Abstract
Ecdysone is the major steroid hormone in insects and plays essential roles in coordinating developmental transitions such as larval molting and metamorphosis through its active metabolite 20-hydroxyecdysone (20E). Although ecdysone is present throughout life in both males and females, its functions in adult physiology remain largely unknown. In this study we demonstrate that ecdysone-mediated signaling in the adult is intimately involved in transitions between the physiological states of sleep and wakefulness. First, administering 20E to adult Drosophila melanogaster promoted sleep in a dose-dependent manner, and it did so primarily by altering the length of sleep and wake bouts without affecting waking activity. Second, mutants for ecdysone synthesis displayed the “short-sleep phenotype,” and this was alleviated by administering 20E at the adult stage. Third, mutants for nuclear ecdysone receptors showed reduced sleep, and conditional overexpression of wild-type ecdysone receptors in the adult mushroom bodies resulted in an isoform-specific increase in sleep. Finally, endogenous ecdysone levels increased after sleep deprivation, and mutants defective for ecdysone signaling displayed little sleep rebound, suggesting that ecdysone is involved in homeostatic sleep regulation. In light of the recent finding that lethargus—a period at larval-stage transitions in the nematode worm Caenorhabditis elegans—is a sleep-like state, our results suggest that sleep is functionally and mechanistically linked to a genetically programmed, quiescent behavioral state during development.
STEROID hormones have a wide variety of effects on the development, physiology and behavior of evolutionarily diverse organisms. The major steroid hormone in the fruit fly Drosophila melanogaster is ecdysone. Extensive studies in Drosophila and other insects have revealed that ecdysone plays vital roles in orchestrating major transitions during development—for example, during larval molting and metamorphosis—through its active metabolite 20-hydroxyecdysone (20E) (Truman and Riddiford 2002). The actions of ecdysone are primarily mediated by ecdysone receptors (EcRs), members of an evolutionarily conserved nuclear hormone receptor family. EcRs form heterodimers with the retinoid X receptor homolog ultraspiracle (USP), and these EcR/USP complexes function as ligand-activated transcription factors to regulate the expression of downstream genes in a tightly coordinated manner (Evans 1988; Aranda and Pascual 2001). Homozygous loss-of-function mutations in the EcR gene (EcR) are lethal, demonstrating that nuclear receptor-mediated ecdysone signaling is indispensable for development. Ecdysone is present throughout life in both males and females (Handler 1982). In adult females, ecdysone signaling is critical for reproduction, as it mediates egg-chamber maturation during mid-oogenesis (Buszczak et al. 1999). However, other functions of ecdysone in mature adult flies have received less attention and remain largely elusive.
Unlike flies homozygous for loss-of-function mutations in EcR, those that are heterozygous for such mutations (EcR/+) develop into adult flies with no apparent deficit in morphology, activity, or fertility. Interestingly, EcR/+ adult flies show enhanced resistance to oxidative stress, heat, and dry starvation (Simon et al. 2003). In addition, under regular laboratory conditions, EcR/+ adult flies exhibit remarkable increases in life span, surviving 40–50% longer than wild-type controls (Simon et al. 2003). Other studies have demonstrated that the levels of 20E are increased in adult flies when they are exposed to unfavorable environmental conditions, such as nutrient restriction and heat treatment (Rauschenbach et al. 2000; Terashima et al. 2005). These findings indicate that ecdysone signaling indeed occurs in adult flies and that it is likely involved in endocrinologic regulation of their physiological and behavioral states in response to the internal and external environments. In support of this possibility, we recently found that the levels of 20E are increased in adult male flies following extensive social interaction with nonvirgin female flies, the condition under which long-term courtship memory is formed (Ishimoto et al. 2009). We have also found that mutants with impaired ecdysone signaling have defects in long-term courtship memory, suggesting that the experience-dependent activation of ecdysone signaling influences the states of the adult brain such that courtship memory is effectively stabilized to a long-lasting form (Ishimoto et al. 2009).
In mammals, the endocrine system regulates sleep—a distinctive physiological and behavioral state that is controlled by a homeostatic drive and a circadian pacemaker. Various hormones and hormone-like molecules, including biogenic amines, peptide/protein factors, and steroids, have significant effects on different aspects of sleep (Steiger 2003). Recent studies have revealed that sleep-like states are conserved among evolutionarily divergent animal species (Zimmerman et al. 2008a) and that Drosophila is a valuable genetic model system for the study of sleep (Hendricks et al. 2000; Shaw et al. 2000; Cirelli and Bushey 2008; Mackiewicz et al. 2008). In Drosophila, monoamines (i.e., dopamine and serotonin) have arousal and sleep-promoting effects that are similar to those observed in mammals (Andretic et al. 2005; Kume et al. 2005; Yuan et al. 2006). It is also known that signaling pathways mediated by neurosecretory protein factors, such as pigment-dispersing factor (PDF) (Parisky et al. 2008; Chung et al. 2009) and epidermal growth factor (EGF) (Foltenyi et al. 2007), play a role in sleep regulation in Drosophila. The effects of steroid hormones on Drosophila sleep, however, have not been investigated to date.
Considering that ecdysone is implicated in the regulation of physiological and behavioral states of adult flies and that the sleep–wake dichotomy likely corresponds to transitions of distinct states of the brain, we hypothesized that ecdysone signaling is likely important for the regulation of sleep and wakefulness in adult flies. In the study presented here, we employed pharmacological and genetic approaches to this problem and demonstrate that the molting steroid hormone ecdysone participates in the regulation of Drosophila sleep. Our findings reveal a novel role for this developmental steroid hormone in sleep and suggest that genetically programmed developmental processes are functionally and mechanistically linked to the regulation of sleep–wake transitions in mature adults.
MATERIALS AND METHODS
Fly strains and culture conditions:
Flies were reared at 25°, 65% humidity, under a 12-hr-light:12-hr-dark regimen, and on a conventional cornmeal/glucose/yeast/agar medium supplemented with the mold inhibitor methyl 4-hydroxybenzoate (0.05%). The Canton-S (CS) strain was used as the wild-type control unless otherwise indicated. The Dominant temperature sensitive 3 (DTS-3) mutant and the Ecdysone receptor (EcR) mutants EcRF288y, EcRA483T, and EcRV559fs were obtained from Dr. A. F. Simon (York College, The City University of New York, New York, NY). DTS-3 was induced in the wild-type strain Samarkand (Holden and Suzuki 1973). EcRF288y, EcRA483T, and EcRV559fs were generated by ethyl methane sulfonate (EMS) mutagenesis on the cn bw background (Bender et al. 1997). Samarkand and cn bw flies were used as controls in experiments involving the DTS-3 and EMS-induced EcR mutants, respectively. These control flies were also obtained from Dr. Simon. EcRNP5219 was generated by a P-element transposition on an i(5) background (Yoshihara and Ito. 2000). EcRNP5219 and i(5) were obtained from the Kyoto Stock Center (Drosophila Genetic Resource Center; Kyoto institute of technology, Kyoto, Japan) and from Dr. K. Ito (The University of Tokyo, Tokyo, Japan), respectively. MB-GS–Gal4, UAS–EcR-A, UAS–EcR-B1, and UAS–EcR-B2 were obtained from the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN) and outcrossed to our control w strain for 10 generations before use in experiments.
20E administration and quantification:
To examine the effects of the administration of exogenous 20E on sleep, we fed flies housed in glass tubes (5 [W] × 65 [L] mm) standard food medium containing 20E (Sigma-Aldrich, St. Louis, MO) at various concentrations (0.01, 0.1, and 1 mm). The control food contained 0.02–2% of vehicle ethanol instead.
To determine the titer of 20E in the whole body or the head, we performed enzyme immunoassays (ACE Enzyme Immunoassay, Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocol. Briefly, samples were extracted from 10 adult flies or heads by homogenization in 70% methanol. The homogenates were dried using a rotary evaporator at room temperature and then dissolved in assay buffer. Calibration curves were generated using the commercially obtained 20E.
Sleep analysis:
Three- to 5-day-old adult flies were individually housed in a glass tube (5 [W] × 65 [L] mm) with regular fly food and monitored while subjected to 12-hr-light and 12-hr-dark cycles at 25° with 65% humidity. The locomotor activity of individual flies was analyzed using the Drosophila Activity Monitoring (DAM) system (Trikinetics, Waltham, MA). Flies were acclimated to the experimental conditions for 1 day before sleep was analyzed. Locomotor activity data were collected at 1-min intervals for 3 days, and analyzed with a Microsoft (Redmond, WA) Excel-based program as described previously (Hendricks et al. 2003; Kume et al. 2005). A sleep bout was defined as 5 min or more of behavioral immobility. The waking activity was calculated by dividing the total activity counts during the observation period by the length of the wake period.
To further evaluate sleep phenotypes of certain genotypes of flies, their movements were directly monitored and analyzed using the video-based system. Newly eclosed flies were collected and kept for 4 days on standard cornmeal agar media and then kept in groups of 10 on standard fly media under 12-hr-light and12-hr-dark cycles at 25° with 65% humidity for 4 days. Each fly was loaded into a glass tube used for the DAM analysis 24 hr before video recording. The glass tubes containing single flies were placed on the light box (11 × 16 cm), which was manually constructed with 12 white LED (wavelength 480 + 550 nm) and a light diffuser. A web camera (Logicool Quickcam IM, Logitech, Fremont, CA) attached with a telephoto lens (HLM35V8E, Honeywell, Morristown, NJ) was mounted 20 cm above the glass tubes. Images were captured every 5 sec (0.2 frames/sec) from Zeitgeber Time (ZT) 4 to ZT 8 and analyzed using pySolo, a multiplatform software for analysis of Drosophila sleep (Gilestro and Cirelli 2009) to calculate total sleep time and mean sleep-bout length. Sleep was defined as a minimum of 5 min quiescence (no pixels moved).
The sleep deprivation assay was carried out using the DAM system as described previously (Huber et al. 2004) with minor modifications. Baseline sleep was measured for 3 days prior to sleep deprivation. Flies set in the DAM board were deprived of sleep using the rotating apparatus (Huber et al. 2004), which gives a random mechanical shock to flies in DAM boards for 12 hr (ZT 12–24). The increases in sleep amount (Δtotal sleep) and sleep-bout duration (Δsleep-bout duration) after sleep deprivation were calculated by subtracting each baseline sleep parameter for an individual fly from the corresponding sleep parameter after the deprivation of nighttime sleep (ZT 12–24). When sleep was not deprived under otherwise identical experimental conditions, Δtotal sleep and Δsleep-bout duration were negligibly small (data not shown). The ratios of Δtotal sleep and the sleep loss (gain/lost ratio) were used to estimate the degree of sleep homeostasis.
Statistical analysis:
Normality of the data was tested using the Kolmogorov–Smirnov test. One-way analyses of variance (ANOVAs) followed by post-hoc comparisons using the Bonferroni method were carried out for multiple comparisons of data with a normal distribution. For multiple group analysis of the nonparametric data, we applied the Krusal–Wallis test followed by the Bonferroni post-hoc test. The significance of the differences between two groups of normally distributed data were analyzed using the two-tailed parametric Student's t-test for data with equal variance and the unpaired t-test for data with unequal variance. Variance equality was tested using the Levene median test. The Mann–Whitney U test was used for analyses comparing two nonparametric data sets.
RESULTS
Administering 20E to wild-type flies promotes sleep:
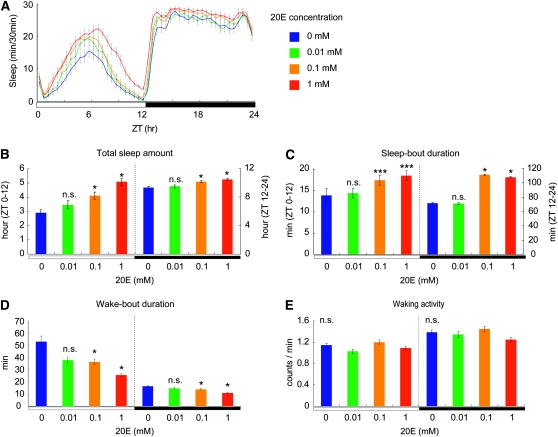
To investigate the possible involvement of ecdysone signaling in the regulation of Drosophila sleep, we examined the effects of exogenous 20E on baseline sleep. Three- to five-day-old wild-type (Canton-S) adult females were fed food containing different concentrations of 20E, and their sleep was analyzed using the DAM system. We found that administering 20E increased the total sleep time in a dose-dependent manner, during both the day and night (Figure 1, A and B). The sleep-promoting effect of 20E was more significant for daytime than nighttime sleep. This is likely due to a ceiling effect of 20E on nighttime sleep, as female flies normally sleep 77.5% (9.3 hr) of a 12-hr night and 24.2% (2.9 hr) of a 12-hr day. When exposed to 0.1 mm and 1 mm 20E, the total daytime sleep was increased by 41.4 and 75.9%, respectively, relative to that in the control flies (no 20E treatment, Figure 1, A and B). The increase in total sleep time was caused, in part, by an increase in the duration of each sleep bout. Under the same conditions, the average daytime sleep bout increased in length by 25.2% (0.1 mm 20E) and 33.1% (1 mm 20E), and the nighttime sleep bout increased by 55.6% (0.1 mm 20E) and 50.7% (1 mm 20E; Figure 1C). Wake-bout durations were also influenced by 20E. The average daytime wake bout decreased by 32.1% (0.1 mm 20E) and 51.7% (1 mm 20E) and the average nighttime wake bout by 12.5% (0.1 mm 20E) and 31.9% (1 mm 20E; Figure 1D). In contrast to the sleep- and wake-bout durations, waking activity was not significantly affected by the administration of 20E (Figure 1E). Therefore, the sleep-promoting effect of 20E is not due simply to a general suppression of locomotor activity. Rather, 20E likely influences the molecular and cellular pathways that contribute to the regulation of transitions between sleep and wakefulness.
Figure 1.—
Both daytime and nighttime sleep increase in a dose-dependent manner in response to treatment with exogenous 20E. (A) Sleep patterns of flies treated with 20E at various concentrations are represented by green (0.01 mm), orange (0.1 mm), and red (1 mm) lines, and the baseline sleep pattern in untreated flies is represented by blue lines. Average values for the total amount of sleep (B), sleep-bout duration (C), wake-bout duration (D), and waking activity (E) at each concentration of 20E were calculated separately for daytime and nighttime sleep data. N = 93 for each 20E concentration. *, P < 0.05; ***, P < 0.001; n.s., no significant difference. Error bars represent the SEM.
Steroid hormones often exhibit significant sexual dimorphism in their actions (Lavranos et al. 2006). Consistent with this, the effects of 20E on Drosophila sleep differed between males and females. Like females, males exhibited an increase in total sleep time in response to the administration of 20E. However, the sleep-promoting effect was observed only at night and at lower concentrations of 20E (0.01 and 0.1 mm) (supporting information, Figure S1, A and B); under these conditions, sleep-bout duration increased without a concomitant change in waking activity (Figure S1, C and D). At 1 mm 20E, however, the nighttime sleep-promoting effect of the hormone was not observed (Figure S1, A–C). Moreover, 20E did not affect wake-bout duration in the male flies at low concentration and even led to a reduction when administered at 1 mm (Figure S1E).
An ecdysone-synthesis mutant DTS-3 exhibits a short-sleep phenotype:
We next examined the effect of reduced 20E levels on Drosophila sleep, using the DTS-3 mutants. At 29°, DTS-3 displays dominant lethality during development. This is due to a low 20E titer, as this phenotype is rescued by exogenous administration of 20E to the mutants (Walker et al. 1987). DTS-3 was recently identified as a mutant allele of the molting defective (mld) gene (P. Maroy, personal communication, University of Szeged, Szeged, Hungary), which encodes a nuclear zinc finger protein required for ecdysone biosynthesis (Neubueser et al. 2005).
Flies heterozygous for DTS-3 (DTS-3/+) can develop into adult flies at 25°, although the 20E levels in adult DTS-3/+ females remain lower than those in wild-type females (Walker et al. 1987). Like the EcR heterozygous mutants (EcR/+), the DTS-3/+ females were found to be more resistant to various stresses than control flies and to exhibit increased longevity at 25° (Simon et al. 2003). The DTS-3 mutant phenotypes exhibit sexual dimorphism, as DTS-3/+ adult males show neither a decrease in the basal levels of 20E (Ishimoto et al. 2009; Walker et al. 1987) nor a significant increase in life span (Simon et al. 2003) at 25°. We thus analyzed DTS-3/+ females at 25° to examine the effect of reduced levels of 20E on sleep.
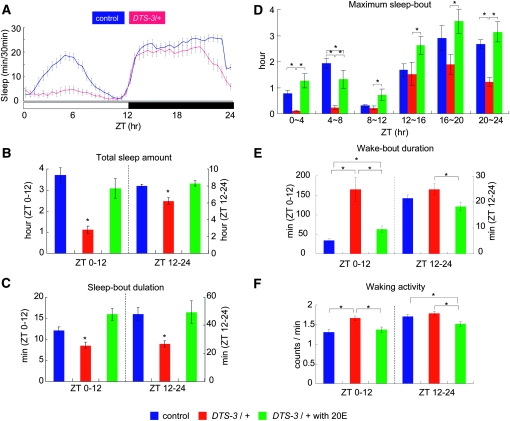
Consistent with the sleep-promoting effect of the application of exogenous 20E, DTS-3/+ females slept significantly less than the control flies at 25° (Figure 2, A and B). The effect of DTS-3 on daytime sleep was particularly pronounced, with total daytime sleep reduced by 64.7% in DTS-3/+ flies compared to control flies and nighttime sleep decreased by 15.7%. We also observed that the sleep pattern of DTS-3/+ flies overlapped significantly with that of control flies during the first 1 hr after the onset of darkness (Figure 2A), suggesting that the regulation of sleep onset in response to the switch from light to dark was not significantly affected in the DTS-3/+ flies. The reduction in total sleep in the DTS-3/+ flies was partly due to shortened sleep-bout duration, with the average sleep-bout duration reduced by 29.7% during the day and 44.0% during the night (Figure 2C). When the maximum sleep-bout length over a consecutive 4-hr period in DTS-3/+ and control flies was compared, the difference was most significant in the early/middle hours of the day (ZT 0–8) and in the late-night hours (ZT 20–24) (Figure 2D). There was no statistical difference in the maximum sleep-bout length between the DTS-3/+ and control flies during ZT 8–20 (Figure 2D).
Figure 2.—
Both daytime and nighttime sleep are reduced in the ecdysteroid-deficient mutant DTS-3. (A) Sleep patterns of control Samarkand and DTS-3/+ flies. Total sleep amount (B), average sleep-bout duration (C), maximum sleep-bout duration (D), wake-bout duration (E), and average waking activity (F) were calculated separately for daytime and nighttime sleep data. Data for control flies, DTS-3/+ and DTS-3/+ mutants treated with 20E, are presented in blue, red, and green, respectively. N = 51 for control and DTS-3/+, N = 36 for DTS-3/+ treated with 20E. *, P < 0.05. Error bars represent the SEM.
Interestingly, DTS-3/+ flies displayed a remarkable increase in wake-bout duration during the day; the average daytime wake-bout duration in DTS-3/+ flies was almost fivefold (485.3%) greater than that in control flies (Figure 2E). There was no significant increase in average wake-bout duration during the night. Waking activity increased during the day but not during the night in DTS-3 females (Figure 2F).
These sleep-related aspects of the DTS-3/+ phenotype (i.e., shorter total sleep time, shorter sleep bouts, longer wake bouts, and greater waking activity) were rescued almost to the levels of controls by the application of exogenous 20E (0.1 mm) to adult DTS-3/+ flies (Figure 2, B–F). This result strongly indicates that the observed sleep abnormalities in DTS-3/+ flies are not primarily due to developmental defects, but can be attributed to lower 20E levels at the adult stage.
Reducing the level of functional EcR results in a short-sleep phenotype:
The EcRs play essential roles in the actions of ecdysone during development, and homozygosity for an EcR loss-of-function mutation causes developmental lethality. We therefore used viable heterozygous EcR mutants to study the roles of EcRs in sleep regulation. EcRF288Y, EcRA483T, and EcRV559fs are all EMS-induced EcR mutant alleles (Bender et al. 1997). EcRF288Y has a point mutation in the DNA-binding domain, whereas EcRA483T and EcRV559fs carry a point mutation and a small deletion in the ligand-binding domain, respectively (Figure S2A). Because sleep-related parameters vary significantly across wild-type strains, our analyses were carried out in mutant fly strains and corresponding controls whose genetic backgrounds had been carefully matched (see Simon et al. 2003, supporting online material). All three EcR mutant strains were derived from a common strain that carries cn bw (Bender et al. 1997) and was used as a control in our experiments involving the EMS-induced EcR mutant alleles. These fly strains had previously been used to investigate the effects of EcR mutations on life span (Simon et al. 2003), a parameter that is known to be significantly affected by genetic background.
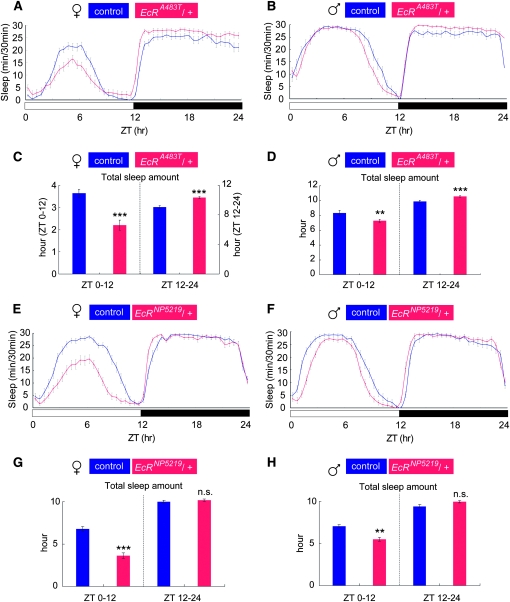
Total daytime sleep in flies heterozygous for EcRA483T (EcRA483T/+) was reduced compared to that in control flies (Figure 3, A–D). The effect of the heterozygous EcRA483T mutation was greater in females than in males, with EcR/+ females displaying a 39.5% reduction in daytime sleep and males a 12.5% reduction (Figure 3, C and D). In contrast to daytime sleep, nighttime sleep was not decreased but rather increased in these flies (Figure 3, C and D). Two other EMS-induced EcR mutations, EcRF288Y and EcRV559fs, had the same effects on sleep as EcRA483T (Figure S3, A–H). To confirm the effect of EcR mutations on sleep, we examined EcRNP5219, a homozygous-lethal EcR allele in which the loss-of-function phenotype is a consequence of the insertion of a P-element transposon in the EcR locus (Figure S2B) (Yoshihara and Ito 2000; Ishimoto et al. 2009). Using the antibody that binds to all EcR subtypes, we previously found that the total EcR protein level in adult head homogenates of EcRNP5219 heterozygotes (EcRNP5219/+) is approximately 50% of the wild-type (+/+) level (Ishimoto et al. 2009). This finding indicates that no or little EcR protein is produced from the EcRNP5219 allele. The sleep-related phenotypes of EcRNP5219/+ flies were similar to those observed in the chemically induced EcR mutants (Figure 3, E–H), except that EcRNP5219/+ flies did not show an increase in nighttime sleep (Figure 3, G and H).
Figure 3.—
Daytime sleep is reduced in heterozygous loss-of-function EcR mutants. Sleep patterns (A, B, E, and F) and total sleep amount (C, D, G, and H) are shown for EMS-induced (EcRA483T) or transposon P-element-induced (EcRNP5219) EcR mutants. All mutants were examined as heterozygotes. The total amount of sleep during the day (ZT 0–12) and during the night (ZT 12–24) were calculated separately, for flies of the indicated genotype and sex. Data for control flies and mutants are presented in blue and red, respectively. N = 65 (control and EcRA483T/+ female), N = 66 (control and EcRA483T/+ male), N = 66 (control and EcRNP5219/+). **, P < 0.01; ***, P < 0.001; n.s., no significant difference. Error bars represent the SEM.
The observation that a 50% reduction in the level of functional EcR caused a decrease in daytime sleep is consistent with our aforementioned findings that applying 20E promotes sleep and that the ecdysone synthesis mutant DTS-3 exhibits reduced sleep. To examine the effect of a further reduction in EcR activity on sleep, we utilized a temperature-sensitive EcR allele, EcRA483T, for which 18° and 25° are permissive and restrictive temperatures, respectively (Bender et al. 1997, 1998). Trans-heterozygous flies carrying EcRA483T and EcRNP5219 (EcRA483T/EcRNP5219) exhibit lethality during development if raised at 25°, whereas they survive to adulthood at 18°. In our experiment, EcRA483T/EcRNP5219 flies were raised to adulthood at 18° and transferred to 25° 3 days after eclosion, after which their sleep was analyzed at 25°.
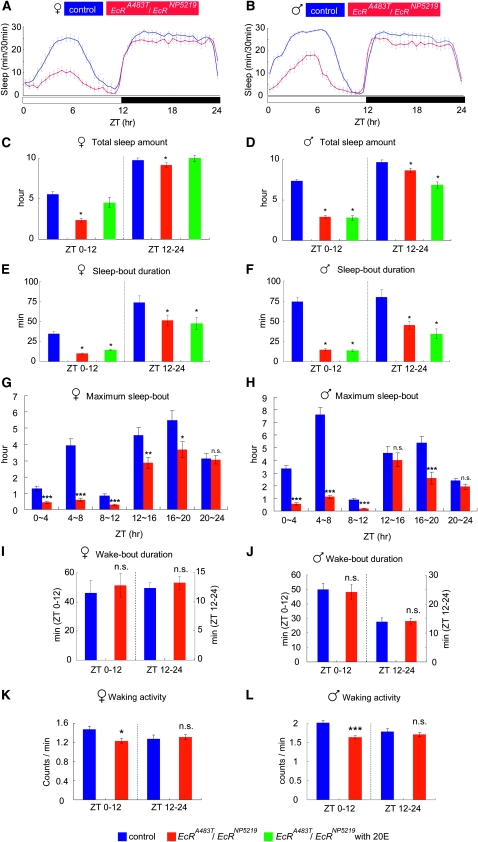
At 25°, EcRA483T/EcRNP5219 flies exhibited stronger sleep phenotypes than either EcRA483T/+ or EcRNP5219/+ flies. The total daytime sleep in the EcRA483T/EcRNP5219 flies was drastically reduced—by 56.0 and 61.0% in females and males, respectively—relative to those in the control animals (Figure 4, A–D). Unlike EcR/+ flies, the EcRA483T/EcRNP5219 trans-heterozygotes displayed a reduction in nighttime sleep, with total nighttime sleep decreased by 7.0 and 11.0% in females and males, respectively. The sleep-bout duration was also significantly decreased in both males and females, during the day and also at night (Figure 4, E and F). The reduction in sleep-bout duration was apparently the primary cause of the reduction in the total sleep in the EcRA483T/EcRNP5219 flies. The maximum sleep-bout length in EcRA483T/EcRNP5219 flies was extremely short, particularly during the day (Figure 4, G and H), further indicating the significance of EcR-mediated ecdysone signaling in the maintenance of sleep. In contrast to their DTS-3/+ counterparts, the EcRA483T/EcRNP5219 flies did not exhibit a significant increase in wake-bout duration (Figure 4, I and J). General hyperactivity was not the cause of the reduced sleep phenotype of EcRA483T/EcRNP5219 flies because the waking activity was not increased in the mutant flies. Rather, it was decreased during the day, in both EcRA483T/EcRNP5219 males and females (Figure 4, K and L).
Figure 4.—
Sleep deficiency is severe in EcR-mutant trans-heterozygotes. Sleep patterns (A and B), total sleep amount (C and D), sleep-bout duration (E and F), maximum sleep-bout length (G and H), wake-bout duration (I and J), and waking activity (K and L) were calculated from sleep data for EcRA483T/ EcRNP5219 trans-heterozygotes. Data for control flies, EcRA483T/ EcRNP5219 and EcRA483T/EcRNP5219 treated with 0.1 mm 20E, are presented in blue, red, and green. Sex is indicated above each graph. N = 58 (male), N = 66 (female). *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., no significant difference. Error bars represent the SEM.
In contrast to DTS-3/+ females where the application of 20E essentially rescued all of the sleep phenotypes, such application had only a limited influence on the EcRA483T/EcRNP5219 sleep phenotypes. Total sleep time in EcRA483T/EcRNP5219 females was increased to levels seen in controls by the application of 20E (0.1 mm) (Figure 4C). However, 20E did not have any significant rescue effect on the short sleep-bout length in these flies (Figure 4E). In EcRA483T/EcRNP5219 males, neither total sleep time nor sleep-bout length was rescued by the application of 20E (0.1 mm) (Figure 4, D and F).
Overexpression of EcR isoforms in the mushroom bodies of wild-type adult female flies leads to an increase in sleep:
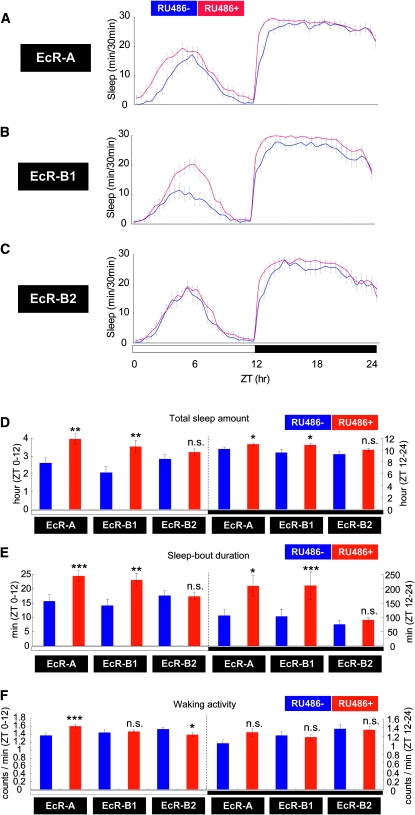
We also examined the effect of EcR overexpression on sleep. In Drosophila, three EcR isoforms—EcR-A, EcR-B1, and EcR-B2—are produced from the single EcR locus through alternative promoter usage and/or alternative splicing (Talbot et al. 1993). These isoforms have the same DNA and ligand-binding domains but differ in their N-terminal regions (Figure S2, A and B), display different expression patterns, and induce different cellular responses (Talbot et al. 1993). We used the GeneSwitch conditional expression system (Osterwalder et al. 2001; Roman et al. 2001) to induce the expression of each EcR isoform in the adult female brain. In this study, we specifically examined the effects of EcR overexpression in the mushroom bodies (MBs) on sleep, because the MBs are known to play a vital role in sleep regulation (Joiner et al. 2006; Pitman et al. 2006). To this end, we crossed the MB-specific GeneSwitch driver, MB-GS–GAL4, to either UAS–EcR-A, UAS–EcR-B1, or UAS–EcR-B2 (Lee et al. 2000) and examined the progeny for sleep in the presence or absence of the GeneSwitch activator, RU486. We found that conditional expression of either EcR-A or EcR-B1 in the adult MBs causes an increase in the total sleep amount, both during the day and at night (Figure 5, A, B, and D). Average sleep-bout duration also increased in response to the overexpression of EcR-A or EcR-B1 in the MBs (Figure 5E). The effects of EcR-A or EcR-B1 overexpression on sleep were largely opposite to those of the EcR mutations, i.e., total daytime sleep as well as daytime and nighttime sleep-bout duration increased. The overexpression of EcR-B2 in the MBs, on the other hand, had little effect on these parameters (Figure 5, C–E). In terms of waking activity, the changes were less dramatic, although overexpression of EcR-A and EcR-B2 led to some increases during the day (Figure 5F). These experiments demonstrated that conditional overexpression of wild-type EcRs in the adult MBs results in an isoform-specific increase in sleep. It should be pointed out, however, that the results of this gain-of-function experiment does not exclude the possibility that cell types other than MB neurons play a significant role in the loss-of-function sleep phenotype observed in EcR mutants.
Figure 5.—
Sleep is promoted by the conditional expression of certain EcR subtypes in the mushroom bodies. The A, B1, and B2 isoform of EcR were expressed in mushroom bodies using the RU486-inducible Gal4 driver MB-GS–Gal4. (A, B, and C) Sleep patterns in RU486-treated and untreated flies. The induced EcR isoforms are indicated to the left of the sleep pattern. The total sleep amount (D), sleep-bout duration (E), and waking activity (F) were calculated from each set of sleep data. Data for RU486-treated and -untreated flies are presented in red and blue, respectively. N = 55 for each genotype. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., no significant difference. Error bars represent the SEM.
Mutants with defective ecdysone signaling exhibit impaired homeostatic sleep regulation:
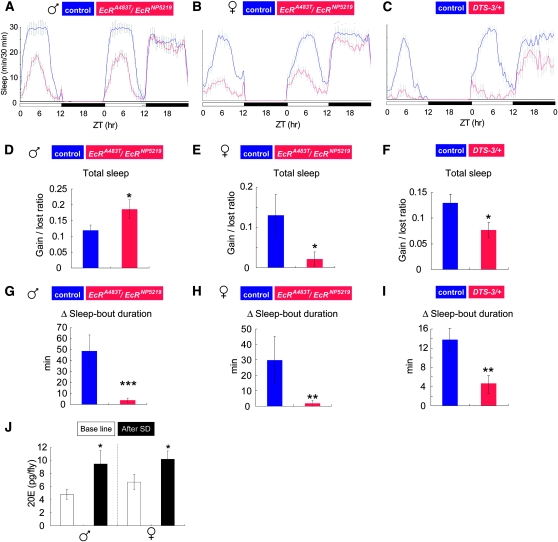
One of the key features of sleep is its homeostatic regulation; the intensity and duration of sleep are dependent on the amount of previous wakefulness (Horne 1985). This is the case in Drosophila sleep as well (Hendricks et al. 2000; Shaw et al. 2000; Huber et al. 2004). To examine whether ecdysone signaling plays a role in sleep homeostasis, we performed sleep deprivation experiments with EcR and DTS-3 mutants, using a mechanical sleep deprivation method that is known to cause a postdeprivation increase in sleep duration and intensity (Huber et al. 2004). EcRA483T/EcRNP5219 and DTS-3/+ mutants, as well as appropriate controls, were kept awake for 12 hr during the night and their sleep was analyzed before and after sleep deprivation. EcRA483T/EcRNP5219 females regained only 2% of the sleep lost during 12 hr sleep deprivation, whereas control females regained 13.0%, during the 12-hr period immediately following sleep deprivation (Figure 6, B and E). Similarly, DTS-3/+ mutants exhibited a smaller sleep rebound (7.6%) than the corresponding controls (13.0%) (Figure 6, C and F). The response of EcRA483T/EcRNP5219 males to sleep deprivation, however, differed from that of females. In fact, they recovered 18.6% of lost sleep during the 12-hr period following sleep deprivation, a level significantly greater than that achieved by their control (wild-type) male counterparts (11.9%) (Figure 6, A and B). The gain of total sleep in control male flies seems to have been restricted by their high basal level of sleep. To circumvent this apparent ceiling effect on sleep rebound in control males with respect to total sleep time, we examined how sleep deprivation affects sleep-bout duration in controls vs. ecdysone signaling mutants. As shown in Figure 6, G–I, the average sleep-bout duration was significantly increased in control flies. Remarkably, neither EcRA483T/EcRNP5219 males nor females showed much change in sleep-bout duration after 12 hr sleep deprivation (Figure 6, G and H). The same tendency was also observed in DTS-3 mutants (Figure 6I). Furthermore, we found that in wild-type flies, both male and female, the levels of 20E were higher following sleep deprivation (Figure 6J). Although there is the possibility that repetitive mechanical stimulation used for sleep deprivation may also contribute to the change in 20E levels, our results suggest that ecdysone signaling is activated by sleep deprivation and that it plays a role in the homeostatic regulation of sleep.
Figure 6.—
The homeostatic sleep response is defective in flies with reduced ecdysone signaling. (A, B, and C) Sleep patterns are depicted for the sleep response after 12 hr sleep deprivation. (D, E, and F) The ratio of the regained sleep/sleep loss was calculated for each genotype. (G, H, and I) The Δ sleep-bout duration indicates the homeostatic response to compensate for the lost sleep. Data for control flies and mutants are presented in blue and red, respectively. Genotypes and sex are indicated above each graph. (J) The total amount of 20E was measured in flies without (baseline, open bar) or with sleep deprivation (with SD, solid bar). N = 42 (EcRA483T/EcRNP5219 and control), N = 36 (DTS-3/+ and control), N = 8 (20E measurement). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent the SEM.
DISCUSSION
The molting steroid hormone ecdysone regulates Drosophila sleep:
In this study, we demonstrate through both genetic and pharmacological approaches that ecdysone is intimately involved in the regulation of Drosophila sleep and that ecdysone has a sleep-promoting effect. These conclusions are based on the sleep analysis using the DAM system. A recent study pointed out that sleep, particularly daytime sleep, could be erroneously defined by the DAM system due to its inability to detect brief movements of flies (Zimmerman et al. 2008b). To evaluate the sleep phenotype in EcRA483T/EcRNP5219 and DTS-3/+ flies independently of the DAM system, we directly observed their movements from ZT4 to ZT8 using a video-recording system (see materials and methods) and sleep parameters were calculated using a Drosophila sleep analysis software, pySolo (Gilestro and Cirelli 2009). The video-based analysis demonstrated that sleep is indeed reduced in EcRA483T/EcRNP5219 and DTS-3/+ flies during the observation period (Figure S4), confirming the original conclusions drawn from the DAM-based sleep analysis.
Previous reports have shown that ecdysone signaling at the adult stage plays a role in the regulation of oogenesis (Buszczak et al. 1999), stress responses, life span (Simon et al. 2003), and formation of long-term memory (Ishimoto et al. 2009). It appears that ecdysone signaling is activated in adults when they are in stressful environments, possibly as a means of urgently managing unfavorable internal conditions caused by these environments. In that sense, ecdysone or 20E might have a function as a stress hormone in adult flies. EcR/+ and DTS-3/+ flies, in which ecdysone signaling is less active, exhibit an increase in life span relative to their wild-type counterparts (Simon et al. 2003), suggesting that frequent or chronic activation of ecdysone signaling is detrimental in adults because it alters metabolic states and leads to increases in the generation of harmful by-products. One of the proposed functions for sleep is to remove undesirable by-products that accumulate during the waking state (Hartmann 1973). Interestingly, 20E levels in wild-type flies tend to increase during daytime (Figure S5), possibly corresponding to the generation of harmful by-products during the major waking period. Flies with suboptimal ecdysone signaling sleep less and fail to exhibit adequate sleep rebound following sleep deprivation. These flies may not accumulate detrimental materials to the same extent as their wild-type counterparts, reducing their sleep need.
Ecdysone signaling controls sleep–wake regulatory processes through EcR-dependent and independent pathways:
The fact that EcRA483T/EcRNP5219 flies show severe defects in sleep indicates that EcR-mediated gene transcription is important for sleep regulation. However, our results suggest that EcR-independent pathways also play a role in the ecdysone-mediated sleep–wake regulatory processes. Specifically, wake-bout duration is likely controlled by such EcR-independent pathways, in light of the following observations. Wake-bout duration during the day is drastically increased in DTS-3/+ mutants and is considerably decreased in 20E-treated wild-type flies; thus ecdysone signaling has significant effects on wake-bout duration. However, EcRA483T/EcRNP5219 females, in which EcR-mediated ecdysone signaling is severely impaired, display normal wake-bout duration. Moreover, administering 20E to EcRA483T/EcRNP5219 females leads to a significant reduction in wake-bout duration (daytime 34.0% and nighttime 30.8%) as is seen in the 20E-treated control flies. Together, these results suggest that EcR-dependent ecdysone signaling pathways are rather dispensable for the regulation of wake-bout duration. Unlike the EcR-dependent transcriptional cascades, which have been well characterized, little is known about the nature of EcR-independent, “nongenomic” ecdysone pathways. One potentially important component of the latter pathways is DopEcR, a novel G protein-coupled receptor with structural similarity to vertebrate β-adrenergic-like receptor (Srivastava et al. 2005). In vitro experiments have demonstrated that the activity of DopEcR can be modulated by both dopamine and ecdysteroids and that DopEcR has effects on multiple intracellular signaling cascades (Srivastava et al. 2005). Future functional studies of DopEcR are expected to provide insights into possible functions of EcR-independent ecdysone signaling in the regulation of sleep and wakefulness.
Ecdysone may have a role in neural modification during sleep and wakefulness:
An intriguing hypothesis for the function of sleep is that it contributes to the modulation of synapses in the brain and thus to neural plasticity (Benington and Frank 2003). Ecdysone has an intrinsic ability to modulate the structure and function of the nervous system during both development and adulthood. It has been shown in Drosophila that ecdysone controls neuronal remodeling during formation of the adult nervous system and that it does so through signaling pathways involving EcRs and TGF-β (Kraft et al. 1998; Lee et al. 2000; Zheng et al. 2003). Ecdysone also plays a role in remodeling of the adult brain in the house cricket (Acheta domesticus), in this case by inhibiting neuroblast proliferation in the MBs and triggering their differentiation into interneurons (Cayre et al. 2000). In honeybees (Apis mellifera L.), ecdysone exposure activates an EcR-mediated transcriptional cascade in the adult MB neurons, suggesting that ecdysone is important for reorganization of the adult brain (Velarde et al. 2009). Moreover, we have recently discovered that long-term courtship memory in Drosophila, which is likely associated with the stable modification of synaptic function and/or structure in the adult brain, is dependent on EcR-mediated ecdysone signaling (Ishimoto et al. 2009). These findings are consistent with the possibility that ecdysone is involved in sleep-associated changes to structure and function in the adult brain. A recent study reported that several synaptic marker proteins in the Drosophila brain show widespread alterations in their expression levels as a function of sleep–wake cycles (Gilestro et al. 2009). Our data suggest that the endocrine system, in particular ecdysone signaling, contributes to such global changes in the adult nervous system and thus plays important roles in the regulation of the brain states during sleep and wakefulness.
A functional and mechanistic link may exist between sleep and developmentally programmed behavioral quiescence:
Insects undergo molting and metamorphosis during development to accommodate changes in their size and morphology. During these critical and drastic developmental processes, the physiological and behavioral states of the animals are controlled by genetically determined developmental programs, so that the molecular, cellular, and behavioral events that are essential for developmental transitions are completed in a highly organized fashion. Ecdysone plays key roles in triggering and orchestrating these events. Our findings in this study suggest that sleep is related to developmental processes through ecdysone, and it is possible that the ecdysone-dependent molecular cascades that are activated during development may be recurrently activated in adults to regulate sleep and wakefulness. Interestingly, the quiescent state exhibited by the silkworm prior to each molt is referred to as “min” in Japanese, which literally means “sleep.” More importantly, studies in the nematode Caenorhabditis elegans have shown that the quiescence associated with lethargus—a developmental period that coincides with larval-stage transitions—has various sleep-like features (Raizen et al. 2008). This discovery further supports a link between developmental processes and sleep. The fact that the phenomenon of sleep is conserved in evolutionarily diverse animals (Campbell and Tobler 1984; Hendricks et al. 2000; Shaw et al. 2000; Greenspan et al. 2001) indicates that sleep is of ancient origin and functional significance—and it is possible that sleep and other sleep-like states in the adult may have originated from a genetically programmed behavioral state that facilitates homeostatic regulation during development. Thus, understanding the genetics underlying ecdysone-mediated sleep regulation is expected to lead to a better understanding of the function and evolutionary origin of sleep, as well as of the mechanisms that control this characteristic physiological and behavioral state.
Acknowledgments
We thank Dr. Simon (York College, The City University of New York, NY) for fly stocks and Dr. Kume (Kumamoto University, Kumamoto, Japan) for the sleep-analysis program. We also thank Dr. Maroy (University of Szeged, Szeged, Hungary) for sharing his unpublished data regarding the identity of DTS-3 and Dr. Gilestro and Dr. Cirelli (University of Wisconsin, Madison, WI) for the sleep analysis software, pySolo. This study was supported partly by grants from the National Institutes of Health (R01 MH62684 and MH085081), National Alliance for Research on Schizophrenia and Depression (Young Investigator Award), and the University of Iowa (Biological Sciences Funding Program) to T.K., and by a fellowship from the Uehara Memorial Foundation to H.I.
Supporting information available online at http://www.genetics.org/cgi/content/full/genetics.110.114587/DC1.
References
- Andretic, R., B. van Swinderen and R. J. Greenspan, 2005. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15 1165–1175. [DOI] [PubMed] [Google Scholar]
- Aranda, A., and A. Pascual, 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 81 1269–1304. [DOI] [PubMed] [Google Scholar]
- Bender, M., F. B. Imam, W. S. Talbot, B. Ganetzky and D. S. Hogness, 1997. Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell 91 777–788. [DOI] [PubMed] [Google Scholar]
- Bender, M., G. E. Carney, A. A. Wade, T. R. Li, J. W. Truman et al., 1998. Mutational dissection of the Drosophila ecdysone receptor (EcR) gene: EcR is required maternally for normal oogenesis and EcR-B isoforms are required for neuronal remodeling during metamorphosis. Dev. Biol. 198 221. [Google Scholar]
- Benington, J. H., and M. G. Frank, 2003. Cellular and molecular connections between sleep and synaptic plasticity. Prog. Neurobiol. 69 71–101. [DOI] [PubMed] [Google Scholar]
- Buszczak, M., M. R. Freeman, J. R. Carlson, M. Bender, L. Cooley et al., 1999. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 126 4581–4589. [DOI] [PubMed] [Google Scholar]
- Campbell, S. S., and I. Tobler, 1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8 269–300. [DOI] [PubMed] [Google Scholar]
- Cayre, M., C. Strambi, A. Strambi, P. Charpin and J. P. Ternaux, 2000. Dual effect of ecdysone on adult cricket mushroom bodies. Eur. J. Neurosci. 12 633–642. [DOI] [PubMed] [Google Scholar]
- Chung, B. Y., V. L. Kilman, J. R. Keath, J. L. Pitman and R. Allada, 2009. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr. Biol. 19 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli, C., and D. Bushey, 2008. Sleep and wakefulness in Drosophila melanogaster. Ann. NY Acad. Sci. 1129 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, R. M., 1988. The steroid and thyroid hormone receptor superfamily. Science 240 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltenyi, K., R. J. Greenspan and J. W. Newport, 2007. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat. Neurosci. 10 1160–1167. [DOI] [PubMed] [Google Scholar]
- Gilestro, G. F., and C. Cirelli, 2009. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics 25 1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro, G. F., G. Tononi and C. Cirelli, 2009. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 324 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan, R. J., G. Tononi, C. Cirelli and P. J. Shaw, 2001. Sleep and the fruit fly. Trends Neurosci. 24 142–145. [DOI] [PubMed] [Google Scholar]
- Handler, A. M., 1982. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev. Biol. 93 73–82. [DOI] [PubMed] [Google Scholar]
- Hartmann, E., 1973. Functions of Sleep. Yale Univ. Press, New Haven, CT.
- Hendricks, J. C., S. M. Finn, K. A. Panckeri, J. Chavkin, J. A. Williams et al., 2000. Rest in Drosophila is a sleep-like state. Neuron 25 129–138. [DOI] [PubMed] [Google Scholar]
- Hendricks, J. C., S. Lu, K. Kume, J. C. Yin, Z. Yang et al., 2003. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J. Biol. Rhythms 18 12–25. [DOI] [PubMed] [Google Scholar]
- Holden, J. J., and D. T. Suzuki, 1973. Temperature-sensitive mutations in Drosophila melanogaster. XII. The genetic and developmental effects of dominant lethals on chromosome 3. Genetics 73 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne, J. A., 1985. Sleep function, with particular reference to sleep deprivation. Ann. Clin. Res. 17 199–208. [PubMed] [Google Scholar]
- Huber, R., S. L. Hill, C. Holladay, M. Biesiadecki, G. Tononi et al., 2004. Sleep homeostasis in Drosophila melanogaster. Sleep 27 628–639. [DOI] [PubMed] [Google Scholar]
- Ishimoto, H., T. Sakai and T. Kitamoto, 2009. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 106 6381–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner, W. J., A. Crocker, B. H. White and A. Sehgal, 2006. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441 757–760. [DOI] [PubMed] [Google Scholar]
- Kraft, R., R. B. Levine and L. L. Restifo, 1998. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J. Neurosci. 18 8886–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume, K., S. Kume, S. K. Park, J. Hirsh and F. R. Jackson, 2005. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25 7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavranos, G., R. Angelopoulou, P. Manolakou and M. Balla, 2006. Hormonal and meta-hormonal determinants of sexual dimorphism. Coll. Antropol. 30 659–663. [PubMed] [Google Scholar]
- Lee, T., S. Marticke, C. Sung, S. Robinow and L. Luo, 2000. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28 807–818. [DOI] [PubMed] [Google Scholar]
- Mackiewicz, M., N. Naidoo, J. E. Zimmerman and A. I. Pack, 2008. Molecular mechanisms of sleep and wakefulness. Ann. NY Acad. Sci. 1129 335–349. [DOI] [PubMed] [Google Scholar]
- Neubueser, D., J. T. Warren, L. I. Gilbert and S. M. Cohen, 2005. molting defective is required for ecdysone biosynthesis. Dev. Biol. 280 362–372. [DOI] [PubMed] [Google Scholar]
- Osterwalder, T., K. S. Yoon, B. H. White and H. Keshishian, 2001. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA 98 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky, K. M., J. Agosto, S. R. Pulver, Y. Shang, E. Kuklin et al., 2008. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman, J. L., J. J. McGill, K. P. Keegan and R. Allada, 2006. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441 753–756. [DOI] [PubMed] [Google Scholar]
- Raizen, D. M., J. E. Zimmerman, M. H. Maycock, U. D. Ta, Y. J. You et al., 2008. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 451 569–572. [DOI] [PubMed] [Google Scholar]
- Rauschenbach, I. Y., M. Z. Sukhanova, A. Hirashima, E. Sutsugu and E. Kuano, 2000. Role of the ecdysteroid system in the regulation of Drosophila reproduction under environmental stress. Dokl. Biol. Sci. 375 641–643. [DOI] [PubMed] [Google Scholar]
- Roman, G., K. Endo, L. Zong and R. L. Davis, 2001. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98 12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. J., C. Cirelli, R. J. Greenspan and G. Tononi, 2000. Correlates of sleep and waking in Drosophila melanogaster. Science 287 1834–1837. [DOI] [PubMed] [Google Scholar]
- Simon, A. F., C. Shih, A. Mack and S. Benzer, 2003. Steroid control of longevity in Drosophila melanogaster. Science 299 1407–1410. [DOI] [PubMed] [Google Scholar]
- Srivastava, D. P., E. J. Yu, K. Kennedy, H. Chatwin, V. Reale et al., 2005. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 25 6145–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger, A., 2003. Sleep and endocrinology. J. Intern. Med. 254 13–22. [DOI] [PubMed] [Google Scholar]
- Talbot, W. S., E. A. Swyryd and D. S. Hogness, 1993. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell 73 1323–1337. [DOI] [PubMed] [Google Scholar]
- Terashima, J., K. Takaki, S. Sakurai and M. Bownes, 2005. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J. Endocrinol. 187 69–79. [DOI] [PubMed] [Google Scholar]
- Truman, J. W., and L. M. Riddiford, 2002. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 47 467–500. [DOI] [PubMed] [Google Scholar]
- Velarde, R. A., G. E. Robinson and S. E. Fahrbach, 2009. Coordinated responses to developmental hormones in the Kenyon cells of the adult worker honey bee brain (Apis mellifera L.). J. Insect Physiol. 55 59–69. [DOI] [PubMed] [Google Scholar]
- Walker, V. K., K. L. Watson, J. J. Holden and C. G. H. Steel, 1987. Vitellogenesis and fertility in Drosophila females with low ecdysteroid titres; the L(3)3DTS mutation. J. Insect Physiol. 33 137–142. [Google Scholar]
- Yoshihara, M., and K. Ito, 2000. Improved Gal4 screening kit for large-scale generation of enhancer-trap strains. Dros. Inf. Serv. 83 199–202. [Google Scholar]
- Yuan, Q., W. J. Joiner and A. Sehgal, 2006. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol. 16 1051–1062. [DOI] [PubMed] [Google Scholar]
- Zheng, X., J. Wang, T. E. Haerry, A. Y. Wu, J. Martin et al., 2003. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112 303–315. [DOI] [PubMed] [Google Scholar]
- Zimmerman, J. E., N. Naidoo, D. M. Raizen and A. I. Pack, 2008. a Conservation of sleep: insights from non-mammalian model systems. Trends Neurosci. 31 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, J. E., D. M. Raizen, M. H. Maycock, G. Maislin and A. I. Pack, 2008. b A video method to study Drosophila sleep. Sleep 31 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]