein which formed a complex with the wild-type Stn1 protein (Figure 5B).
Finally, we used these diploid strains to examine whether either ten1–D138A or ten1–D138Y could suppress the stn1–L164D mutation, with all mutations integrated at their corresponding genomic loci. Dissection of the stn1–L164D/STN1 ten1–D138A/TEN1 diploid strain never yielded more than two viable spores, and genotype analysis of the viable spores led to the conclusion that the stn1–L164D ten1–D138A haploid strain was inviable. In contrast, dissection of the stn1–L164D/STN1 ten1–D138Y/TEN1 strain resulted in viable stn1–L164D ten1–D138Y haploid strains. The double mutant stn1–L164D ten1–D138Y strain exhibited a growth defect (Figure 5D) and elongated telomeres (data not shown), indicating that suppression was incomplete. However, this partial cosuppression might be simply a consequence of the lower expression of the Ten1–D138Y protein (Figure 5B). To test this, a stn1–L164D ten1–D138Y strain bearing a single copy CEN ten1–D138Y plasmid (generated by dissection of the parental diploid transformed with this plasmid) was examined. As shown in Figure 5D and Figure S5, a modest increase in ten1–D138Y gene dosage conferred a substantial growth advantage on the stn1–L164D ten1–D138Y strain (although still not quite comparable to a wild-type strain). In contrast, dissection of a stn1–L164D/STN1 ten1–D138Y/TEN1 diploid strain transformed with a CEN TEN1 plasmid failed to give rise to viable haploid stn1–L164D ten1–D138Y strains bearing the wild-type TEN1 plasmid, further supporting the specificity of the interaction between the mutant Stn1–164D and Ten1–D138Y proteins.
Thus, a strain bearing inviable mutations in two genes is nevertheless viable. This demonstrates that stn1–L164D and ten1–D138Y are reciprocal cosuppressing mutations, consistent with the ability of these two mutant proteins to form a complex; the correlations between the biochemical and the genetic data (with mutations integrated at their corresponding genomic loci) are summarized in Figure 5E. Collectively, these observations indicate that amino acid L164 of Stn1 and D138 of Ten1 are in close proximity in the S. cerevisiae Stn1–Ten1 complex, as schematically depicted in Figure 6.
Figure 6.—
The proposed interaction between Stn1 and Ten1. A schematic representation is shown for the S. cerevisiae and S. castellii wild-type proteins, as well as for the double mutant S. cerevisiae stn1–L164D ten1–D138Y proteins. The position of residue L164 of Stn1 is shown on the basis of structure predictions of this region of the S. cerevisiae Stn1 protein (see Figure S1 and Figure S6); in contrast, the structure of the proposed Ten1 α-helix, and the proposed location of D138 on this helix in particular, is hypothetical, due to our inability to generate a reliable structural prediction for the S. cerevisiae Ten1 protein.
DISCUSSION
In this study, a structural prediction for the S. cerevisiae Stn1 protein has been the basis for testing whether Stn1 and Ten1 interact through an interface which is analogous to the interaction between the comparable subunits of the conventional RPA complex. These experiments originated with our previous observation that the OB-fold of Rpa2 could be substituted with the predicted OB-fold of Stn1, resulting in an Rpa2–OBStn1 chimera that was capable of rescuing an rpa2-Δ strain (Gao et al. 2007). This chimera retained a key feature of the Rpa2 OB-fold, which was an α-helix that had been previously proposed to be essential for RPA complex formation (Bochkareva et al. 2002; Deng et al. 2007). The in vivo behavior of two additional Rpa2–OBStn1 chimeras described in this study indicates that whether a given Rpa2–OBStn1 chimera can functionally replace either Rpa2 or Stn1 is determined by two α-helices flanking either side of the β barrel of the OB-fold. This suggests that the proposed trimerization interface of the RPA complex may involve more than just the α-helices flanking the C-terminal side of the OB-fold of each RPA subunit, as portrayed in Figure S1.
These chimera experiments also provided the impetus for a more detailed examination of residues required for the formation of the Stn1–Ten1 subcomplex. Mutagenesis of a hydrophobic surface of the proposed Stn1 α-helix resulted in an inability to bind Ten1, although the only mutations that resulted in lethality (and thus would be amenable to a search for suppressing mutations in TEN1) were due to introduction of aspartic acid at amino acids L164 or I168. Introducing a charged residue into a hydrophobic surface would normally be expected to disrupt protein structure in a nonspecific manner. Nevertheless, we successfully recovered a mutation in TEN1 (ten1–D138Y) which was capable of suppressing stn1–L164D. Thus, as depicted schematically in Figure 6, residue 164 of Stn1 can be either L (in S. cerevisiae) or F (in S. castellii), paired with residue D138 of Ten1, in the wild-type situation. The reciprocal suppression data showed that this interaction could still be maintained if residue 164 of Stn1 was replaced with D and residue 138 of Ten1 with Y. These observations are consistent with the prediction that the S. cerevisiae Stn1 and Ten1 proteins interact through α-helices that flank the C terminus of the OB-fold found in each protein, as portrayed in Figure 6.
The completion of this current study coincided with the publication of the structures for the N-terminal Stn1 domain complexed with Ten1, from S. pombe and C. tropicalis (Sun et al. 2009). Therefore, this provided an opportunity to evaluate the validity of the structural prediction used in our experiments, as well as the resulting cosuppression data. As shown in Figure S6, a comparison of the S. cerevisiae Stn1 prediction with the actual structure of the C. tropicalis Stn1 protein revealed a very similar overall topology, with regard to both the OB-fold and the position of the α-helix that was the focus of our experiments (see the legend of Figure S6 for further discussion). The structural characterization of the S. pombe and C. tropicalis Stn1–Ten1 subcomplexes by Lei and colleagues has also demonstrated that the Stn1–Ten1 interface is mediated by contacts between α-helices located C terminal to OB-folds present in both proteins (Sun et al. 2009). The S. cerevisiae Stn1–Ten1 interface presumably relies on a similar structural architecture, although we have been unable to generate a reliable prediction for the structure of the S. cerevisiae Ten1 protein (E. K. Mandell, unpublished observations). Thus, while structural predictions place amino acid L164 on the α-helix of Stn1, we are unable to similarly determine the position of Ten1–D138 (although the location of the ten1–D138Y mutation in the 160-amino-acid Ten1 protein is at least consistent with the premise that D138 is in/near the predicted Ten1 α-helix).
In vivo phenotypes of stn1− and ten1− mutations are exquisitely dosage sensitive:
Several results presented in this current study suggest that prior observations about the requirements for interactions between Cdc13, Stn1, and Ten1 may need to be reevaluated. In particular, the comparison between biochemical assessments of the ability of mutant Stn1 and Ten1 proteins to interact vs. the behavior of these same proteins in two-hybrid assays indicates that the threshold for a positive result in a Stn1–Ten1 two-hybrid test is very low. For example, Ten1–D138A and Ten1–D138Y cannot be distinguished from each other on the basis of a two-hybrid test; however, these two proteins exhibit strikingly different abilities to interact with Stn1+ vs. Stn1–L164D when assessed by both co-immunoprecipitation and in vivo criteria. This caveat potentially extrapolates to the ability of Stn1 (including truncation alleles of Stn1) to interact with Cdc13 in a two-hybrid test. In such an assay, Cdc13 interacts with the C terminus of Stn1, which is dispensable for viability, but not with the N terminus of Stn1, an observation which has been used to argue that Cdc13 is dispensable for the essential capping function performed by this protein complex (Petreaca et al. 2007; Puglisi et al. 2008). However, if this Stn1–Cdc13 two-hybrid test is similarly assessing only a minimal level of protein function, this leaves open the question of whether the determinant(s) that dictates the interaction between Stn1 and Cdc13 has been adequately analyzed.
The in vivo phenotype of strains expressing certain stn1− and ten1− mutations also appears to be strikingly sensitive, with differences in viability observed even when a given allele is present on a single copy CEN vector over the respective null mutation vs. integrated into the genome. This suggests that the selective pressure for viability may alter expression levels of plasmid-borne alleles, potentially by driving up plasmid copy number. Thus, conclusions about the biological role of Stn1 or Ten1, on the basis of the viability of particular mutant alleles, particularly alleles that are overexpressed, should be viewed with some caution (Puglisi et al. 2008).
Further perspectives:
Elucidation of in vivo function through the analysis of phenotypes following mutagenesis has been described as “blindly smashing many cars with a hammer and then determining which broken parts matter by attempting to drive each machine” (Endy 2008). This potentially turns into a demolition derby, if randomly generated mutations become the starting point for the identification of reciprocal cosuppressing mutations in prospective binding partners. Although a fortuitous mutation in CDC13, cdc13-2, led to a reciprocal mutation in EST1 (Pennock et al. 2001), such successes are rare. In contrast, reliance on a structure prediction for Stn1 rapidly directed our attention to a limited set of mutations (stn1–L164D and stn1–I168D) as the basis for a search for reciprocal mutations in TEN1. This suggests that structure-based genetics can be an experimentally tractable approach for probing predicted protein–protein interactions through cosuppressing mutations.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.111922/DC1.
References
- Anderson, E. M., W. A. Halsey and D. S. Wuttke, 2002. Delineation of the high-affinity single-stranded telomeric DNA-binding domain of Saccharomyces cerevisiae Cdc13. Nucleic Acids Res. 30 4305–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuch, A. A., and V. Lundblad, 2003. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol. Cell. Biol. 23 8202–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, A., S. Negrini and D. Shore, 2004. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol. Cell 16 139–146. [DOI] [PubMed] [Google Scholar]
- Bochkarev, A., E. Bochkareva, L. Frappier and A. M. Edwards, 1999. The crystal structure of the complex of replication protein A subunits RPA32 and RPA14 reveals a mechanism for single-stranded DNA binding. EMBO J. 18 4498–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkareva, E., S. Korolev, S. P. Lees-Miller and A. Bochkarev, 2002. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 21 1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti, D., M. Martina, M. Clerici, G. Lucchini and M. P. Longhese, 2009. Multiple pathways regulate 3′ overhang generation at S. cerevisiae telomeres. Mol. Cell 35 70–81. [DOI] [PubMed] [Google Scholar]
- Chandra, A., T. R. Hughes, C. I. Nugent and V. Lundblad, 2001. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X., J. E. Habel, V. Kabaleeswaran, E. H. Snell, M. S. Wold et al., 2007. Structure of the full-length human RPA14/32 complex gives insights into the mechanism of DNA binding and complex formation. J. Mol. Biol. 374 865–876. [DOI] [PubMed] [Google Scholar]
- Endy, D., 2008. Genomics. Reconstruction of the genomes. Science 319 1196–1197. [DOI] [PubMed] [Google Scholar]
- Evans, S. K., and V. Lundblad, 2002. The Est1 subunit of Saccharomyces cerevisiae telomerase makes multiple contributions to telomere length maintenance. Genetics 162 1101–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, C. J., M. Hyde and C. W. Greider, 2006. Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol. Cell 24 423–432. [DOI] [PubMed] [Google Scholar]
- Gao, H., R. B. Cervantes, E. K. Mandell, J. H. Otero and V. Lundblad, 2007. RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 14 208–214. [DOI] [PubMed] [Google Scholar]
- Garvik, B., M. Carson and L. Hartwell, 1995. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 15 6128–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas, A. D., M. Paschini, F. E. Reyes, A. Heroux, R. T. Batey et al., 2009. Telomere capping proteins are structurally related to RPA with an additional telomere-specific domain. Proc. Natl. Acad. Sci. USA 106 19298–19303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin, N., S. I. Reed and M. Charbonneau, 1997. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11 512–527. [DOI] [PubMed] [Google Scholar]
- Grandin, N., C. Damon and M. Charbonneau, 2001. Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 20 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira, G., A. Pellicioli, A. Balijja, X. Wang, S. Fiorani et al., 2004. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., J. Halladay and E. A. Craig, 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus, K., S. Katzman, G. Shackleford, M. Koeva, J. Draper et al., 2005. SAM-T04: what is new in protein-structure prediction for CASP6. Proteins 61(Suppl 7): 135–142. [DOI] [PubMed] [Google Scholar]
- Lendvay, T. S., D. K. Morris, J. Sah, B. Balasubramanian and V. Lundblad, 1996. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics 144 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydall, D., and T. Weinert, 1995. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science 270 1488–1491. [DOI] [PubMed] [Google Scholar]
- Martin, V., L. L. Du, S. Rozenzhak and P. Russell, 2007. Protection of telomeres by a conserved Stn1-Ten1 complex. Proc. Natl. Acad. Sci. USA 104 14038–14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou, E. P., and L. S. Symington, 2008. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent, C. I., T. R. Hughes, N. F. Lue and V. Lundblad, 1996. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274 249–252. [DOI] [PubMed] [Google Scholar]
- Pennock, E., K. Buckley and V. Lundblad, 2001. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104 387–396. [DOI] [PubMed] [Google Scholar]
- Petreaca, R. C., H. C. Chiu and C. I. Nugent, 2007. The role of Stn1p in Saccharomyces cerevisiae telomere capping can be separated from its interaction with Cdc13p. Genetics 177 1459–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi, A., A. Bianchi, L. Lemmens, P. Damay and D. Shore, 2008. Distinct roles for yeast Stn1 in telomere capping and telomerase inhibition. EMBO J. 27 2328–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söding, J., A. Biegert and A. N. Lupas, 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33 W244–W248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J., E. Y. Yu, Y. Yang, L. A. Confer, S. H. Sun et al., 2009. Stn1-Ten1 is an Rpa2-Rpa3-like complex at telomeres. Genes Dev. 23 2900–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodenicharov, M. D., and R. J. Wellinger, 2006. DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (CDC28/Clb) cell cycle kinase. Mol. Cell 24 127–137. [DOI] [PubMed] [Google Scholar]
- Weinert, T. A., and L. H. Hartwell, 1993. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics 134 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold, M. S., 1997. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Ann. Rev. Biochem. 66 61–92. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., and J. Skolnick, 2005. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33 2302–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Z., W. H. Chung, E. Y. Shim, S. E. Lee and G. Ira, 2008. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]