Abstract
Prolonged exposure of quiescent Swiss 3T3 cells to vasopressin prevents mitogenic stimulation on subsequent addition of bombesin. This heterologous desensitization is selective and can be mimicked by vasopressin agonists, including [Lys8]vasopressin and oxytocin but not by the V1-type-specific vasopressin receptor antagonist [Pmp1,O-Me-Tyr2,Arg8]vasopressin [where Pmp is 1-(beta-mercapto-beta,beta-cyclopenthamethylene propionic acid)]. Furthermore, vasopressin-induced loss of responsiveness to bombesin can be blocked by addition of this antagonist, indicating that heterologous desensitization is mediated through the vasopressin receptor. Desensitization requires prolonged incubation (half-maximal desensitization occurring after approximately 20 hr of pretreatment) and continuous protein synthesis. Bombesin responsiveness is restored by incubation in the absence of vasopressin. Pretreatment does not alter the number, affinity, or internalization capacity of the bombesin receptors. However, the induction of the protooncogene c-fos by bombesin is profoundly inhibited after vasopressin pretreatment. We suggest that the coupling of the activated bombesin receptor to the generation of its early signals is impaired in desensitized cells.
Full text
PDF

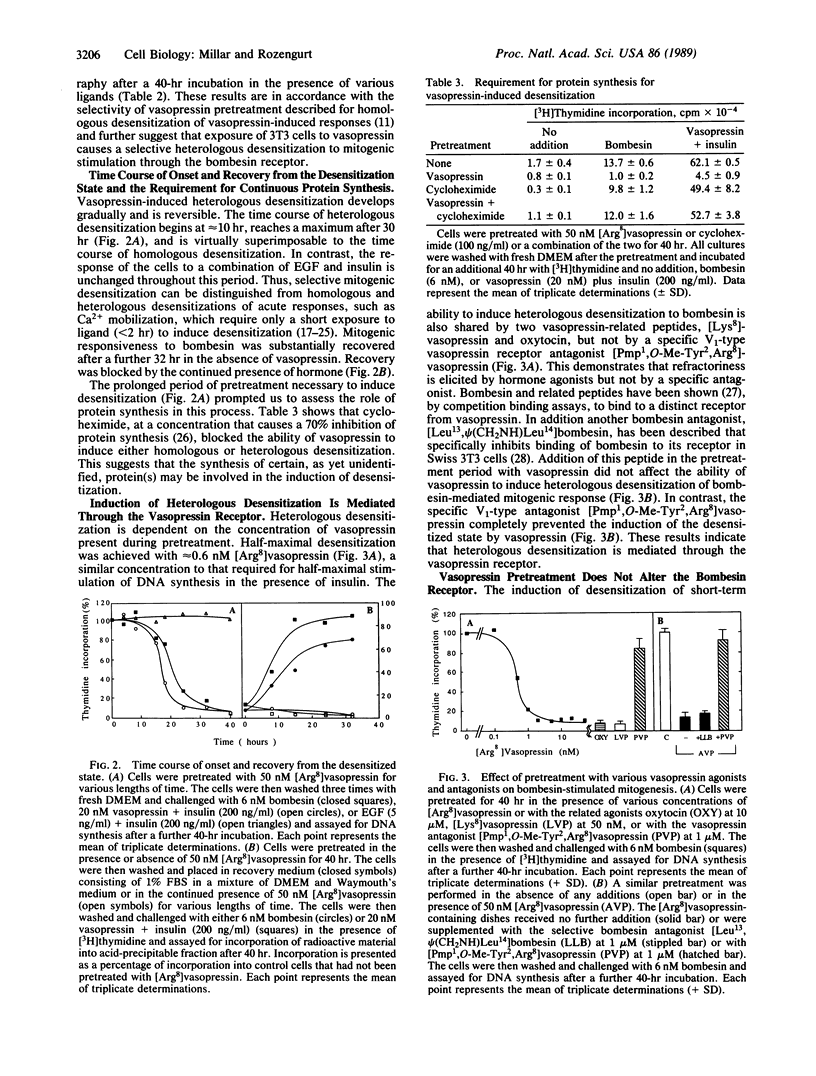
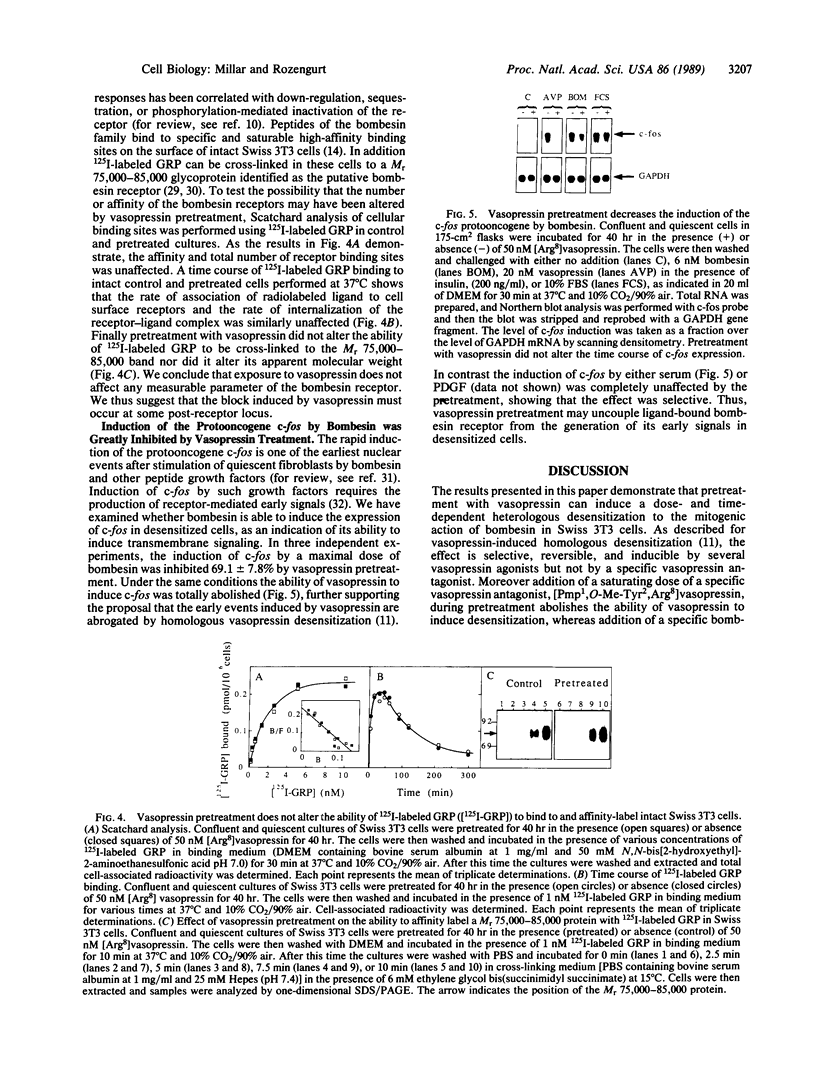

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantau B., Guillon G., Alaoui M. F., Chicot D., Balestre M. N., Devilliers G. Evidence of two steps in the homologous desensitization of vasopressin-sensitive phospholipase C in WRK1 cells. Uncoupling and loss of vasopressin receptors. J Biol Chem. 1988 Jul 25;263(21):10443–10450. [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Vasopressin induces selective desensitization of its mitogenic response in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1924–1928. doi: 10.1073/pnas.80.7.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Coll K. E., Williamson J. R. Differential effects of phorbol ester on phenylephrine and vasopressin-induced Ca2+ mobilization in isolated hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3281–3288. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Morris J. D., Moore J. P., Metcalfe J. C. Ca2+ and pH responses to sequential additions of mitogens in single 3T3 fibroblasts: correlations with DNA synthesis. J Biol Chem. 1988 Aug 25;263(24):11879–11886. [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Adelberg E. A., Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Talamo B. R. Rapid desensitization of substance P- but not carbachol-induced increases in inositol trisphosphate and intracellular Ca++ in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1017–1024. doi: 10.1016/s0006-291x(87)80233-4. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Phorbol esters and diacylglycerol inhibit vasopressin-induced increases in cytoplasmic-free Ca2+ and 45Ca2+ efflux in Swiss 3T3 cells. Exp Cell Res. 1986 Jun;164(2):536–545. doi: 10.1016/0014-4827(86)90051-0. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Mufson R. A. Phorbol ester inhibition of the hormone-stimulated phosphoinositide cycle in WRK-1 cells. Biochem J. 1986 May 15;236(1):171–175. doi: 10.1042/bj2360171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Paris S., Magnaldo I., Pouysségur J. Homologous desensitization of thrombin-induced phosphoinositide breakdown in hamster lung fibroblasts. J Biol Chem. 1988 Aug 15;263(23):11250–11256. [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. W. Bombesin induction of c-fos and c-myc proto-oncogenes in Swiss 3T3 cells: significance for the mitogenic response. J Cell Physiol. 1987 May;131(2):218–225. doi: 10.1002/jcp.1041310211. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Early signals underlying the induction of the c-fos and c-myc genes in quiescent fibroblasts: studies with bombesin and other growth factors. Prog Nucleic Acid Res Mol Biol. 1988;35:261–295. doi: 10.1016/s0079-6603(08)60616-9. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J., Zachary I., Rozengurt E. Characterization of a bombesin receptor on Swiss mouse 3T3 cells by affinity cross-linking. J Cell Biochem. 1988 Dec;38(4):237–249. doi: 10.1002/jcb.240380403. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Stabel S., Rodriguez-Pena A., Young S., Rozengurt E., Parker P. J. Quantitation of protein kinase C by immunoblot--expression in different cell lines and response to phorbol esters. J Cell Physiol. 1987 Jan;130(1):111–117. doi: 10.1002/jcp.1041300116. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Graham W. R. The fos oncogene. Adv Cancer Res. 1987;49:29–52. doi: 10.1016/s0065-230x(08)60793-9. [DOI] [PubMed] [Google Scholar]
- Woll P. J., Coy D. H., Rozengurt E. [Leu13-psi(CH2NH)Leu14]bombesin is a specific bombesin receptor antagonist in Swiss 3T3 cells. Biochem Biophys Res Commun. 1988 Aug 30;155(1):359–365. doi: 10.1016/s0006-291x(88)81093-3. [DOI] [PubMed] [Google Scholar]
- Woll P. J., Rozengurt E. [D-Arg1,D-Phe5,D-Trp7,9,Leu11]substance P, a potent bombesin antagonist in murine Swiss 3T3 cells, inhibits the growth of human small cell lung cancer cells in vitro. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1859–1863. doi: 10.1073/pnas.85.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. A substance P antagonist also inhibits specific binding and mitogenic effects of vasopressin and bombesin-related peptides in Swiss 3T3 cells. Biochem Biophys Res Commun. 1986 May 29;137(1):135–141. doi: 10.1016/0006-291x(86)91186-1. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. High-affinity receptors for peptides of the bombesin family in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7616–7620. doi: 10.1073/pnas.82.22.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Identification of a receptor for peptides of the bombesin family in Swiss 3T3 cells by affinity cross-linking. J Biol Chem. 1987 Mar 25;262(9):3947–3950. [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Internalization and degradation of peptides of the bombesin family in Swiss 3T3 cells occurs without ligand-induced receptor down-regulation. EMBO J. 1987 Aug;6(8):2233–2239. doi: 10.1002/j.1460-2075.1987.tb02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Kozma M., Sinnett-Smith J., Rozengurt E. Vasoactive intestinal peptide synergistically stimulates DNA synthesis in mouse 3T3 cells: role of cAMP, Ca2+, and protein kinase C. Exp Cell Res. 1988 May;176(1):155–161. doi: 10.1016/0014-4827(88)90129-2. [DOI] [PubMed] [Google Scholar]




