Abstract
Endoreplication, also known as endoreduplication, is a phyogenetically widespread modified version of the cell cycle in which DNA replication is not followed by cell division. The SIAMESE (SIM) gene of Arabidopsis thaliana encodes the founding member of a novel class of plant-specific cyclin-dependent kinase (CDK) inhibitors and is a key regulator of endoreplication during the development of trichomes (shoot epidermal hairs). Here, we have identified mutations in the CCS52A1 gene as genetic modifiers of the multicellular trichome phenotype of sim mutants. Loss-of-function ccs52A1 mutations dramatically enhance the multicellularity of sim mutants trichomes in double mutants, whereas overexpression of CCS52A1 completely suppresses the sim mutant phenotype. CCS52A1 encodes a CDH1/FZR-like protein, a class of proteins that function as activators of the anaphase-promoting complex. Unicellular ccs52A1 trichomes become multicellular upon overexpression of B-type cyclin, consistent with repression of the accumulation of mitotic cyclins in the developing trichome by CCS52A1. As these M-phase-specific cyclins are known to accumulate in sim mutant trichomes, our data suggest that CCS52A1 and SIM cooperate in repressing accumulation of mitotic cyclins to establish the trichome endocycle. Comparison with endoreplication pathways in Drosophila and mammals indicates that while these organisms all use similar components to initiate endoreplication, the components are deployed differently in each organism.
IN multicellular eukaryotes, development requires close coordination of cell division, cell growth, and cell differentiation to produce the organs and tissues of the mature individual. Because plants are sessile and can respond to environmental challenges only by regulation of growth, these processes must be tightly, yet flexibly, regulated. An important aspect of this coordination is achieved via control of the key cell-cycle regulatory proteins, cyclin-dependent kinases (CDKs), and their respective cyclin (CYC) partners by plant hormones, environmental stimuli, and nutritional signals (Dewitte and Murray 2003; Ramirez-Parra et al. 2005). In the archetypal model of the cell cycle, DNA replication is followed by mitosis and cytokinesis, and the replicated DNA is shared equally between the two daughter cells. As a result, the DNA content of cells oscillates between 2C and 4C, with C representing DNA content of the haploid genome. Often, however, the cell cycle is modified to meet the needs of specific developmental contexts. One common developmental variant of the cell cycle is called endoreplication or endoreduplication, in which the cell undergoes several rounds of DNA replication without mitosis or cytokinesis, resulting in a cell with DNA content greater than 4C (Edgar and Orr-Weaver 2001; Larkins et al. 2001).
Endoreplication is developmentally regulated and occurs in specific stages and tissues in both plants and animals. In animals, endoreplication occurs in Drosophila larval tissues, mammalian megakaryocytes, and placental trophoblasts, among other instances (Edgar and Orr-Weaver 2001). The nuclear DNA content of endoreplicated cells is often, although not always, correlated with cell size (Melaragno et al. 1993), and endoreplication may occur when natural selection favors production of large cells. In spite of the common occurrence of this altered cell-cycle variant in plants and its role in economically important processes, such as fruit development (Kowles and Phillips 1985; Joubes et al. 1999; Cheniclet et al. 2005) and nitrogen fixation (Vinardell et al. 2003), the control of the switch from mitotic cycles to endoreplication remains poorly understood.
In animals, the study of endoreplication is most advanced in Drosophila (Lilly and Duronio 2005). Endoreplication requires downregulation of mitotic A-type and B-type cyclins, as well as the Drosophila CDC25 homolog String and other factors that promote mitosis, coupled with the upregulation of CDH1/FZR. CDH1/FZR is an activator of the anaphase-promoting complex/cyclosome (APC/C activator), a ubiquitin E3 ligase that targets mitotic cyclins for degradation by the 26S proteasome. Repeated rounds of DNA replication are driven by cycling of CYCE/CDK kinase activity mediated by the CDK inhibitor Dacapo, which enforces low CDK activity between rounds of replication during endocycles to promote replication licensing (Hong et al. 2007). As in Drosophila, endoreplication in mammalian trophoblasts requires expression of a CDK inhibitor, p57KIP, and CDH1/FZR, but surprisingly does not require downregulation of mitotic cyclins (Garcia-Higuera et al. 2008; Ullah et al. 2008).
In many respects, the control of endoreplication in plant cells resembles that in animals. Plants lack the CDC25 phosphatase (Boudolf et al. 2006; Dissmeyer et al. 2009), but employ both CDH1/FZR-like APC/C activators and CDK inhibitors in the control of their endocycle (reviewed in Inzé and De Veylder 2006). Plants have two types of CDH1/FZR proteins, termed CCS52A and CCS52B (Tarayre et al. 2004). On the basis of time of expression during the cell cycle, the CCS52A proteins seem likely to be the most similar in function to CDH1/FZR proteins (Fülop et al. 2005). Arabidopsis has two CCS52A homologs, CCS52A1 and CCS52A2, and one CCS52B homolog. Expression of the plant CDH1 homolog CSS52 in Medicago sativa is correlated with both the degree of endoreplication and with endoreplication onset, and endoreplication levels are decreased by expression of an antisense CDH1/CCS52 construct (Vinardell et al. 2003). CDK inhibitors of the ICK/KRP class, which are distantly related to the mammalian p57KIP and Drosophila Dacapo proteins, can also affect endoreplication in a dose-dependant fashion when overexpressed, blocking mitosis, and promoting endoreplication at moderate levels and inhibiting both mitosis and endoreplication at high levels (Verkest et al. 2005b; Weinl et al. 2005). However, these proteins have not yet been shown to play a role in naturally occurring endoreplication.
Arabidopsis trichomes, or shoot epidermal hairs, are a model for the study of endoreplication and other aspects of plant cell differentiation (Larkin et al. 2003, 2007). These trichomes are large branched single cells that protrude from the epidermis and have a DNA content of 16C to 32C (Melaragno et al. 1993; Hülskamp et al. 1994). One key regulator of the transition from mitosis to endoreplication in plants, the Arabidopsis SIAMESE (SIM) gene, was originally identified by mutants that produce multicellular trichomes that undergo mitosis instead of endoreplication (Walker et al. 2000). Overexpression of SIM results in large, highly endoreplicated cells in leaves, and the gene encodes a protein that binds to CDKA and D-type cyclins (CYCDs) and defines a family of plant-specific CDK inhibitors distinct from the ICK/KRP family (Churchman et al. 2006; Peres et al. 2007). The sim loss-of-function mutations result in misexpression of mitotic B-type cyclins (CYCBs) in developing trichomes, suggesting that SIM acts upstream of CYCB transcript accumulation to prevent mitosis and promote endoreplication in developing trichomes (Schnittger et al. 2002; Churchman et al. 2006). To date, SIM is the only plant protein known to function primarily to regulate the transition to the endocycle.
In this work, a screen for genetic modifiers of the sim-1 mutant trichome phenotype, we have identified loss-of-function mutations in the gene encoding the CDH1-like protein CCS52A1 that dramatically enhance the multicellular trichome phenotype resulting from mutations in sim. Conversely, overexpression of CCS52A1 completely suppresses the sim mutant phenotype. These results, as well as interactions between ccs52a1 and B-type cyclin overexpressing constructs, suggest that CCS52A1 and SIM play complementary roles in establishing the endocycle.
MATERIALS AND METHODS
Plant material and growth conditions:
Arabidopsis thaliana genotype Col-0 was used as the wild-type control. The sim-1 mutation has been previously described (Churchman et al. 2006). The T-DNA insertion lines SALK_083656 and SALK_101689 were obtained from the Arabidopsis Biological Resource Center, and the locations of the inserts were confirmed by sequencing of T-DNA-genomic border fragments recovered by PCR. These T-DNA lines have been characterized previously and shown to express little or no full-length transcript of the gene AT4G22910 (Lammens et al. 2008; Larson-Rabin et al. 2009; Vanstraelen et al. 2009). Transgenic lines overexpressing the cyclins CYCB1;1, CYCB1;2 from the GL2 promoter (proGL2) were provided by Dr. Arp Schnittger (University of Strasbourg, Strasbourg, France; Schnittger et al. 2002; Schnittger et al. 2005). Plants were grown as previously described (Larkin et al. 1999).
Isolation of mutants:
Homozygous sim-1 seeds were mutagenized with ethyl methanesulfonate (EMS) and collected as 37 pools of 300 M1 plants each for a total of 11,100 M1 plants. Approximately 2000 M2 plants per pool were screened for enhancement of the multicellular or clustered trichome phenotype of the sim-1 mutant. Putative supressors were also obtained in the initial screening, but none were genetically well behaved in subsequent generations. Mutants with similar phenotypes were considered independent only if isolated from different pools. Putative mutants were backcrossed three times to the sim-1 mutant and allowed to self. These double mutants with the enhanced phenotype were crossed to Col-0 to generate an F2 population segregating for both sim-1 and the new mutation. In this segregating population, plants with a new phenotype, unicellular trichomes with reduced branching, were observed for each independent mutant. This new trait was confirmed to be recessive and monogenic. Pairwise crosses among the independently isolated mutants demonstrated allelism. Isolated single mutants were then crossed to sim-1 to reconstruct the double-mutant phenotype (enhanced multicellularity), confirming that the enhanced sim phenotype resulted from the same mutation that caused the reduced trichome branching phenotype as a single mutant.
Genetic mapping and identification of the gene:
Bulked segregant analysis (Lukowitz et al. 2000) was used to map the ccs52a1-3 mutation to a region on chromosome VI near the molecular marker ciw7 on BAC clone F18E5, based on the reduced trichome branching phenotype. Genomic DNA samples of 883 mutant plants from an F2 population of a cross between the single mutant (Col background) and Landsberg erecta (Ler) were tested by PCR with molecular markers generated using the Cereon database of Columbia (Col)–Ler polymorphisms (http://www.arabidopsis.org/browse/Cereon/index.jsp). The mutation was localized to a region of ∼140 kb between two cleaved amplified polymorphic sequences (CAPs) markers (supporting information, Table S1), F7H19-3a and T12H17-2 that contained portions of two overlapping BAC clones, F7H19 and T12H17. The mutation in this allele (ccs52a1-3) was identified by sequencing the putative candidate gene AT4G22910, and the alleles ccs52a1-4, ccs52a1-5, ccs52a1-6, ccs52a1-7, and ccs52a1-8 were subsequently shown to contain mutations in this same gene.
Generation of transgenic lines and growth conditions:
For molecular complementation of the ccs52a1-3 mutation, the genomic coding sequence of AT4G22910 (CCS52A1) as well as 1607-bp upstream sequence and 617-bp downstream sequence was PCR amplified from the BAC clone F7H19 using primers ENS2-1CF and ENS2-1CR3 (Table S1). This fragment was inserted into the GATEWAY entry vector pENTR D-TOPO (Invitrogen, Carlsbad, CA), followed by recombination into the promoterless GATEWAY destination binary vector pMDC100 (Curtis and Grossniklaus 2003), to create pMDC100/ENS2C-5.2, which was used to transform ccs52a1-3 plants.
The full-length coding sequence of CCS52A1 was PCR amplified from the BAC clone F7H19 using primers ENS2-1F and ENS2-1R1 (Table S1), inserted into pENTR D-TOPO, and recombined into the GATEWAY-compatible destination vector pLEELA-pGL2 (a gift of A. Schnittger, University of Strasbourg, Strasbourg, France) for expression from the GLABRA2 promoter (proGL2). A previously described pDONR221/CCS52A2 coding-region construct (Lammens et al. 2008) was used for recombination of the CCS52A2 coding region into pLEELA-pGL2.
All PCR-generated constructs were confirmed by sequencing. The resulting plasmids were introduced into Agrobacterium tumefaciens either by electroporation or by the freeze–thaw method (Weigel and Glazebrook 2002) and then into plants via floral dip method (Clough and Bent 1998), selected on medium containing the relevant antibiotics, and transferred to soil.
Characterization of phenotypes:
Trichomes were examined by scanning electron microscopy (SEM) as previously described (Larkin et al. 1999). Trichome branch numbers were counted using the SEM for all trichomes on the adaxial side of the first or second leaf from at least 10 plants of each genotype. The number of cells per trichome initiation site (TIS) was determined by fluorescence microscopy of 4′,6-diamidino-2-phenylindole (DAPI)-stained leaves (Walker et al. 2000).
Nuclear DNA measurements:
In situ measurement of trichome nuclear DNA contents were measured and normalized as previously described (Brininstool et al. 2008). Flow cytometric analysis of nuclear DNA contents was performed as previously described (Verkest et al. 2005a). Statistical analysis was performed using SigmaStat software (Systat software Inc., San Jose, CA). Nuclear DNA contents were compared using the nonparametric Kruskal–Wallis One Way ANOVA test.
RESULTS
Isolation of phenotypic enhancers of sim:
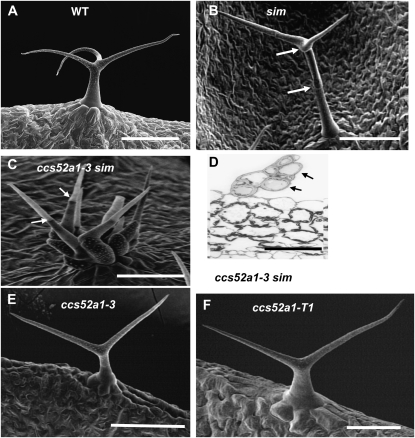
Wild-type trichomes are unicellular and typically have three or four branches (Figure 1A). The sim-1 mutation is a loss-of-function mutation resulting in multicellular and/or clustered trichomes (Figure 1B). In a screen for modifiers of the sim-1 mutant trichome phenotype, we isolated recessive mutants that dramatically enhanced the multicellular trichome phenotype of sim-1. Six of these mutations were allelic, defining a single gene (Figure 1C). In homozygous double mutants of sim and this enhancer, the number of nuclei per TIS (a site containing one cell or several contiguous cells phenotypically recognizable as trichome cells) was increased nearly fivefold relative to the sim-1 single mutant (Figure 1D; Table 1). Putative homozygous single mutants of each of the six alleles separated from sim-1 exhibited unicellular trichomes with reduced branching, producing predominantly two-branched trichomes on their leaves (Figure 1, E and F; Table 2). Crossing these putative single mutants back to sim-1 reconstructed the enhanced multicellular phenotype in the F2, confirming that the mutations causing reduced trichome branching were identical to the mutations enhancing the sim-1 phenotype.
Figure 1.—
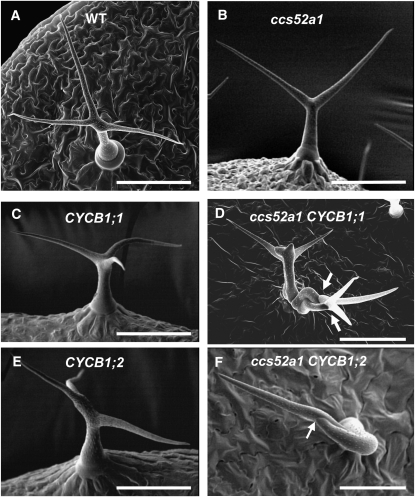
Identification of a genetic enhancer of the sim multicellular phenotype. (A) Scanning electron micrograph (SEM) of wild-type unicellular Col-0 trichome. (B) SEM of a sim-1 mutant trichome consisting of three cells. Arrows indicate cell junctions. (C) SEM of a ccs52a1-3 sim-1 double-mutant trichome initiation site (TIS). All cells (at least 11) extending from the epidermis would be considered part of the same TIS. Arrows indicate two cell junctions. (D) Cross section through a ccs52a1-3 sim-1 TIS. Arrows point to two of the eight cells visible. (E) SEM of a ccs52a1-3 single-mutant trichome. (F) SEM of a ccs52a1-T1 T-DNA insertion mutant trichome. Scale bars in A, B, C, and E are 100 μm; in D and F, they are 50 μm.
TABLE 1.
Mutations in ccs52a1 enhance the multicellularity of sim mutant trichomes
| Genotype | Nuclei per TISa | Total TIS countedb |
|---|---|---|
| Col | 1.0 ± 0.0 | 55 |
| ccs52a1-3 | 1.0 ± 0.0 | 50 |
| sim-1 | 2.5 ± 1.3 | 50 |
| ccs52a1-3 sim-1 | 12.0 ± 3.8 | 52 |
| ccs52a1-4 sim-1 | 11.9 ± 3.7 | 50 |
The number of DAPI-stained nuclei per TIS. Data shown as mean ± SD.
TIS were counted on fully expanded first or second leaves.
TABLE 2.
Expression of either CCS52A1 or CCS52A2 from the GL2 promoter increases trichome branching
| No. of branch points (% of total)a |
|||||||
|---|---|---|---|---|---|---|---|
| Genotype | 0 | 1 | 2 | 3 | 4 | 5 | # of trichomes |
| Col | 0.0 | 0.0 | 87.0 | 12.8 | 0.3 | 0.0 | 392 |
| ccs52a1-3 | 0.0 | 78.1 | 21.9 | 0.0 | 0.0 | 0.0 | 343 |
| ccs52a1-3 proGL2:CCS52A1 | 0.0 | 0.0 | 30.0 | 34.4 | 29.4 | 6.1 | 180 |
| ccs52a1-3 proGL2:CCS52A2 | 0.0 | 0.0 | 32.5 | 37.2 | 17.7 | 12.6 | 317 |
| proGL2:CCS52A1 | 0.0 | 0.0 | 16.7 | 39.3 | 27.6 | 16.3 | 257 |
| proGL2:CCS52A2 | 0.0 | 0.0 | 13.7 | 32.3 | 33.0 | 21.6 | 317 |
Unbranched trichomes have zero branch points, two-branched trichomes have one branch point, etc. Counts were made on fully expanded first or second leaves. For transgenics, >10 lines with a similar increased branching phenotype were obtained. These data are based on counts from a single representative line.
A phenotypic enhancer of sim encodes a CDH1-like activator of the APC/C:
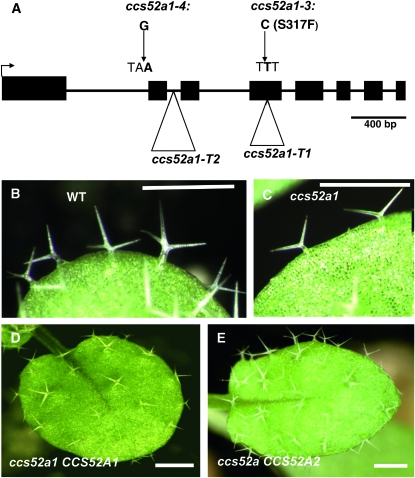
One allele was mapped to a region of ∼114 kb on chromosome IV. The most promising candidate gene in the region was AT4G22910, chosen because it encodes the CDH/FZR-like protein CCS52A1. Sequencing revealed a C to T point mutation (TCT to TTT) in exon 4 that changes a conserved serine to phenylalanine at codon 316 in the allele used for mapping (Figure 2A), which was designated ccs52a1-3. Another allele, designated ccs52a1-4, has a G to A point mutation (AG to AA) at the splice acceptor site of the first intron of AT4G22910 (Figure 2A). The remaining four alleles, although presumed to be isolated independently on the basis of the design of our screen, contained the same mutation as ccs52a1-4 and will not be considered further. Two T-DNA insertion lines in AT4G22910 were also available, one with an insertion in the fourth exon and the other with an insertion in the second intron (Figure 2A, ccs52a1-T1 and ccs52a1-T2, respectively). Recently, it was shown that neither T-DNA insertion expresses full-length transcripts of AT4G22910 (Lammens et al. 2008; Larson-Rabin et al. 2009). Plants homozygous for either of these T-DNA alleles have trichome phenotypes indistinguishable from that of ccs52a1-3 (Figure 1, E and F), and both fail to complement the ccs52a1-3 trichome phenotype. Double mutants of sim-1 with either of these T-DNA alleles showed the same enhanced multicellular trichome phenotype of the ccs52a1-4 sim-1 double mutant (Figure S1, B and C), and double mutants of either ccs52a1-3 or ccs52a1-4 and the sim-3 deletion allele also exhibited the enhanced phenotype (Figure S1, E and F), demonstrating that the interaction between these two genes is not allele specific. Given that the interaction occurs with apparent complete loss-of-function alleles of sim or ccs52a1, it is unlikely that the enhanced phenotype in the double mutant is due to a direct interaction between the proteins. Finally, the ccs52a1-3 mutant phenotype was rescued by specific expression in trichomes of the AT4G22910 coding sequence under the control of the GL2 promoter (Figure 2D) (Weinl et al. 2005) and by a genomic DNA fragment including 1607 bp upstream of the AT4G22910 start codon, the entire coding region, plus 617 bp downstream of the stop codon. Together this evidence established that the gene identified by genetic enhancement of sim in our screen is AT4G22910, which encodes the CDH1/FZR homolog CCS52A1. CCS52A1 and CCS52A2 are functionally equivalent, but only CCS52A1 is required for endoreplication in trichomes.
Figure 2.—
Identification of the CCS52A1 gene as the genetic enhancer. (A) Structure of the AT4G22910 locus, showing the location of the ccs52a1 mutations referred to in the text. Solid boxes are exons. (B) Wild-type Col-0 trichomes. (C) ccs52a1 trichomes. (D) Complementation of ccs52a1-3 by proGL2:CCS52A1. (E) Suppression of ccs52a1-3 phenotype by proGL2:CCS52A2. Scale bars are 4 mm in B and C, and 2 mm in D and E.
CDH1/FZR proteins are substrate-specific activators of the APC/C that regulate M/G1-phase-specific proteolysis and are required for endoreplication in a variety of organisms (Sigrist and Lehner 1997; Cebolla et al. 1999; Sorensen et al. 2000). Among the proteins targeted by the APC/C when activated by these proteins are the mitotic B-type cyclins. Arabidopsis has three genes encoding CDH1/FZR-like proteins, the closely related CCS52A1 and CCS52A2 genes and the more divergent CCS52B1 gene (Tarayre et al. 2004; Fülop et al. 2005). During the mitotic cycle, CCS52A1 and CCS52A2 are expressed predominantly in G1/S, while CCS52B1 is primarily expressed during G2/M (Fülop et al. 2005; Lammens et al. 2008), suggesting that of these genes, CCS52A1 and CCS52A2 are most likely to have overlapping functions.
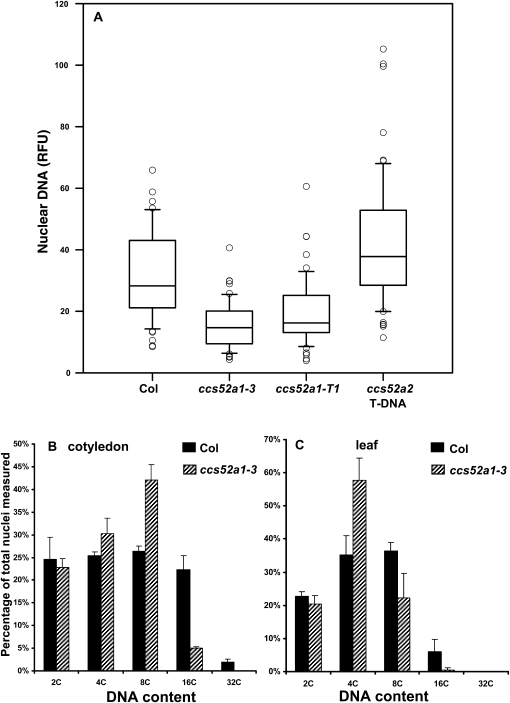
Recent work has shown that ccs52a1-T1 plants have reduced endoreplication in leaves and trichomes (Lammens et al. 2008; Larson-Rabin et al. 2009), and a similar reduction in DNA content in trichomes (Figure 3A), leaves (Figure 3B) and cotyledons (Figure 3C) was observed in our ccs52a1-3 plants. The role of the closely related CCS52A2 gene in trichome endoreplication had not been previously investigated. In contrast to the situation in whole leaves or cotyledons, where mutations in either gene affect endoreplication levels (Lammens et al. 2008), the T-DNA-induced loss-of-function allele ccs52a2-1 did not affect endoreplication in trichomes (Figure 3A).
Figure 3.—
Effect of ccs52a1 and ccs52a2 mutations on endoreplication in trichomes. (A) DNA content of DAPI-stained trichome nuclei of the indicated genotypes measured in situ, presented as relative fluorescence units (RFU). Data were normalized to an assumed wild-type mean of 32C for Col trichomes. Data are presented as box plots, where the box encompasses the 25th through the 75th percentile of the data, the line within the box is the median (50th percentile), and the error bars represent the 5th (bottom bar) and 95th (top bar) percentiles. All pairwise comparisons are significant at P < 0.05 except ccs52a1-3 vs. ccs52a1-T2 and Col-0 vs. ccs52a2 T-DNA. (B) Nuclear DNA content of Col-0 and ccs52a1-3 21-day-old cotyledons. (C) Nuclear DNA content of Col-0 and ccs52a1-3 21-day-old leaves. For B and C, DNA content was determined by flow cytometry.
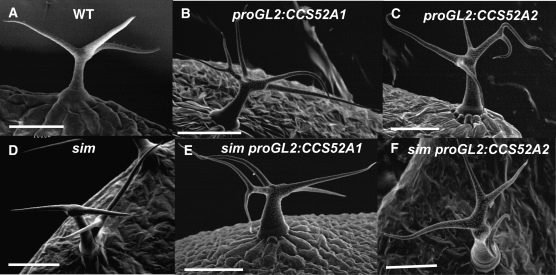
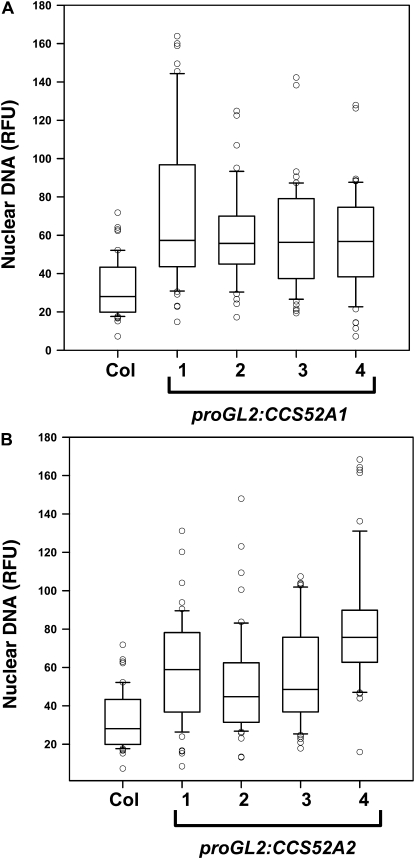
To further examine the role of these two homologous genes in trichome endoreplication, CCS52A1 and CCS52A2 were expressed specifically in trichomes from the GL2 promoter (proGL2). Wild-type trichomes typically have three or four branches, equivalent to two or three branch points (Figure 4A; Table 2). Expression of either gene from the GL2 promoter resulted in a similar degree of increased trichome branching to four, five, or more branches (Figure 4, B and C; Table 2). Expression of either gene also increased the trichome nuclear DNA content a similar amount relative to wild-type (Figure 5, A and B). Thus both genes are capable of triggering increased endoreplication in trichomes, even though only the ccs52a1 loss-of-function mutations affect endoreplication in trichomes. Furthermore, expression of CCS52A2 from the GL2 promoter was capable of complementing the ccs52a1 mutant phenotype (Figure 2E). These results are consistent with microarray results indicating that CCS52A1 transcripts are detectable in trichomes in microarray experiments, while CCS52A2 transcripts are not (Marks et al. 2009).
Figure 4.—
Expression of either CCS52A1 or CCS52A2 in trichomes from the GL2 promoter results in increased trichome branching and suppression of the multicellular trichome phenotype of sim. (A) Wild-type Col-0 trichome. (B) proGL2:CCS52A1 trichome. (C) proGL2:CCS52A2 trichome. (D) sim-1 multicellular trichome. (E) sim-1 proGL2:CCS52A1 trichome. (F) sim-1 proGL2:CCS52A2 trichome. All images are SEMs. Scale bars, 100 μm.
Figure 5.—
Expression of either CCS52A1 or CCS52A2 in trichomes from the GL2 promoter increases the level of endoreplication. DNA content of DAPI-stained trichome nuclei of the indicated genotypes measured in situ, presented as RFU, normalized to an assumed wild-type mean of 32C for Col-0 trichomes. Data are presented as box plots, where the box encompasses the 25th through the 75th percentile of the data, the line within the box is the median (50th percentile), and the error bars represent the 5th (bottom bar) and 95th (top bar) percentiles. (A) Col-0 wild-type and four independent proGL2:CCS52A1 transgenic lines. Pairwise comparisons of Col-0 with each of the four transgenic lines were significant at P < 0.05. (B) Col-0 wild-type and four independent proGL2:CCS52A2 lines. Pairwise comparisons of Col-0 with each of the four transgenic lines were significant at P < 0.05.
CCS52A1 can suppress the sim-1 phenotype when overexpressed:
As noted earlier, wild-type trichomes are unicellular and occur singly at every TIS (Figure 4A). In the sim-1 mutant, most trichomes are multicellular, often occurring in clusters of adjacent trichomes arising by mitotic division of a single precursor cell (Figure 4D; Walker et al. 2000). To investigate the role of the Arabidopsis CDH1/FZR-like proteins in relation to SIM, we expressed either CCS52A1 or CCS52A2 in sim-1 mutant plants under the control of the GL2 promoter. These sim-1 plants expressing either proGL2:CCS52A1 or proGL2:CCS52A2 had enlarged unicellular trichomes with supernumerary branches and no trichome clusters (Figure 4, E and F; Table 3). The trichome phenotypes produced by overexpression of CCS52A1 or CCS52A2 in sim-1 mutant trichomes were essentially identical to those produced by expression of these transgenes in wild type (Figure 4, B and C), indicating that overexpression of either CCS52A1 or CCS52A2 completely suppressed the sim-1 phenotype.
TABLE 3.
Suppression of sim-1 multicellular trichome phenotype by expression of CCS52A1 or CCS52A2 in trichomes
| Genotype | Nuclei per TISa | Total TIS countedb |
|---|---|---|
| Col | 1.0 ± 0.0 | 54 |
| sim-1 | 2.2 ± 1.2 | 64 |
| sim-1 proGL2:CCS52A1 | 1.0 ± 0.0 | 55 |
| sim-1 proGL2:CCS52A2 | 1.0 ± 0.0 | 50 |
The number of DAPI-stained nuclei per TIS. Data shown as mean ± SD.
TIS were counted on fully expanded first or second leaves.
Overexpressing B-type cyclins in ccs52a1 mutants results in multicellular trichomes:
Previous work demonstrated that CYCB1;1 and CYCB1;2 are ectopically expressed in sim mutant trichomes (Schnittger et al. 2002; Churchman et al. 2006). The N termini of both CYCB1;1 and CYCB1;2 contain destruction box (DBox) motifs that target them for degradation by the APC/C. Furthermore, overexpression in trichomes of full-length CYCB1;2 in wild-type plants results in unicellular trichomes, but expression of an N-terminal deletion of CYCB1;2 lacking the Dbox results in multicellular trichomes (Schnittger et al. 2002; Schnittger et al. 2005). Thus, a possible explanation of the genetic interaction between sim and ccs52a1 is that sim CCS52A1+ plants producing functional CCS52A1 have an active APC that degrades most of the ectopic mitotic cyclins, limiting the potential for division. In contrast, sim ccs52a1 double mutants would not have an active APC/C, and promotion of mitosis by ectopic expression of mitotic cyclins would be unrestricted. This predicts that trichome-specific expression of mitotic cyclins in a SIM+ ccs52a1 mutant background should promote division in developing trichomes.
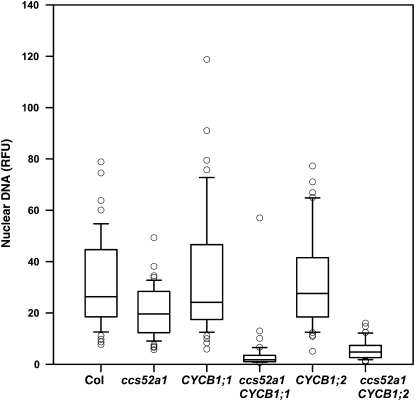
To test this prediction, plants overexpressing CYCB1;1 or CYCB1;2 under the control of the GL2 promoter were examined. Trichomes of both wild-type and ccs52a1 plants are unicellular (Figure 6, A and B; Table 4). Trichomes of plants overexpressing either of these B cyclins in a wild-type background are unicellular (Figure 6, C and E; Table 4) but nearly half of ccs52a1-3 proGL2:CYCB1;1 trichomes are multicellular (Figure 6D; Table 4), and approximately 20% of ccs52a1-3 proGL2:CYCB1;2 trichomes are multicellular (Figure 6F; Table 4). We also observed that plants overexpressing these cell-cycle regulators in the ccs52a1-3 mutant background had greatly reduced endoreplication when compared either to wild type or to ccs52a1-3 alone (Figure 7). Note that since only a fraction of trichomes were multicellular, endoreplication was strongly inhibited even in unicellular trichomes. These data indicate that high levels of B cyclins are incompatible with endoreplication, even in cells that fail to divide.
Figure 6.—
Expression of B-type cyclins in ccs52a1 mutant trichomes results in multicellular trichomes. (A) Wild-type Col-0 trichome. (B) ccs52a1-3 trichome. (C) proGL2:CYCB1;1 trichome. (D) ccs52a1-3 proGL2:CYCB1;1 multicellular trichome. (E) proGL2:CYCB1;2 trichome (F) ccs52a1-3 proGL2:CYCB1;2 multicellular trichome.
TABLE 4.
Overexpression of B-type cyclins in ccs52a1 mutant trichomes results in multicellular trichomes
| Nuclei per TISa | Total TIS countedb | |
|---|---|---|
| Col | 1.0 ± 0.0 | 55 |
| ccs52a1-3 | 1.0 ± 0.0 | 50 |
| proGL2:CYCB1;1 | 1.0 ± 0.0 | 54 |
| proGL2:CYCB1;2 | 1.0 ± 0.0 | 50 |
| ccs52a1-3 proGL2:CYCB1;1 | 1.5 ± 0.0 | 61 |
| ccs52a1-3 proGL2CYCB1;2 | 1.2 ± 0.0 | 63 |
The number of DAPI-stained nuclei per TIS. Data shown as mean ± SD.
TIS were counted on fully expanded first or second leaves.
Figure 7.—
Expression of B-type cyclins in ccs52a1-3 mutant trichomes strongly inhibits endoreplication. DNA content of DAPI-stained trichome nuclei of the indicated genotypes measured in situ, presented as RFU, normalized to an assumed wild-type mean of 32C for Col-0 trichomes. The CYCB1;1 and CYCB1;2 transgenes were expressed from the GL2 promoter. Data are presented as box plots, where the box encompasses the 25th through the 75th percentile of the data, the line within the box is the median (50th percentile), and the error bars represent the 5th (bottom bar) and 95th (top bar) percentiles. Two-way comparisons of CYCB1;1 vs. ccs52a1-3 CYCB1;1 and CYCB1;2 vs. ccs52a1-3 CYCB1;2 were significant at P < 0.05.
DISCUSSION
The discovery of the Arabidopsis SIM gene and its involvement in trichome endoreplication provided crucial clues regarding the switch between the mitotic cell cycle and the endocycle, but the mechanism by which the SIM protein regulates this switch remains unclear in spite of insights into the molecular interactions of the protein. SIM interacts with, and likely inhibits, the kinase activity of CYCD/CDK complexes (Churchman et al. 2006; Peres et al. 2007), which are generally considered to trigger S phase, yet SIM functions to block mitosis while allowing multiple rounds of DNA replication. Recent work indicates that SIM is rapidly upregulated in trichomes and is a direct target of the trichome-initiating transcription factors GL1 and GL3 (Churchman et al. 2006; Jakoby et al. 2008; Morohashi and Grotewold 2009), suggesting that SIM is the key regulator of entry into the endocycle during trichome development. The demonstration here that altering the level of functional CCS52A1 can enhance or suppress the sim mutant phenotype sheds light on the mechanism by which SIM regulates the transition to the endocycle during trichome development.
The Arabidopsis CCS52A1 gene product is a homolog of the CDH1/FIZZY-RELATED class of activators of the APC/C ubiquitin E3 ligase. During the mitotic cell cycle, APC/CCDH1 targets proteins containing the Dbox motif, and in some cases other amino acid sequence motifs, for proteolysis (Harper et al. 2002). Well-characterized APC/CCDH1 targets include the mitotic cyclins and geminin. Geminin is an inhibitor of replication origin licensing and blocks DNA replication if not degraded (Mcgarry and Kirschner 1998; Nishitani et al. 2001). During the mitotic cycle, the function of APC/CCDH1 is to maintain low levels of CDK activity during late stages of mitosis and during G1, preventing reentry into mitosis and allowing replication origin licensing. In mammals (Garcia-Higuera et al. 2008), Drosophila (Sigrist and Lehner 1997), and plants (Vinardell et al. 2003), CDH1/FZR is also necessary for endoreplication, where it presumably functions in APC/CCDH1 to enforce a G1-like cell-cycle phase between S phases. In Drosophila, compromising APC/CCDH1 activity after entry into the endocycle results in accumulation of mitotic cyclins and geminin and inhibits DNA replication (Narbonne-Reveau et al. 2008). The APC/CCDH1 remains active in differentiated cells, regulating in axon growth and synapse function (Kim and Bonni 2007) in animals and vascular development in plants (Marrocco et al. 2009). The genome of Arabidopsis encodes two CCS52A proteins, termed CCS52A1 and CCS52A2, both of which can associate with APC components and with DBox-containing cyclins (Fülop et al. 2005). Both proteins have been implicated in endoreplication in leaves on the basis of reduced endoreplication in mutant plants (Lammens et al. 2008; Larson-Rabin et al. 2009).
Our work demonstrates that CCS52A1 promotes endoreplication in trichomes, while CCS52A2 does not (Figure 3A). Nonetheless, expression of either gene in trichomes can complement the ccs52A1 mutant phenotype (Figure 2, D and E), and expression of either gene in wild-type trichomes results in a similar increase in the level of endoreplication (Figure 5, A and B). These results suggest that the two proteins are functionally equivalent and differ only in the regulation of their transcription. We have not examined the expression pattern of the two CCS52A genes in trichomes. However, in the data from a recent study of the trichome transcriptome, CCS52A1 expression was detectable in all trichome data sets analyzed, including wild-type trichomes and those of several mutant genotypes, while CCS52A2 was undetectable in all trichome data sets (Marks et al. 2009). These results are consistent with those of a recent study demonstrating that differences in the roles of CCS52A1 and CCS52A2 in the root meristem were due to differences at the transcriptional level rather than to functional differences between the proteins (Vanstraelen et al. 2009).
The dramatic enhancement of the sim multicellular trichome phenotype seen in sim ccs52a1 double mutants was unexpected, given the unicellular reduced endoreplication phenotype of ccs52a1 single mutants. However, previous work demonstrated that expression of the mitotic cyclins CYCB1;1 and CYCB1;2 is reactivated in sim mutant trichomes, suggesting that SIM is involved in suppression of M-phase gene expression (Schnittger et al. 2002; Churchman et al. 2006). Both of these mitotic cyclins contain DBox sequences, and thus are potential targets of the APC/C. Furthermore, expression in trichomes of an N-terminal truncation of CYCB1;2 that removes the destruction box promotes cell division in trichomes, while expression of the DBox-containing full-length CYCB1;2 does not (Schnittger et al. 2002, 2005). Here, we have shown that expression of full-length CYCB1;1 or CYCB1;2 results in cell division in ccs52a1 mutant trichomes (Table 4; Figure 6, D and F), but not in wild-type trichomes (Table 4; Figure 6, D and E). These results demonstrate that wild-type CCS52A1 function can suppress the effects of ectopic CYCB expression in developing trichomes, consistent with activation of the APC in trichomes by CCS52A1.
Perhaps the most significant result from our work is the complete suppression of the sim phenotype by overexpression of either CCS52A1 or CCS52A2 in developing trichomes (Table 3, Figure 4, E and F). This result strongly suggests that the sim mutant phenotype is largely or entirely due to ectopic expression of M-phase-promoting targets of the APC/CCCS52A ubiquitin E3 ligase complex, which in a ccs52a1 mutant would not be targeted for proteolysis and thus lead to a higher frequency of mitosis. The mechanism by which SIM suppresses transcription of mitotic cyclins is unknown. Because SIM targets CYCD/CDK complexes, which are thought to primarily function during late G1 and S phases, it is possible that this suppression is indirect. However, the three-repeat MYB transcription factors (MYB3Rs) that activate transcription of some G2/M-phase genes (Ito et al. 2001; Haga et al. 2007) are activated by phosphorylation (Araki et al. 2004). Transcription of these MYB3Rs appears to begin in late S phase, prior to G2/M CYCB transcription. It is possible that SIM could inhibit phosphorylation and activation of these or other G2/M transcription factors by a CYCD/CDK complex.
The APC/CCCS52A must also have another function during endoreplication separate from the pathway in which it cooperates with SIM to suppress accumulation of mitotic cyclins. Trichomes of ccs52a1 SIM+ plants have a very different phenotype from either sim CCS52A1+ or sim ccs52a1 double-mutant plants. Not only do ccs52a1 SIM+ trichomes fail to divide, they also exhibit reduced endoreplication (Figure 3A). Additionally, in the absence of ccs52a1, high levels of B-type cyclins, presumably resulting in high levels of CDK activity, strongly inhibit endoreplication, even in trichome cells that do not divide (Figure 7). The simplest interpretation of these observations is that there is another target of the APC/CCCS52A, not regulated by SIM, that must be degraded during the endocycle G1 phase for replication licensing or entry into S phase to occur. One candidate for such a factor is CYCA2;3, which has recently been shown to be a target of the APC/CCCS52A in roots (Imai et al. 2006; Boudolf et al. 2009). Another possibility would be a geminin-like protein. Although no clear geminin homolog has been found in plants, a possible functional equivalent has recently been suggested (Caro et al. 2007; Caro and Gutierrez 2007).
Although control of entry into endocyles from the mitotic cycle is not completely understood in any organisms, the general outlines of the mechanisms involved in the switch are becoming clear in several models. Endocycles in Drosophila appear to be triggered by upregulation of CDH1/FZR (Sigrist and Lehner 1997; Schaeffer et al. 2004; Narbonne-Reveau et al. 2008), although the CDK inhibitor Dacapo is also needed to enforce low levels of CDK activity during G1 to allow replication licensing (Hong et al. 2007). In contrast, recent work in mice demonstrates that endoreplication in mammal tropoblast stem cells is triggered by the CDK inhibitor p57/Kip2, which inhibits CDK activity in the continued presence of B-type mitotic cyclins (Ullah et al. 2008), although CDH1 is also required for endoreplication (Garcia-Higuera et al. 2008).
The results presented here, along with other recent results, suggest the following model for entry into the endocycle during Arabidopsis trichome development. The key event triggering endoreplication is likely to be greatly increased expression of SIM. Recent work shows that the trichome cell fate transcription factors GL1 and GL3 promote the rapid upregulation of SIM transcripts upon initiation of trichome development (Morohashi and Grotewold 2009). The accumulation of SIM then blocks induction of the G2/M transcriptional program, and CCS52A1 expression both reinforces the SIM block that prevents induction of the G2/M program and enforces low CDK levels to allow replication licensing during the G1-like phase of the endocycle. Microarray studies indicate that CCS52A1 transcripts are not upregulated in trichomes in comparison to other tissues (Marks et al. 2009; Morohashi and Grotewold 2009), so it seems unlikely that transcriptional upregulation of CDH1-like proteins plays a role in trichome endoreplication, in contrast to the situation in Drosophila. Thus, while Drosophila, mammals, and Arabidopsis use APC/C activators and CDK inhibitors to initiate endocycles, it appears that each organism deploys these factors in slightly different ways to trigger endoreplication.
Acknowledgments
The authors acknowledge Dr. Arp Schnittger (University of Strasbourgh, Strasbourgh, France) for the gift of plasmids and Arabidopsis strains and Dr. James Moroney and Dr. Kirsten Prüfer for critical reading of the manuscript. The authors also acknowledge Cindy Henk, Ying Zhao, and Matt Brown of the Socolofsky Microscopy Facility at Louisiana State University for expert technical assistance. This work was supported by National Science Foundation grant IOS 0744566 to J.C.L. and by a fellowship from the Research Foundation Flanders to L.D.V.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.113274/DC1.
References
- Araki, S., M. Ito, T. Soyano, R. Nishihama and Y. Machida, 2004. Mitotic cyclins stimulate the activity of c-Myb-like factors for transactivation of G2/M phase-specific genes in tobacco. J. Biol. Chem. 279 32979–32988. [DOI] [PubMed] [Google Scholar]
- Boudolf, V., D. Inze and L. De Veylder, 2006. What if higher plants lack a CDC25 phosphatase? Trends Plant Sci. 11 474–479. [DOI] [PubMed] [Google Scholar]
- Boudolf, V., T. Lammens, J. Boruc, J. Van Leene, H. Van Den Daele et al., 2009. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiol. 150 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brininstool, G., R. Kasili, L. A. Simmons, V. Kirik, M. Hulskamp et al., 2008. Constitutive expressor of pathogenesis-related Genes5 affects cell wall biogenesis and trichome development. BMC Plant Biol. 8 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro, E., and C. Gutierrez, 2007. A green GEM: intriguing analogies with animal geminin. Trends Cell Biol. 17 580–585. [DOI] [PubMed] [Google Scholar]
- Caro, E., M. M. Castellano and C. Gutierrez, 2007. A chromatin link that couples cell division to root epidermis patterning in Arabidopsis. Nature 447 213–217. [DOI] [PubMed] [Google Scholar]
- Cebolla, A., J. M. Vinardell, E. Kiss, B. Olah, F. Roudier et al., 1999. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. Embo J. 18 4476–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniclet, C., W. Rong, M. Causse, N. Frangne, L. Bolling et al., 2005. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 139 1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman, M. L., M. L. Brown, N. Kato, V. Kirik, M. Hulskamp et al., 2006. SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell 18 3145–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S. J., and A. F. Bent, 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Curtis, M. D., and U. Grossniklaus, 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte, W., and J. A. Murray, 2003. The plant cell cycle. Annu. Rev. Plant Biol. 54 235–264. [DOI] [PubMed] [Google Scholar]
- Dissmeyer, N., A. K. Weimer, S. Pusch, K. De Schutter, C. L. Kamei et al., 2009. Control of cell proliferation, organ growth, and DNA damage response operate independently of dephosphorylation of the Arabidopsis Cdk1 homolog CDKA;1. Plant Cell 21 3641–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A., and T. L. Orr-Weaver, 2001. Endoreplication cell cycles: more for less. Cell 105 297–306. [DOI] [PubMed] [Google Scholar]
- Fülop, K., S. Tarayre, Z. Kelemen, G. Horvath, Z. Kevei et al., 2005. Arabidopsis anaphase-promoting complexes: multiple activators and wide range of substrates might keep APC perpetually busy. Cell Cycle 4 1084–1092. [PubMed] [Google Scholar]
- Garcia-Higuera, I., E. Manchado, P. Dubus, M. Canamero, J. Mendez et al., 2008. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat. Cell Biol. 10 802–811. [DOI] [PubMed] [Google Scholar]
- Haga, N., K. Kato, M. Murase, S. Araki, M. Kubo et al., 2007. R1R2R3-Myb proteins positively regulate cytokinesis through activation of KNOLLE transcription in Arabidopsis thaliana. Development 134 1101–1110. [DOI] [PubMed] [Google Scholar]
- Harper, J. W., J. L. Burton and M. J. Solomon, 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16 2179–2206. [DOI] [PubMed] [Google Scholar]
- Hong, A., K. Narbonne-Reveau, J. Riesgo-Escovar, H. Fu, M. I. Aladjem et al., 2007. The cyclin-dependent kinase inhibitor Dacapo promotes replication licensing during Drosophila endocycles. EMBO J. 26 2071–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülskamp, M., S. Miséra and G. Jürgens, 1994. Genetic dissection of trichome cell development in Arabidopsis. Cell 76 555–566. [DOI] [PubMed] [Google Scholar]
- Imai, K. K., Y. Ohashi, T. Tsuge, T. Yoshizumi, M. Matsui et al., 2006. The A-type cyclin CYCA2;3 is a key regulator of ploidy levels in Arabidopsis endoreduplication. Plant Cell 18 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé, D., and L. de Veylder, 2006. Cell cycle regulation in plant development. Annu. Rev. Genet. 40 77–105. [DOI] [PubMed] [Google Scholar]
- Ito, M., S. Araki, S. Matsunaga, T. Itoh, R. Nishihama et al., 2001. G2/M-phase-specific transcription during the plant cell cycle is mediated by c-Myb-like transcription factors. Plant Cell 13 1891–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby, M. J., D. Falkenhan, M. T. Mader, G. Brininstool, E. Wischnitzki et al., 2008. Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiol. 148 1583–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubes, J., T. H. Phan, D. Just, C. Rothan, C. Bergounioux et al., 1999. Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol. 121 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, A. H., and A. Bonni, 2007. Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system. Mol. Cell Neurosci. 34 281–287. [DOI] [PubMed] [Google Scholar]
- Kowles, R., and R. Phillips, 1985. DNA amplification patterns in maize endosperm nuclei during kernel development. Proc. Natl. Acad. Sci. USA 82 7010–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens, T., V. Boudolf, L. Kheibarshekan, L. P. Zalmas, T. Gaamouche et al., 2008. Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset. Proc. Natl. Acad. Sci. USA 105 14721–14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J. C., J. D. Walker, A. C. Bolognesi-Winfield, J. C. Gray and A. R. Walker, 1999. Allele-specific interactions between ttg and gl1 during trichome development in Arabidopsis thaliana. Genetics 151 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J. C., M. L. Brown and J. Schiefelbein, 2003. How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 54 403–430. [DOI] [PubMed] [Google Scholar]
- Larkin, J. C., M. L. Brown and M. L. Churchman, 2007. Insights into the endocycle from trichome development, pp. 249–268 in Cell Cycle Control and Plant Development, edited by D. Inzé. Blackwell Scientific, Oxford.
- Larkins, B. A., B. P. Dilkes, R. A. Dante, C. M. Coelho, Y. M. Woo et al., 2001. Investigating the hows and whys of DNA endoreduplication. J. Exp. Bot. 52 183–192. [PubMed] [Google Scholar]
- Larson-Rabin, Z., Z. Li, P. H. Masson and C. D. Day, 2009. FZR2/CCS52A1 expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiol. 149 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly, M. A., and R. J. Duronio, 2005. New insights into cell cycle control from the Drosophila endocycle. Oncogene 24 2765–2775. [DOI] [PubMed] [Google Scholar]
- Lukowitz, W., C. S. Gillmor and W. R. Scheible, 2000. Positional cloning in Arabidopsis: why it feels good to have a genome initiative working for you. Plant Physiol. 123 795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, M. D., J. P. Wenger, E. Gilding, R. Jilk and R. A. Dixon, 2009. Transcriptome analysis of arabidopsis wild-type and gl3-sst sim trichomes identifies four additional genes required for trichome development. Mol. Plant 2 803–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco, K., A. Thomann, Y. Parmentier, P. Genschik and M. C. Criqui, 2009. The APC/C E3 ligase remains active in most post-mitotic Arabidopsis cells and is required for proper vasculature development and organization. Development 136 1475–1485. [DOI] [PubMed] [Google Scholar]
- McGarry, T. J., and M. W. Kirschner, 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93 1043–1053. [DOI] [PubMed] [Google Scholar]
- Melaragno, J., B. Mehrota and A. Coleman, 1993. Relationship between endoploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi, K., and E. Grotewold, 2009. A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 5 e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne-Reveau, K., S. Senger, M. Pal, A. Herr, H. E. Richardson et al., 2008. APC/CFzr/Cdh1 promotes cell cycle progression during the Drosophila endocycle. Development 135 1451–1461. [DOI] [PubMed] [Google Scholar]
- Nishitani, H., S. Taraviras, Z. Lygerou and T. Nishimoto, 2001. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J. Biol. Chem. 276 44905–44911. [DOI] [PubMed] [Google Scholar]
- Peres, A., M. L. Churchman, S. Hariharan, K. Himanen, A. Verkest et al., 2007. Novel plant-specific cyclin-dependent kinase inhibitors induced by biotic and abiotic stresses. J. Biol. Chem. [DOI] [PubMed]
- Ramirez-Parra, E., B. Desvoyes and C. Gutierrez, 2005. Balance between cell division and differentiation during plant development. Int. J. Dev. Biol. 49 467–477. [DOI] [PubMed] [Google Scholar]
- Schaeffer, V., C. Althauser, H. R. Shcherbata, W. M. Deng and H. Ruohola-Baker, 2004. Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells. Curr. Biol. 14 630–636. [DOI] [PubMed] [Google Scholar]
- Schnittger, A., U. Schobinger, Y. D. Stierhof and M. Hulskamp, 2002. Ectopic B-type cyclin expression induces mitotic cycles in endoreduplicating Arabidopsis trichomes. Curr. Biol. 12 415–420. [DOI] [PubMed] [Google Scholar]
- Schnittger, A., U. Schöbinger, Y. D. Stierhof and M. Hülskamp, 2005. Erratum: ectopic B-type cyclin expression induces mitotic cycles in endoreduplicating Arabidopsis trichomes. Curr. Biol. 15 980. [DOI] [PubMed] [Google Scholar]
- Sigrist, S. J., and C. F. Lehner, 1997. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90 671–681. [DOI] [PubMed] [Google Scholar]
- Sorensen, C. S., C. Lukas, E. R. Kramer, J. M. Peters, J. Bartek et al., 2000. Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell. Biol. 20 7613–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarayre, S., J. M. Vinardell, A. Cebolla, A. Kondorosi and E. Kondorosi, 2004. Two classes of the CDh1-type activators of the anaphase-promoting complex in plants: novel functional domains and distinct regulation. Plant Cell 16 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, Z., M. J. Kohn, R. Yagi, L. T. Vassilev and M. L. DePamphilis, 2008. Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev. 22 3024–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen, M., M. Baloban, O. Da Ines, A. Cultrone, T. Lammens et al., 2009. APC/CCCS52A complexes control meristem maintenance in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 106 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest, A., C. L. Manes, S. Vercruysse, S. Maes, E. Van Der Schueren et al., 2005. a The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. Plant Cell 17 1723–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest, A., C. Weinl, D. Inze, L. De Veylder and A. Schnittger, 2005. b Switching the cell cycle. Kip-related proteins in plant cell cycle control. Plant Physiol. 139 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinardell, J. M., E. Fedorova, A. Cebolla, Z. Kevei, G. Horvath et al., 2003. Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15 2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J. D., D. G. Oppenheimer, J. Concienne and J. C. Larkin, 2000. SIAMESE, a gene controlling the endoreduplication cell cycle in Arabidopsis thaliana trichomes. Development 127 3931–3940. [DOI] [PubMed] [Google Scholar]
- Weigel, D., and J. Glazebrook, 2002. Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Woodbury, NY.
- Weinl, C., S. Marquardt, S. J. Kuijt, M. K. Nowack, M. J. Jakoby et al., 2005. Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. Plant Cell 17 1704–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]