Abstract
Genome stability in fission yeast requires the conserved S-phase kinase Hsk1 (Cdc7) and its partner Dfp1 (Dbf4). In addition to their established function in the initiation of DNA replication, we show that these proteins are important in maintaining genome integrity later in S phase and G2. hsk1 cells suffer increased rates of mitotic recombination and require recombination proteins for survival. Both hsk1 and dfp1 mutants are acutely sensitive to alkylation damage yet defective in induced mutagenesis. Hsk1 and Dfp1 are associated with the chromatin even after S phase, and normal response to MMS damage correlates with the maintenance of intact Dfp1 on chromatin. A screen for MMS-sensitive mutants identified a novel truncation allele, rad35 (dfp1-(1–519)), as well as alleles of other damage-associated genes. Although Hsk1–Dfp1 functions with the Swi1–Swi3 fork protection complex, it also acts independently of the FPC to promote DNA repair. We conclude that Hsk1–Dfp1 kinase functions post-initiation to maintain replication fork stability, an activity potentially mediated by the C terminus of Dfp1.
THE Hsk1 protein kinase, the fission yeast ortholog of Saccharomyces cerevisiae Cdc7, is a conserved protein essential for the initiation of DNA replication (Masai et al. 1995; Brown and Kelly 1998; Snaith et al. 2000). Data from many systems suggest that the kinase functions at individual replication origins to activate the prereplication complex (preRC) through phosphorylation of the MCM helicase and other subunits (reviewed in Forsburg 2004). In fission yeast, Hsk1 kinase activity is limited to S phase by its regulatory subunit Dfp1, which is transcriptionally and post-translationally regulated to restrict its peak of activity to S phase (Brown and Kelly 1999; Takeda et al. 1999). The requirement for Dfp1 (in S. cerevisiae, Dbf4) is similar to the dependence of CDK kinases on cyclin activity; thus, the Ccd7 kinase family has been dubbed DDK (Dbf4-dependent kinases) (Johnston et al. 1999; Duncker and Brown 2003). Hsk1 is a target of the Cds1 checkpoint kinase and undergoes Cds1-dependent phosphorylation during hydroxyurea (HU) treatment in vivo and in vitro (Snaith et al. 2000). Interestingly, deletion of Δcds1 partly rescues hsk1–1312 temperature sensitivity, which suggests that Hsk1 is negatively regulated by the replication checkpoint. In turn, Cds1 is poorly activated in hsk1 mutants after HU treatment, indicating that there may be a feedback loop linking these two kinases (Snaith et al. 2000; Takeda et al. 2001). hsk1 mutants are sensitive to HU treatment, with a phenotype suggesting a specific defect in recovery (Snaith et al. 2000).
DDK kinases have substrates outside of the replication initiation pathway. Functional dissection of Schizosaccaromyces pombe Dfp1 identifies separate regions that are required for checkpoint response (N-terminal domain; Takeda et al. 1999; Fung et al. 2002), for centromere cohesion and replication (MIR domain; Bailis et al. 2003; Hayashi et al. 2009) and for proper response to alkylation damage during S phase (C-terminal domain; Takeda et al. 1999; Fung et al. 2002). Recent studies indicate that the DDK kinase is required for initiation of programmed double-strand breaks in meiosis (Sasanuma et al. 2008; Wan et al. 2008) and meiotic chromosome orientation (Lo et al. 2008; Matos et al. 2008). The different domains of Dfp1 are presumed to target the Hsk1 kinase to different substrates. Because kinase activity is limited to S phase, these results suggest that the cell uses the DDK kinase to link various cell-cycle events to S-phase passage.
MMS causes alkylation damage that affects replication forks (Wyatt and Pittman 2006; Kaina et al. 2007). This results in Cds1-dependent slowing of DNA replication forks (Lindsay et al. 1998; Marchetti et al. 2002). However, Δcds1 mutants are only modestly sensitive to MMS treatment (Lindsay et al. 1998; Marchetti et al. 2002), suggesting at least partial independence from the replication checkpoint. In contrast, hsk1 and dfp1 C-terminal mutants are extremely MMS sensitive (Snaith et al. 2000; Takeda et al. 2001; Fung et al. 2002; Matsumoto et al. 2005; Sommariva et al. 2005). It has been suggested that this reflects Hsk1 association with the fork protection complex (FPC), which consists of the nonessential proteins Swi1/ScTof1 and Swi3/ScCsm3, which are required for replication fork pausing (Noguchi et al. 2003, 2004; Krings and Bastia 2004; Matsumoto et al. 2005; Sommariva et al. 2005). In budding yeast, tof1 mutants treated with HU show uncoupling of replication machinery from the fork (Katou et al. 2003), which underscores the importance of maintaining replication fork stability at sites of pausing or damage. This uncoupling suggests that one function of the FPC, and perhaps Hsk1, is holding together the stalled replisome to facilitate replication fork restart.
However, the FPC may not be the only way Hsk1 contributes to MMS response. Alkylation damage during S phase is repaired by several mechanisms, including homologous recombination, template switching, and translesion synthesis pathways controlled by the Rad6/Rad18 (SpRhp6/SpRhp18) epistasis group (reviewed in Verkade et al. 2001, Barbour and Xiao 2003; Wyatt and Pittman 2006; Branzei and Foiani 2007; Andersen et al. 2008). While activation of translesion synthesis may be coupled to a polymerase switching event at the fork, evidence suggests that it occurs behind the replication fork as well [reviewed in Branzei and Foiani (2007); Lambert et al. (2007)]. Several studies suggest that checkpoint proteins may be intimately involved in the decision between recombination, template switching, and translesion synthesis (Paulovich et al. 1998; Kai and Wang 2003; Liberi et al. 2005; Kai et al. 2007). An intriguing observation links DDK kinases specifically to translesion synthesis. Induced mutagenesis is the result of error-prone bypass of lesions following DNA damage (reviewed in Barbour and Xiao 2003; Andersen et al. 2008), and budding yeast Cdc7 is one of the few proteins required for induced mutagenesis, outside of the specialized translesion synthesis (TLS) polymerases (Njagi and Kilbey 1982a,b). Recent data suggest that ScCdc7 participates in TLS (Pessoa-Brandao and Sclafani 2004), although the mechanism is not clear.
In this study, we investigate the contributions of Hsk1 and Dfp1 to replication recovery mechanisms post-initiation by analyzing its contributions to fork stability and repair. Our data suggest that Hsk1–Dfp1 functions at the replication fork after initiation to promote appropriate modes of recovery independent of the FPC. Mutations that destabilize the replication fork are particularly sensitive to attenuation of Hsk1 activity. hsk1–1312 phenotypes overlap with, but can be distinguished from, phenotypes associated with FPC components swi1 and swi3, indicating that they perform distinct functions in the response to DNA damage. Hsk1 is likely to perform multiple functions as it has pleiotropic effects: first, in the maintenance of genome integrity, and second, the response to alkylation damage. hsk1–1312 cells suffer DNA damage even under permissive conditions and this causes increased rates of mitotic recombination and increased recruitment of Rad22 (ScRad52). We show that both hsk1+ and dpf1+ are required for induced mutagenesis in response to alkylation, and epistasis suggests this is through the error-prone TLS pathway. We isolated a novel allele of dfp1+ in a screen for MMS-sensitive mutations. Our data suggest that the effect is mediated by the C terminus of Dfp1, and we propose that this domain is required to maintain Hsk1 and Dfp1 on the chromatin during alkylation damage to promote appropriate repair and contribute to genome stability after replication initiation.
MATERIALS AND METHODS
Yeast manipulation:
S. pombe strains were grown in Edinburgh minimal medium (EMM) or Pombe glutamate medium (PMG) and supplemented with adenine, histidine, leucine, and uracil as required (Moreno et al. 1991). Crosses were performed as described (Moreno et al. 1991). All strains were derived from 972 h−. Strain genotypes are shown in supporting information, Table S1. In experiments with temperature-sensitive strains, cultures were grown at 25° and shifted to 36° for 4 hr (approximately one cell cycle). Arrests of temperature-sensitive strains confirmed by flow cytometry were performed as described (Dolan et al. 2004; data not shown). Synthetic lethal mutants were those unable to generate a viable double mutant compared to formation of >20 nonparental wild-type colonies from the same cross.
Construction of dfp1v5∷ura4+ strains:
A XhoI–NotI fragment from pmyc42X6his–dfp1 (gift of Grant Brown) was cloned into pJAH1172, a LEU2 vector with a C-terminal 3xv5 epitope tag expressed by nmt (J. A. Hodson and S. L. Forsburg, unpublished data), to create pWPD12. A 2-kb XhoI–SmaI fragment was excised from WPD12 and cloned into pJK210 (Keeney and Boeke 1994) to create pWPD35. pWPD35 was digested with EcoRI, and the resultant 6-kb fragment was used to transform strain FY528 by electroporation (Kelly et al. 1993). Ura+ transformants were streaked to yeast extract with supplements and single colonies restreaked to EMM lacking uracil to ensure stable Ura+ transformants.
UV survival analysis:
Strains were grown overnight at 25° to mid-log phase in YES. Cultures were diluted in YES, plated, allowed to dry, and exposed to UV light. Plates were wrapped in aluminum foil and incubated at 25° for 3 to 5 days. Experiments were performed three times with duplicate plates for each experiment.
Mitotic recombination analysis:
Single colonies were taken from EMM His− plates, inoculated directly into YES, and grown at 25° for 20 to 30 hr. Cell density was counted on a hemacytometer immediately after incubation and before plating 104 cells to two EMM Ade− plates. Ade+ cells were patched to EMM Ade− and EMM Ade− His− to determine auxotrophies. Mitotic recombination frequency was determined per generation (Stewart et al. 1997). Data are the averages of seven or eight independent cultures. Significance was assessed by using Mann–Whitney U-test available online at http://elegans.swmed.edu/∼leon/stats/utest.html
Rad22YFP microscopy:
Logarithmic phase cultures growing at 25° in supplemented EMM were split and HU was added to one culture for a final concentration of 12 mm. Cultures were grown for 3 hr. Cells were collected, washed twice in PBS, and resuspended in PBS. Cells were spotted on slides with poly-l-lysine and air dried. Cells were viewed at 60× on a DeltaVision Spectris microscope (Applied Precision, Issaquah, WA) and eight 0.5 μm sections were taken, deconvolved, and projected to one image with softWoRx (Applied Precision, Issaquah, WA). These images were viewed and contrast adjusted in Canvas 8-10 (ACD Systems, Victoria, BC, Canada). For two experiments, 100–500 cells were counted per experiment.
In situ chromatin binding assays:
Assays were modified from (Dolan et al. 2004) as follows: hsk1HA, dfp1HA, and dfp1-(1–459)HA strains were viewed with a 1:250 dilution of monoclonal anti-HA 16B12 (BabCO, Berkeley, CA). dfp1v5 strains were immunostained with a 1:500 dilution of mouse anti-v5 antibody (Invitrogen, Carlsbad, CA). The above samples were incubated with a 1:250 dilution of goat anti-mouse∷AlexaFluor 546 secondary antibody (Molecular Probes, Eugene, OR). Fixed, stained cells were spotted on microscope slides treated with poly-l-lysine (Sigma-Aldrich, St. Louis, MO). Cells were viewed at 60× on a DeltaVision Spectris microscope and images were taken with softWoRx (Applied Precision, Issaquah, WA). hsk1HA assays were performed three times; others were performed at least twice. One hundred cells were scored per sample per experiment.
Induced mutagenesis:
The fluctuation analysis protocol was adapted from Liu et al. (1999). Strains were grown on EMM–uracil at 25°. For each strain, at least 12 independently chosen colonies were inoculated into 5 ml PMG–uracil for overnight culture and incubated at 25°. From each overnight culture, cells cultures at about 0.8 OD595 were diluted in YES liquid for 6.5 generations at 25° to mid-logarithmic phase (OD595 = 0.8). Half the culture was incubated with 0.0025% MMS for 1 hr while the other half was left untreated. Cells were counted using a hemocytometer and equal numbers were washed twice in 10 ml PMG–uracil, and resuspended in 1 ml PMG–uracil. Twenty-five microliters of a 1:2000 dilution was plated onto YES plates to determine the number of cells surviving after MMS treatment. Cells 2 × 105 were plated onto PMG–FOA plates and incubated at 25° for 10 days. Cells plated onto YES plates were incubated at 25° for 5 days before counting. The uracil reversion rate was calculated according to the following formula: 1 − e(1/x)×ln((y−z)/(y)), where x is the duration of incubation in YES measured in number of generations, y is the total number of cells plated, and z is the number of colonies growing on PMG–FOA plates.
The R software package [Version 2.7.2 (2008-08-25)/The R Foundation for Statistical Computing] was used to determine the statistics and to generate the box plot of the relative mutation rate. The relative mean forward mutation rate was calculated by dividing the mean forward mutation rate for each strain and condition by that of the untreated wild-type strain. Two-sided 95% confidence intervals were calculated from the one sample t-test. To test whether there is a difference in the mean reversion rate, the P-values were calculated using the Welch two-sample t-test to accommodate the differences in sample sizes.
UV mutagenesis:
The strain HE686 (h90 smt-0 leu1–32 ura4–D18) was mutagenized with UV to about 10% viability. Approximately 40,000 treated cells were plated on YES agar (YEA) (about 200 cells per plate) and the resulting colonies were replicated on YEA + 0.01% MMS. Sensitive mutants were picked from the master plates and retested for MMS sensitivity. Ninety-two mutants proved to be MMS sensitive on YEA + 0.01% MMS. Ten of the clearly sensitive strains were analyzed further.
RESULTS
hsk1–1312 sensitivity to replication fork disruption:
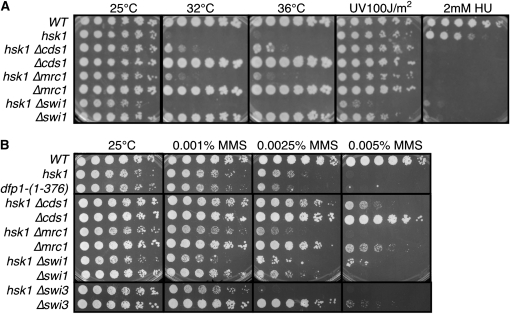
To dissect the contributions of Hsk1 to replication fork stability, we compared the damage sensitivity of hsk1–1312 double mutants with mutations in the replication checkpoint (Δcds1, Δmrc1) and the fork protection complex (Δswi1, Δswi3). Since Δcds1 partly suppresses hsk1 temperature sensitivity (Snaith et al. 2000), and Mrc1 contributes to Cds1 activity (Tanaka and Russell 2001; Xu et al. 2006), we were not surprised to see a similar partial rescue of hsk1–1312 by Δmrc1 (Figure 1). Interestingly, deletion of Δcds1 also partly rescues the MMS sensitivity associated with hsk1–1312, consistent with previous genetic analysis suggesting that Cds1 negatively regulates Hsk1 (Snaith et al. 2000). Although Δcds1 is much less MMS sensitive than hsk1–1312 (e.g., Figure 1), the most parsimonious explanation for the result is that Cds1 actively restrains some aspect of Hsk1 activity during the MMS response that is required for resistance. But it is also possible that rapid collapse of replication forks after stalling at alkylated bases facilitates survival because it leads more efficiently into recombination–mediated repair or bypass pathways.
Figure 1.—
Synthetic interactions of hsk1–1312 with replication checkpoint and fork protection complex mutations. (A) hsk1 temperature sensitivity is suppressed by deletion of Δcds1 or Δmrc1 but not Δswi1. (B) MMS sensitivity of hsk1 and dfp1, Δswi1, and Δswi3 single and double mutants. Cells were diluted fivefold on YES under the indicated conditions.
Although hsk1 and swi1 are reported to be in the same epistasis group for MMS response (Matsumoto et al. 2005; Sommariva et al. 2005), we observed that the double mutants between swi1 or swi3 with hsk1 are more sensitive to UV irradiation damage compared to either single mutant (Figure 1 and data not shown). We also observed that there is a slightly increased sensitivity to low-dose MMS in the hsk1 Δswi3 mutant compared to either single mutation.
We next examined the phenotype of hsk1–1312 when combined with mutations directly affecting replication fork stability. hsk1–1312 has negative synthetic interactions (reduced growth rate and reduced permissive temperature) with mutations in the MCM helicase that cause replication fork collapse (Snaith et al. 2000; Bailis et al. 2008). The double mutant mcm2ts hsk1–1312 has a reduced permissive temperature (Snaith et al. 2000). However, mcm2ts is synthetic lethal with Δswi1 and synthetic sick with Δswi3 (Table 1). This suggests that the fork protection complex is particularly important when MCM helicase activity is abrogated and is consistent with recent work showing replication fork collapse in mcm2ts alleles (Bailis et al. 2008). A temperature-sensitive mutant of the GINS subunit psf2 completes a first round of replication at restrictive temperature (Gómez et al. 2005), but is also synthetic lethal with Δswi1 and synthetic sick with Δswi3 (Table 1). In contrast, hsk1–1312, Δswi1, and Δswi3 show little synthetic interaction with replication mutants that arrest prior to replication fork activation: sna41goa1/cdc45ts (Uchiyama et al. 2001) and pol1–1 (D'Urso et al. 1995) (Table 1). Together, these interactions suggest that mutants that disrupt early steps of initiation are less sensitive to loss of Swi1 and Swi3 than mutants that carry out substantial DNA synthesis.
TABLE 1.
Summary of genetic interactions
| Interactions with | Strain | Interaction | Reference |
|---|---|---|---|
| Checkpoint mutants | |||
| hsk1-1312 Δrad3 | Synthetic lethal | Snaith et al. (2000) | |
| hsk1-1312 Δchk1 | Synthetic lethal | Snaith et al. (2000) | |
| hsk1-1312 Δrad26 | Synthetic lethal | This work | |
| orp1-4 Δrad1 | None | This work | |
| hsk1-1312 Δrad1 | Synthetic lethal | This work | |
| orp1-4 Δrad17 | None | This work | |
| hsk1-1312 Δrad17 | Synthetic lethal | This work | |
| hsk1-1312 Δcrb2 | Severely reduced growth (microcolonies) | This work | |
| Replication mutants | |||
| hsk1-1312 mcm2ts | Decreased restrictive temperature | Snaith et al. (2000) | |
| Δswi1 mcm2ts | Synthetic lethal | This work | |
| Δswi3 mcm2ts | Slow growth and decreased restrictive temperature | This work | |
| Δswi1 psf2ts | Synthetic lethal | This work | |
| Δswi3 psf2ts | Slow growth and decreased restrictive temperature | This work | |
| hsk1-1312 cdc45ts | Decreased restrictive temperature | Dolan et al. (2004) | |
| Δswi1 cdc45ts | None | This work | |
| Δswi3 cdc45ts | None | This work | |
| Δswi1 pol1-1 | None | This work | |
| Δswi3 pol1-1 | None | This work | |
| Recombination regulators | |||
| hsk1-1312 Δrqh1 | Synthetic lethal | Snaith et al. (2000) | |
| Δswi1 Δrqh1 | Slow growth; increased sensitivity to MMS and TBZ | This work | |
| Δswi3 Δrqh1 | Slow growth; increased sensitivity to MMS and TBZ | This work | |
| hsk1-1312 Δsrs2 | No interaction | This work | |
| hsk1-1312 Δrhp51 | Synthetic lethal | ||
| Centromere proteins | |||
| hsk1-1312 rad21-K1 | Synthetic lethal | Snaith et al. (2000) | |
| Δswi1 rad21-K1 | Synthetic lethal | This work | |
| Δswi3 rad21-K1 | Synthetic lethal | This work | |
| hsk1-1312 Δswi6 | Viable | Bailis et al. (2003) | |
| Δswi1 Δswi6 | Increased sensitivity to MMS and TBZ | This work |
Since Hsk1 and Swi1/Swi3 (the FPC) function together (Matsumoto et al. 2005; Sommariva et al. 2005), and since Hsk1 is required for proper centromere cohesion and chromosome segregation (Bailis et al. 2003), we asked whether the FPC overlaps with Hsk1 in this activity. We compared the interactions between FPC mutants and hsk1 when combined with mutations in the cohesin subunit Rad21, and the centromeric heterochromatin protein Swi6, which is required for centromere cohesion (Bernard et al. 2001; Nonaka et al. 2002; Bailis et al. 2003). We observed that Δswi1 and Δswi3 are synthetic lethal with the cohesin mutant rad21–K1 (Table 1), similar to hsk1–1312 rad21–K1 (Snaith et al. 2000). In contrast, Δswi1 Δswi6 double mutants were viable although they are more sensitive to thiabendazole treatment than either parent (Table 1).
Previously, we showed that at the permissive temperature, hsk1–1312 is synthetic lethal with damage checkpoint mutations Δrad3 and Δchk1 (Snaith et al. 2000). This contrasts with the suppression of hsk1–1312 by Δcds1. Rad3 is the fission yeast ATR homolog and functions as the master kinase for checkpoint activation, and Chk1 is activated by Rad3 in response to DNA damage (reviewed in Harrison and Haber 2006). We also found that hsk1–1312 was synthetic lethal with other damage checkpoint response mutants, including Δrad26, which encodes the fission yeast homolog of ATRIP, which recruits ATR to RPA-coated single-strand DNA (ssDNA) (Edwards et al. 1999; Cortez et al. 2001; Zou and Elledge 2003); Δrad17, encoding the RFC alternative required for DNA repair (Griffiths et al. 1995); and Δrad1, which deletes a component of the 9-1-1 clamp (Kostrub et al. 1998; Kaur et al. 2001) (Table 1). Double mutants between hsk1–1312and Δcrb2 are viable, but extremely slow growing and able to form only microcolonies; Crb2 is a mediator of Chk1 activity (reviewed in Harrison and Haber 2006).
The dependence on the damage checkpoint is not seen in mutations that affect replication initiation only; orp1–4, a mutant defective for prereplicative complex formation and replication initiation (Grallert and Nurse 1996; Dolan et al. 2004), is viable in combination with Δrad17 and Δrad1 (Table 1). The dependency of hsk1 cells on an intact damage checkpoint suggests that the hsk1–1312 itself generates DNA damage. The acute sensitivity of hsk1–1312 to MMS suggests that this damage might result from aberrant repair of replication associated lesions, subsequent to initiation. We investigated these observations in turn.
Increased DNA damage and recombination in hsk1–1312:
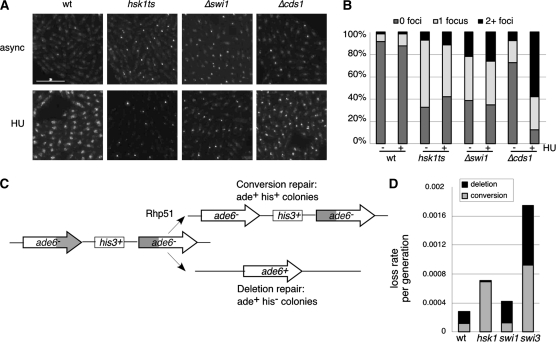
Previously, we showed that hsk1–1312 has a low, but detectable, level of Chk1 phosphorylation even at the permissive temperature, consistent with a chronic activation of the damage checkpoint (Snaith et al. 2000). To determine whether this reflects active damage, we employed a YFP-tagged Rad22 (ScRad52) to visualize repair foci (Lisby et al. 2001, 2003, 2004; Du et al. 2003; Meister et al. 2003, 2005). We observed that 67.3% of hsk1–1312 cells and 61.6% of Δswi1 cells growing asynchronously at 25° had at least one Rad22YFP focus, vs. only 8.5% of wild-type cells (Figure 2). This is consistent with both hsk1–1312 and Δswi1 mutants having some level of constitutive DNA damage that recruits recombination proteins, although in hsk1 at least, there is no evidence for extensive chromosome breakage by PFGE analysis (Snaith et al. 2000). These results also agree with previously published data showing that both Δswi1 and Δswi3 mutants have increased populations of cells with Rad22 foci in asynchronously growing cultures (Noguchi et al. 2003, 2004) and is consistent with increased damage phenotypes associated with other alleles of hsk1 (Matsumoto et al. 2005; Sommariva et al. 2005).
Figure 2.—
Analysis of recombination in hsk1–1312. (A) rad22YFP (FY2878), hsk1–1312 rad22YFP (FY3285), Δswi1 rad22YFP (FY3287), and Δcds1 rad22YFP (FY3286) cultures were grown overnight at 25°. Cultures were split and either no hydroxyurea (async) or 12 mm HU (HU) was added. After 3 hr of growth, cells were washed twice in PBS, and spotted on slides with poly-l-lysine. Images were collected, deconvolved, and counted. Scale bar, 15 μm. (B) Quantification of cell counts averaged from two experiments. (C) Scheme of ade6–his3+–ade6 cassette used in mitotic recombination assay. (D) Quantification of mitotic recombination frequency per generation relative to wild type. Wild-type (FY2132), hsk1-1312 (FY3101), swi1(FY3098), and swi3 (FY3100) strains were grown in YES, and two plates of 104 cells were plated to EMM Ade−. Survivors were patched to Ade− and Ade− His− to determine histidine prototrophy. Data are the averages of seven or eight independent cultures. Statistical analysis is presented in the text.
HU treatment leads to replication fork collapse in mutants lacking the Cds1 checkpoint kinase, which can be visualized by an increase in the Rad22–YFP foci in these cells following addition of HU (Figure 2; Bailis et al. 2008). To determine whether hsk1–1312 or Δswi1 mutations result in replication fork collapse in HU, we compared the fraction of cells with Rad22–YFP foci ± HU under otherwise permissive growth conditions (Figure 2B). We observed no significant increase in Rad22–YFP foci in hsk1or Δswi1 strains, suggesting that the replication fork does not collapse to generate additional breaks in response to HU treatment in these mutants.
Consistent with the increase in recombination centers in hsk1–1312 under permissive conditions, we observed that hsk1–1312 is lethal combined with the mutation Δrhp51 (RAD51; Table 1). This suggests that hsk1–1312 causes intrinsic damage that requires the recombination apparatus for repair, even at permissive temperature. Therefore, we investigated whether hsk1–1312 shows evidence for increased recombination. Previous work showed that alleles of hsk1 and dfp1 mutants have elevated levels of gene conversion in diploids (Snaith et al. 2000; Fung et al. 2002), consistent with the increase in breaks suggested by the elevated frequency of Rad22–YFP foci. We examined mitotic crossovers frequency in haploids, using a ade6 tandem heteroallele flanking the his3+ gene (Osman et al. 2000) to examine the nature of spontaneous recombination in hsk1–1312, compared to swi1–111 or swi3–146 mutations. The heteroallele can be converted to ade6+ by gene conversion, which retains the intervening his3+ allele and is thought to result from strand exchange and Holliday junction intermediates, or by deletion events, which lose the his3+ marker, and are thought to result from single-strand annealing, replication slippage, or unequal sister chromatid crossing over (Osman et al. 2000, 2002; Catlett and Forsburg 2003). Efficient conversion repair in this system (Ade+ His+) requires homologous recombination proteins including Rhp51 and Rhp54, although mutations in these proteins lead to increased rates of deletion products (Osman et al. 2000). In contrast, we observed that hsk1–1312 mutants had a 5.9-fold increase in conversion repair (P < 0.05) but an 11.6-fold decrease in deletion repair (P < 0.05) (Figure 2B) relative to wild-type strains.
Unlike hsk1–1312, swi1 mutants had little effect with just a 1.6-fold increase in mitotic recombination (P < 0.05; not statistically significant). However, swi3 mutants showed a 6.3-fold increase of both types of repair (P < 0.05) (Figure 2B), consistent with published data showing increased mitotic recombination in these mutants (Sommariva et al. 2005).
Hsk1 association with the chromatin in G2 phase cells and after damage:
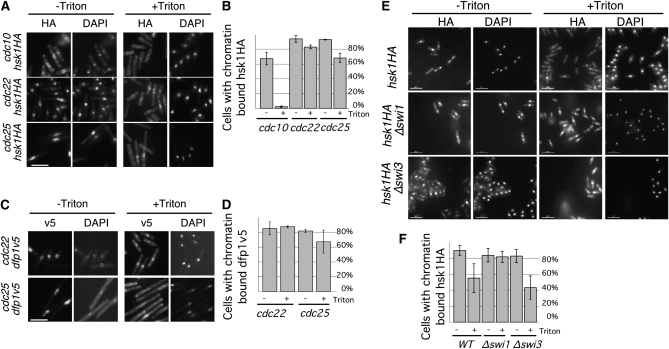
The genetic interactions between hsk1 and mutations that cause replication fork instability suggested that there may be a role for Hsk1 not just in replication fork activation, but during replication fork progression. If the Hsk1–Dfp1 complex functions after initiation, then these proteins should be associated with the chromatin after replication initiation. To examine the timing of chromatin association, we used an in situ chromatin binding assay to analyze the association of Hsk1 and Dfp1 with chromatin (Kearsey et al. 2000). We used a panel of cell-cycle mutants to arrest cells in G1 (cdc10), S (cdc22), and G2 (cdc25) phases and tested the chromatin binding of tagged proteins Hsk1HA and Dfp1V5. We found that Hsk1HA was present in the nucleus throughout the cell cycle, but bound only to chromatin in 2% of G1 cells (Figure 3). Hsk1HA bound chromatin in 82.7% of S-phase cells and, surprisingly, in 68% of G2 cells (Figure 3). Dfp1V5 was chromatin associated in fewer than 10% of cells arrested by cdc10 in G1 phase (data not shown). However, Dfp1V5 bound chromatin in 87% of S-phase cells and 67% of G2 cells (Figure 3). These data indicate that the Hsk1–Dfp1 complex is bound to chromatin not only in S phase, but also in G2, which could allow it to act after initiation or even after the conclusion of bulk DNA replication.
Figure 3.—
Hsk1 and Dfp1 bind chromatin during S and G2 phases. (A) Cultures of cdc10 hsk1HA (FY1000), cdc22 hsk1HA (FY1011), and cdc25 hsk1HA (FY1006) were grown at 25°, shifted to 36° for 4 hr, and processed for in situ chromatin binding. Scale bar, 10.7 μm. (B) Quantification of data from A; averages of three experiments are presented and error bars show standard deviations. (C) Cultures of cdc22 dfp1v5 (FY3281) and cdc25 dfp1v5 (FY3282) were grown at 25°, shifted to 36° for 4 hr, and processed for in situ chromatin binding. Scale bar, 10.7 μm. (D) Quantification of data from (C); averages of two experiments are presented and error bars are standard deviations. (E) Hsk1–Dfp1 and Swi1–Swi3 bind chromatin independently. hsk1HA (FY1077), Δswi1 hsk1HA (FY3249), and Δswi3 hsk1HA (FY3251) cultures were grown at 32° and processed for in situ chromatin binding. Scale bar, 10.7 μm. (F) Quantification of data from E; averages of three experiments are presented and error bars show standard deviations.
We repeated the experiment in Δswi1 mutant cells. We found chromatin-bound Hsk1HA in 79.2% of asynchronously growing Δswi1 cells and 44.5% of Δswi3 cells, compared to 55% of wild-type cells (Figure 3). Thus, Hsk1 recruitment to the chromatin is independent of Swi1. Since Δswi1 alone causes DNA damage (Figure 2 and (Matsumoto et al. 2005; Sommariva et al. 2005), we reasoned that the modest enhancement of Hsk1 on the chromatin in Δswi1 mutants might mean that Hsk1 is responding to that damage. If this were the case, we predicted that alleles of the Hsk1 kinase that are defective in the damage response might be defective in chromatin binding.
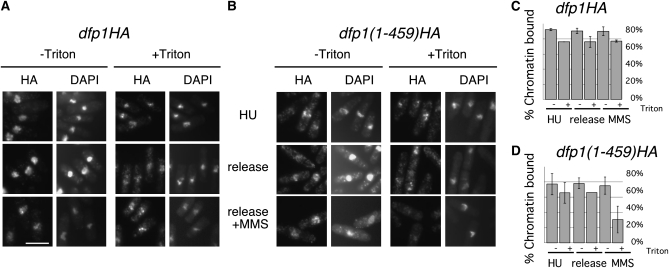
Previously, strains with C-terminal truncations of the Dfp1 protein, dfp1-(1–376) and dfp1-(1–459), were shown to be sensitive to MMS but not HU or UV, which led to the identification of the Dfp1 “C-motif” as necessary for response to alkylation damage (Fung et al. 2002). We arrested dfp1–HA or dfp1-(1–459)–HA strains with HU for 3 hr and released them either to fresh medium or fresh medium containing 0.03% MMS for 1 hr. We found that Dfp1–HA and Dfp1-(1–459)HA were bound to chromatin in HU-arrested cells and cells released into plain medium (Figure 4). However, only 30.5% of cells had chromatin-bound Dfp1-(1–459)HA after release to MMS, compared to 77% of cells with chromatin-bound Dfp1–HA (Figure 4). Thus, reduction in Hsk1–Dfp1 chromatin association correlates with increased damage sensitivity, suggesting that Hsk1–Dfp1 maintenance on the chromatin or at stalled forks may be important for the slowed replication forks and/or repair of the MMS lesions.
Figure 4.—
Dfp1ΔC chromatin association with damaged DNA is disrupted. dfp1HA (FY1763) (A) or dfp1ΔCHA (FY1794) (B) cultures were grown at 32°, arrested with 20 mm HU for 3 hr (HU), and released to plain medium (release) or medium with 0.03% MMS (release +MMS) for 1 hr. Cells were then processed for in situ chromatin binding. Scale bar, 10.7 μm. (C) Quantification of data from A and B; averages of two experiments are presented. Error bars show standard deviations.
Isolation of a new allele of dfp1:
To identify additional mutations that affect normal MMS response, we performed a genetic screen in an h90 smt-0 background. This strain is not able to make any DSBs at the mating-type locus, which initiates mating-type switching. We used this mutant because the MMS-sensitive mutant Δrad22 is not viable in a homothallic (h90) wild-type background (see materials and methods). The screen yielded mutations in eight genes, of which six were identified by complementation tests (Table 2). We identified mutations affecting two MRN recombination complex subunits, nbs1+ and rad32/mre11+. MRN is required for end resection and homologous recombination (reviewed in Williams et al. 2007). We also isolated snf22 (two alleles), a SNF2-related ATPase characterized for its role in chromatin remodeling in meiosis (Yamada et al. 2004); swi9/rad16, the ScRAD1 ortholog required for excision repair and some forms of recombination (Carr et al. 1994; Farah et al. 2005); and a mutation in SPBC19G7.10c, encoding an uncharacterized homolog of the topoisomerase-interacting S. cerevisiae Pat1 protein required for mRNA decapping (Wang et al. 1996; Bonnerot et al. 2000). We were unable to identify the genes corresponding to two mutations, rad37 and rad39.
TABLE 2.
Isolation of MMS sensitive mutants
| Mutant | Gene | Function | Reference |
|---|---|---|---|
| 226 | rad32 | Mre11 component of MRN recombination complex | Tavassoli et al. (1995) |
| 253 | rad32 | Mre11 component of MRN recombination complex | Tavassoli et al. (1995) |
| 249 | swi9/rad16 | ScRad1 nuclease ortholog required for excision repair and recombination | Carr et al. (1994) |
| 106 | snf22 | ATP dependent helicase, Snf2 family | Yamada et al. (2004) |
| 261 | snf22 | ATP dependent helicase, Snf2 family | Yamada et al. (2004) |
| 271 | rad35/dfp1 | DDK (Hsk1) regulatory subunit | Brown and Kelly (1998); Takeda et al. (1999) |
| 219 | rad36/SPBC19G7.10c | ortholog of topoisomerase-associated RNA-degreading factor ScPAT1 | Not studied |
| 265 | rad37 | Not cloned | |
| 278 | rad38/nbs1 | Nbs1 component of MRN recombination complex | Chahwan et al. (2003); Ueno et al. (2003) |
| 280 | rad39 | Not cloned |
The rad35–271 mutation proved to be allelic to the Hsk1 regulatory subunit encoded by dfp1+, and we henceforth call it dfp1-(1–519) or dfp1rad35. This mutation is a C-terminal truncation that truncates the protein at amino acid 519. Its phenotype is reminiscent of the dfp1-(1–459) and dfp1-(1–376) mutations analyzed in (Fung et al. 2002), which were shown to be MMS sensitive but competent for replication. C-terminal truncation mutations of Dfp1 function as separation-of-function alleles that allow selective inactivation of just one or two functions of the kinase (Takeda et al. 1999; Fung et al. 2002; Bailis et al. 2003). However, the construction of the dfp1-(1–459) and dfp1-(1–376) alleles was genetically complex, making it comparatively difficult for further genetic analysis with these mutations. Since the MMS phenotypes of dfp1-(1–376), dfp1(1–459), and dfp1(1–519)rad35 are similar (Figure 5; Fung et al. 2002), we continued our analysis using the dfp1(1–519) allele as a separation of function mutation and followed it in genetic crosses using MMS sensitivity.
Figure 5.—
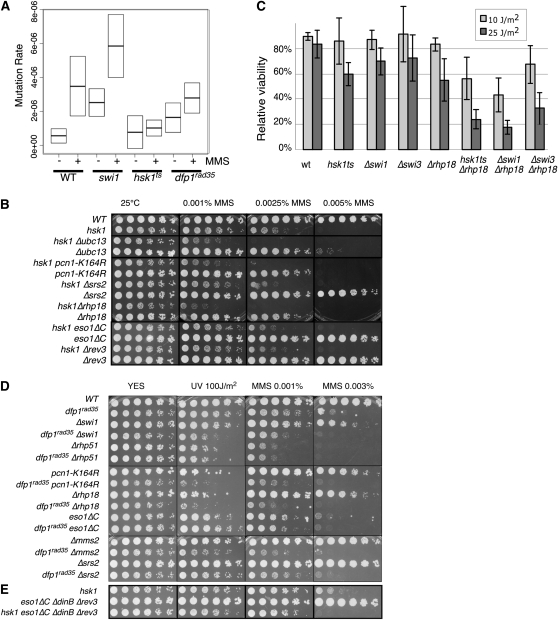
Hsk1–Dfp1 function in the error-prone postreplication repair pathway. (A) hsk1 is required for induced mutagenesis. The graph shows the relative mean forward mutation frequency of the ura4+ gene for untreated samples (−) and samples exposed to 0.0025% MMS for 1 hr (+) in wild-type (FY8), Δswi1∷kanMX (FY4588), hsk1–1312 (FY1418), and dfp1rad35-271 (FY3998) strains. The relative forward mutation frequency is the rate compared to that of wild-type untreated cells. The middle line in each box represents the mean, and the upper and lower limits of the box represent 95% confidence interval calculated from the one-sample t-test. Each confidence interval was calculated from a sample size of at least 12 independently chosen colonies. (B) Synthetic interactions between hsk1 and components of the postreplication repair pathway. Cells were plated in 5× dilutions on YES with the indicated amount of MMS. (C) Synthetic interactions of hsk1–1312, Δswi1, and Δswi3 with Δrhp18 in response to UV. Cultures were grown overnight at 25° into log phase. Cells were plated and plates allowed todry. Plates were exposed to 0, 10, or 25 J/m2 UV light. Plates were incubated at 25° for 3–5 days and counted. Values are the average relative viability of three assays; error bars show standard deviations. (D) Synthetic interactions between dfp1–(1-519) and components of the postreplication repair pathway. (E) Synthetic interactions between hsk1 and deletion of the error-prone polymerases.
To determine whether dfp1(1–519) suffers the same intrinsic damage as hsk1–1312, we analyzed the formation of Rad22–YFP foci in exponentially growing cells. We observed a constitutive level of foci in dfp1-(1–519), about 70% overall, substantially higher than wild type, but similar to the levels observed in hsk1–1312. Following 3.5 hr treatment with HU, we observed 27% cells with no foci, 53% with one focus, and 16% with more then one focus, suggesting that like hsk1–1312, dfp1-(1–519) does not affect replication fork stability during arrest (compare with Figure 2). We also observed that dfp1-(1–519) shows a reduced growth rate with somewhat elongated cells, suggesting an intrinsic level of damage. There is also modest sensitivity to camptothecin, a topoisomerase inhibitor that results in S-phase-specific breaks (Figure S1). However, the dfp1 alleles show no synthetic phenotype with Δrhp51, in contrast to hsk1, which is synthetic lethal (Table 1 and Figure 5; Fung et al. 2002), indicating that the foci we observe do not represent sufficient damage to make the cells dependent upon HR repair. Alternatively, the synthetic lethality in hsk1–1312 could reflect a combination of initiation and postreplicative events and events at replication initiation that are not defective in the dfp1 mutant. We considered dfp1-(1–519) as a separation of function mutation, competent for replication but defective in the MMS response.
Hsk1 and Dfp1 in damage repair:
Previous genetic analysis of dfp1motif C mutations showed that they fall into a separate epistasis group from several known repair pathways (Fung et al. 2002), including those defined by Δrad13 (nucleotide excision repair XPG/ERCC5; Carr et al. 1993), Δrhp51 (Rad51; homologous recombination repair; Muris et al. 1993), Δrad2 (FEN-1 flap endonuclease; Murray et al. 1994), or Δmag1 (base excision repair glycosolase; Memisoglu and Samson 2000), all of which contribute to normal MMS repair (Memisoglu and Samson 2000). These data suggest that Hsk1–Dfp1 is required for survival of alkylation damage in a pathway that is independent of excision repair and homologous recombination pathways. A likely candidate is the postreplication repair pathway dependent upon Rhp18 (ScRad18) that includes error-free and error-prone branches (reviewed in Barbour and Xiao 2003; Andersen et al. 2008). This would be consistent with observations in budding yeast suggesting that ScCdc7 is required for induced mutagenesis in MMS (Njagi and Kilbey 1982a,b), via the error-prone pathway that responds to alkylation damage.
To investigate this in S. pombe, we first examined whether hsk1–1312 or dfp1-(1–519) affect the frequency of induced mutagenesis in fission yeast (Figure 5A). We performed a simple forward mutation assay at the ura4+ locus by calculating the rate of 5-FOA resistance as in (Liu et al. 1999), comparing wild type to hsk1–1312, dfp1-(1–519) and Δswi1mutant strains. We plated cells on selective medium in the absence or presence of prior treatment with MMS. First, we observed that there is a slightly higher mutation rate in hsk1 and dfp1 compared to wild type in the absence of any exogenous treatment. We repeated the experiment following MMS treatment and observed a dramatic increase in the frequency of mutation in wild type (“induced mutagenesis”) as expected. A similar induction was also apparent in Δswi1, even though it has a higher basal level of mutation. In contrast, there was no significant elevation of mutation rate in hsk1–1312 (P = 0.63) and only a modest increase in dfp1-(1–519) above the levels in untreated cells (P = 0.06). This indicates that as in S. cerevisiae, S. pombe Hsk1 and Dfp1 are required for induced mutagenesis, and importantly, this phenotype is independent of Swi1 and the FPC.
We next examined genetic interactions between hsk1, dfp1, and the MMS response pathway defined by the ScRad6/SpRhp6 epistasis group. This pathway relies on the ScRad6–Rad18 (S. pombe Rhp6–Rhp18) ubiquitin ligase, which ubiquitylates PCNA and has several downstream branches required for translesion synthesis and template switching (reviewed in Barbour and Xiao 2003; Andersen et al. 2008). Further ubiquitylation of PCNA by the ubiquitin ligase Sc/SpUbc13 and Sc/SpMms2 and the helicase ScRad5/SpRad8 activates an error-free replication bypass system, while the error-prone branch of pathway relies on translesion synthesis by bypass polymerases Polη (part of a fusion protein encoded by the C terminus of eso1+), the dinB ortholog polκ (mug40/dinB), and Polζ (rev3). Studies in budding yeast suggest that ScCdc7 functions in a branch of the translesion synthesis pathway (Pessoa-Brandao and Sclafani 2004).
To determine whether similar interactions occur in fission yeast, we examined the phenotype of double mutants between hsk1 or dfp1 and several components of this pathway (Figure 5 and Figure S2). Δrhp18 (Verkade et al. 1999) disrupts the E3 ligase that cooperates with the Rhp6/RAD6 E2 ligase for ubiquitylation of PCNA and activation of both error-free and error-prone translesion synthesis (Andersen et al. 2008). An allele of PCNA, pcn1–K164R is proficient for normal replication but defective in damage-induced ubiquitination, disupting both branches of the pathway (Frampton et al. 2006). Δmms2 and Δubc13 are required for the error-free arm of the pathway (Brown et al. 2002). We also examined disruption alleles of the 40 TLS polymerases, eso1ΔC (Δpolη), and Δrev3, and a triple deletion eso1ΔC (Δpolη), ΔdinB Δrev3.
First, we examined combinations with mutant hsk1. All the double mutants were viable, although we observed that hsk1–1312 Δrhp18 had a modestly reduced growth rate compared to the single mutants (Figure 5B). This suggests that any endogenous damage in the double mutant caused by hsk1–1312 does not rely on these damage processing pathways for viability. UV and MMS sensitivity were increased relative to either single parent when hsk1–1312 was combined with pcn1–K164R or Δrhp18, indicating a combinatorial effect (Figure 5, B and C). When we examined relative viability associated with UV treatment in the double mutants, we observed a modest but distinct reduction in viability in hsk1, Δswi1, or Δswi3 mutations combined with Δrhp18. This suggests that Hsk1 and the FPC proteins have some functions in repair independent of the Rhp18 pathway and this would be consistent with a general role in replication fork stability. hsk1 also showed synthetic phenotypes in combination with Δubc13 or Δmms2. By contrast, no synthetic phenotypes were observed when hsk1 was combined with mutations in eso1ΔC or Δrev3, and there was only a slight increase in sensitivity in the quadruple mutant eso1ΔC Δrev3 ΔdinB hsk1 at higher doses (Figure 5E).
Next we examined dfp1-(1–519) (Figure 5D). Again, the double mutants with either Δrhp18 or pcn1–K164R were significantly more sensitive to MMS and UV than the parents, and synthetic phenotypes were also observed with Δmms2. There was only a modest increase in sensitivity with the eso1 mutant.
These observations suggest that Hsk1–Dfp1 is at least partly independent from ScRad6/SpRhp6 dependent postreplication repair in response to MMS and UV, although these proteins may overlap in function in repair or at other points in cell cycle. Additionally, although swi1 and swi3 mutants have little UV sensitivity by themselves (Noguchi et al. 2003, 2004), we found that Δswi1 Δrhp18 and Δswi3 Δrhp18 double mutants were also significantly more sensitive to UV or MMS treatment than the parental strains (Figure 5C and Figure S3). Δswi1 and Δswi3 showed similar defects in combination with Δubc13 and Δmms2 (data not shown). This is consistent with a role for the FPC in replication fork stability independent of PRR activation.
When TLS or template switching pathways are inhibited, homologous recombination pathways are used to repair the lesions (Figure 6). In budding yeast, the ScΔsrs2 mutation suppresses the damage sensitivity of ScΔrad18, presumably because Δsrs2 relieves the inhibition of the HR pathway and allows it to substitute for the PRR pathway; however, this is not observed in fission yeast (Kai et al. 2007). We observed no genetic interactions between Δsrs2 and hsk1–1312 or dfp1-(1–519), and no changes in the damage sensitivity of the double mutants. This suggests that hsk1–1312 is epistatic with Δsrs2. In contrast, hsk1–1312 is lethal in combination with Δrqh1 (Snaith et al. 2000), another helicase that antagonizes recombination by a different mechanism (Doe et al. 2002; Hope et al. 2006). We observed that Δswi1 and Δswi3 are synthetic sick when combined with Δrqh1with increased sensitivity to MMS and, curiously, the spindle poison thiabendazole (Table 2). This suggests that unregulated recombination in the Δrqh1 mutant is deleterious to hsk1, swi1, or swi3 mutants, and particularly so when damage occurs.
Figure 6.—
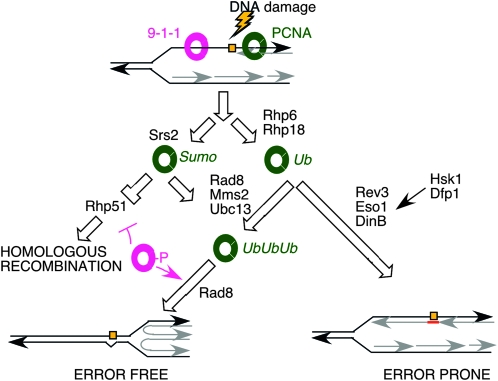
Model for Hsk1 interaction with TLS pathway. The sliding clamp proteins PCNA (green) and 9-1-1 (pink) mediate the choice between repair mechanisms. Homologous recombination is inhibited by the Srs2 helicase and PCNA sumoylation. Polyubiquitination of PCNA by Rhp6/18 and then Mms2/Ubc13/Rad8 drives error free bypass repair. Error prone translesion synthesis by minor polymerases occurs in response to PCNA monoubiquitination. Hsk1 and Dfp1 appear to operate in this pathway independent of Swi1/3 and Rhp6/18. Modified from Kai et al. (2007); Andersen et al. (2008); Branzei et al. (2008).
DISCUSSION
The Hsk1–Dfp1 (DDK) kinase has a well-studied role in promoting replication initiation. In this study, we analyzed the phenotypes associated with hsk1–1312 and alleles of dfp1 to dissect the contributions of the DDK kinase to genome stability after replication initiation. Our work suggests multiple functions for this kinase in promoting replication fork stability and appropriate response to DNA damage caused by alkylating agents during and after S phase.
Hsk1 interacts with Swi1 (ScTof1) and Swi3 (ScCsm3), which constitute the fork protection complex (Matsumoto et al. 2005; Sommariva et al. 2005). The FPC, although not essential for viability, is linked to replication fork pausing and stabilization of the replisome during S-phase arrest (Katou et al. 2003; Noguchi et al. 2003, 2004; Matsumoto et al. 2005; Sommariva et al. 2005) as well as response to alkylating damage (Foss 2001; Noguchi et al. 2003, 2004; Matsumoto et al. 2005; Sommariva et al. 2005). This leads to the model that Hsk1 and the FPC are required for fork stabilization during repair, maintenance of cohesion during repair, and fork recovery required for successful completion of S phase. Consistent with this role at the elongating fork, we observe synthetic phenotypes between hsk1–1312, swi1, or swi3 when combined with mutations that are defective in replication fork stability, such as mcm2ts or psf2. In contrast, we see no synthetic phenotypes in combination with mutations that affect the prereplication complex assembly or initiation, such as orp1 or pol1.
These data suggest that hsk1–1312, like FPC, functions at the replication fork after initiation to promote stability, and therefore is not simply a replication initiation factor. Consistent with this model, we observe that Hsk1 associates with chromatin in G2 phase, and this association is enhanced in MMS-treated cells. Importantly, however, while chromatin association requires the C terminus of Dfp1, it does not require the FPC.
We observe that hsk1–1312 causes intrinsic damage, which is sufficiently severe to activate the DNA damage checkpoint, and hsk1 cells require the checkpoint for viability (Snaith et al. 2000 and this work). There are increased foci corresponding to the recombination protein Rad22–YFP (Rad52) in hsk1–1312 even at the permissive temperature; such foci are characteristic of a range of lesions not limited to double-strand breaks (e.g., Bailis et al. 2008). We also observe that hsk1–1312 is lethal in the absence of Rhp51 (ScRad51). Thus, we conclude that hsk1–1312 generates constitutive damage that renders the cells dependent upon an active recombination system.
Such damage might be expected to increase rates of mitotic recombination, and indeed we observed substantially increased rates of recombination in the form of gene conversion, but reduced levels of deletion repair, measured using an ade6 heteroallele system (Osman et al. 2000, 2002). Previous analysis of this system suggests that the conversion repair arises from break-induced strand exchange and Holliday junction intermediates and depends upon HR genes such as rad22+, while the deletion-type recombination occurs from single-strand annealing, replication slippage, intrachromatid crossing over, or unequal sister chromatid exchange (Osman et al. 2000, 2002). The hsk1 phenotype suggests that the homologous recombination pathway is induced. The mechanism could be indirect, reflecting increased levels of damage due to replication fork instability, or could also reflect a role for Hsk1 directly in negatively regulating this pathway, such that its activity is enhanced in hsk1 mutants. The loss of conversion products could reflect an active downregulation of SSA or other pathways; alternatively, these pathways may be initiated but not resolved, which would result in lethality and thus failure to recover any products. It is interesting to note that Δrhp51 mutants, which cannot carry out HR and have low levels of conversion products, nevertheless have increased levels of deletion repair (Osman et al. 2000). Thus, it is possible that the loss of conversion products in hsk1 implicates the kinase in SSA or other forms of repair.
Although swi1 and swi3 mutants also have increased Rad22YFP foci, we do not see the same spectrum of recombination events in the ade6 heteroallele as we see with hsk1 (Figure 2 and Sommariva et al. 2005). The increase in deletion events in swi3 cells is consistent with a specific function in replication fork instability, and the absence of these events in hsk1 suggests that its defects may be functionally separated from the FPC. Similar phenotypes to those observed with FPC mutants are also reported for other repair mutants including MMS-sensitive repair mutants Δrad16/swi9 (ScRad1) and Δswi10 (ScRad10) (Doe et al. 2000; Osman et al. 2000). These genes encode a nuclease that is involved in excision repair but also associated with recombination (Carr et al. 1994; Farah et al. 2005, 2009). The differences in mutation spectra between hsk1–1312 and swi1 and swi3 suggest that these proteins make nonidentical contributions to genome stability and recombination.
Inappropriate activation of the recombination pathway is antagonized by several helicases, including the Rqh1 (Bloom syndrome) helicase, which blocks formation of recombinogenic structures and is required for replication fork stability, and Srs2, which antagonizes formation of Rad51 filaments (reviewed in Barbour and Xiao 2003; Branzei and Foiani 2007; Lambert et al. 2007). hsk1–1312 is lethal in combination with Δrqh1 (Snaith et al. 2000), but has no additional phenotype combined with Δsrs2 (this work). This is consistent with a general defect in hsk1 in replication fork stability that is additive with the defects in Δrqh1 and suggests that Hsk1 may function in a common genetic pathway with Srs2.
We performed a screen for additional mutations that cause MMS sensitivity. Mutations were identified in the MRN complex subunits nbs1+ and rad32+ (MRE11), in an ortholog to the RNA decapping enzyme S. cerevisiae PAT1, in rad16+encoding a nuclease required for recombination and excision repair (Carr et al. 1994; Farah et al. 2005), and in the snf22+ helicase (Yamada et al. 2004). We also isolated rad35, a novel allele of dfp1 that truncates the extreme C terminus of the protein at residue 519. Additional dfp1-(1–519) serves as a separation of function mutation that specifically affects the MMS response of cells.
Blocked replication forks can be recovered without repair using either homologous recombination or the lesion bypass system. Data from S. cerevisiae suggest that cdc7 mutants disrupt induced mutagenesis and function in the Rad6–Rad18 pathway of bypass repair via translesion synthesis (Njagi and Kilbey 1982a,b). This would be a function consistent with a requirement for DDK in replication fork stability, since lesion bypass requires assembly of nonreplicative polymerases at the fork (Branzei and Foiani 2005; Andersen et al. 2008). Induced mutagenesis following MMS exposure depends largely on the error-prone translesion synthesis pathway (Barbour and Xiao 2003; Andersen et al. 2008). Both hsk1 and dfp1 cells have a slightly higher basal mutation rate than wild-type cells. However, treatment with MMS did not further increase the rate of mutation in hsk1–1312 and only modestly increased the mutation frequency in dfp1-(1–519). In contrast, both Δswi1 and wild-type cells had a robust induction of mutagenesis following treatment with MMS. This indicates first that Hsk1 and Dfp1 are required for induced mutagenesis in response to alkylation damage and, second, that the FPC is not required for induced mutagenesis. Thus, Hsk1–Dfp1 have a separate role from the FPC in promoting this response.
We observed a modest synthetic interaction between hsk1 or dfp1 with Δrhp18 and other components of the PRR pathway including pcn1–K164R, Δmms2, and Δubc13, and the error-prone polymerases Δeso1, ΔdinB (mug40), or Δrev3 (there is no dinB ortholog in budding yeast). These data suggest that the Hsk1–Dfp1 kinase complex affect the error-prone repair pathway independent of the Rhp18 PCNA-ubiquitylation pathway that has been identified previously. This is consistent with observations in budding yeast, which suggest that Cdc7 functions in a distinct epistasis group in the error-prone repair pathway (Pessoa-Brandao and Sclafani 2004).
We propose that the function of Hsk1–Dfp1 in the proper response to MMS depends upon association of the kinase with the chromatin during the MMS response, via the C terminus of Dfp1. In fact, Hsk1 may be recruited by specific damage recognition or repair proteins. For example, in a recent proteomics study, budding yeast Cdc7 was isolated in an affinity purification using MGMT (O6-methylguanine–DNA methyltransferase (Niture et al. 2005). This enzyme is responsible for directly removing methyl groups from DNA damaged by alkylating agents such as N-methyl-N-nitrosourea (MNU) (Kaina et al. 2001, 2007; Wyatt and Pittman 2006); fission yeast uses a different enzyme to deal with these lesions (Pearson et al. 2006). Therefore, we propose that Hsk1 may contribute to the choice of repair mechanism by direct regulation of repair proteins at the replication fork.
There are other examples of Rhp18-independent inputs into the PRR pathway. A recent study suggested that phosphorylation of the Rad9 checkpoint protein on T225 by Rad3/ATR specifically activates the error-free translesion synthesis pathway (Kai et al. 2007). Interestingly, mutation of rad9–T225C combined with mutations of Δrhp18 leads to a dramatic increase of gene conversion, but not deletion recombination (Kai et al. 2007). The authors suggest that loss of the rhp18+-mediated lesion bypass system is synergistic with mutations that inhibit inappropriate recombination, leading to a hyperrecombinant phenotype. Thus, the recombination response is intimately linked to the PRR response. Our data suggest that Hsk1 is required for error-prone repair; at least in genetic terms, this may antagonize the effects of Rad9-phosphoT225. Interestingly, the hyperrecombinant phenotype of hsk1, and the lack of genetic interaction with Δsrs2, could suggest that Hsk1 also inhibits the recombination response to alkylating damage.
Our data suggest several roles for the Hsk1–Dfp1 that contribute to genome stability after the initiation of DNA synthesis. We agree with previous studies that Hsk1 functions in concert with the FPC to promote fork stability during fork pausing. This is consistent with evidence showing that reduction of Cdc7 in murine ES cells causes slowing of replication, and its complete depletion causes p53-dependent apoptosis (Kim et al. 2002, 2003). However, our data show that Hsk1 also fulfills an FPC-independent function that promotes error-prone repair. We suggest that this is dependent on the C terminus of Dfp1, which may directly associate with repair proteins. Further studies will be necessary to identify the target for Dfp1 association and likely substrates for Hsk1 activity in the repair process.
Acknowledgments
We thank Grant Brown, Tony Carr, Jacob Dalgaard, Greg Freyer, Matthew O'Connell, and Paul Russell for yeast strains; Grant Brown, Jacob Dalgaard, Greg Freyer, Matthew O'Connell, and Oscar Aparicio for helpful discussions and sharing unpublished data; and Oscar Aparicio, Julie Bailis, Douglas Dalle Luche, Rebecca Nugent, Lorraine Pillus, Sarah Sabatinos, and Angel Tabancay for helpful comments throughout the course of this work. We thank Cathrin Struck for excellent technical assistance in the screen for MMS sensitive mutants. W.P.D. was supported by training grant GM08666 from the National Institutes of Health (University of California—San Diego). This work was supported by American Cancer Society grant RSG-00-132-04-CCG, and NIH grants R01 GM059321 and R01 GM081418 to S.L.F.
Supporting Information available online at http://www.genetics.org/cgi/content/full/genetics.109.112284/DC1.
References
- Andersen, P. L., F. Xu and W. Xiao, 2008. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 18 162–173. [DOI] [PubMed] [Google Scholar]
- Bailis, J. M., P. Bernard, R. Antonelli, R. Allshire and S. L. Forsburg, 2003. Hsk1/Dfp1 is required for heterochromatin-mediated cohesion at centromeres. Nat. Cell Biol. 5 1111–1116. [DOI] [PubMed] [Google Scholar]
- Bailis, J. M., D. D. Luche, T. Hunter and S. L. Forsburg, 2008. MCM proteins interact with checkpoint and recombination proteins to promote S phase genome stability. Mol. Cell. Biol. 28 1724–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour, L., and W. Xiao, 2003. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat. Res. 532 137–155. [DOI] [PubMed] [Google Scholar]
- Bernard, P., J. F. Maure, J. F. Partridge, S. Genier, J. P. Javerzat et al., 2001. Requirement of heterochromatin for cohesion at centromeres. Science 294 2539–2542. [DOI] [PubMed] [Google Scholar]
- Bonnerot, C., R. Boeck and B. Lapeyre, 2000. The two proteins Pat1p (Mrt1p) and Spb8p interact in vivo, are required for mRNA decay, and are functionally linked to Pab1p. Mol. Cell. Biol. 20 5939–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei, D., and M. Foiani, 2005. The DNA damage response during DNA replication. Curr. Opin. Cell Biol. 17 568–575. [DOI] [PubMed] [Google Scholar]
- Branzei, D., and M. Foiani, 2007. Interplay of replication checkpoints and repair proteins at stalled replication forks. DNA Repair (Amst.) 6 994–1003. [DOI] [PubMed] [Google Scholar]
- Branzei, D., F. Vanoli and M. Foiani, 2008. SUMOylation regulates Rad18-mediated template switch. Nature 456 915–920. [DOI] [PubMed] [Google Scholar]
- Brown, G. W., and T. J. Kelly, 1998. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J. Biol. Chem. 273 22083–22090. [DOI] [PubMed] [Google Scholar]
- Brown, G. W., and T. J. Kelly, 1999. Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl. Acad. Sci. USA 96 8443–8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M., Y. Zhu, S. M. Hemmingsen and W. Xiao, 2002. Structural and functional conservation of error-free DNA postreplication repair in Schizosaccharomyces pombe. DNA Repair (Amst.) 1 869–880. [DOI] [PubMed] [Google Scholar]
- Carr, A. M., H. Schmidt, S. Kirchhoff, W. J. Muriel, K. S. Sheldrick et al., 1994. The rad16+ gene of Schizosaccharomyces pombe: a homolog of RAD1 gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 14 2029–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, A. M., K. S. Sheldrick, J. M. Murray, R. al-Harithy, F. Z. Watts et al., 1993. Evolutionary conservation of excision repair in Schizosaccharomyces pombe: evidence for a family of sequences related to the Saccharomyces cerevisiae RAD2 gene. Nucleic Acids Res. 21 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, M. G., and S. L. Forsburg, 2003. Schizosaccharomyces pombe Rdh54 (TID1) acts with Rhp54 (RAD54) to repair meiotic double-strand breaks. Mol. Biol. Cell 14 4707–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahwan, C., T. M. Nakamura, S. Sivakumar, P. Russell and N. Rhind, 2003. The fission yeast Rad32 (Mre11)–Rad50–Nbs1 complex is required for the S-phase DNA damage checkpoint. Mol. Cell. Biol. 23 6564–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez, D., S. Guntuku, J. Qin and S. J. Elledge, 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294 1713–1716. [DOI] [PubMed] [Google Scholar]
- Doe, C. L., J. S. Ahn, J. Dixon and M. C. Whitby, 2002. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J. Biol. Chem. 277 32753–32759. [DOI] [PubMed] [Google Scholar]
- Doe, C. L., J. Dixon, F. Osman and M. C. Whitby, 2000. Partial supression of the fission yeast rqh1− phenotype by expression of a bacterial Holliday junction resolvase. EMBO J. 19 2751–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan, W. P., D. A. Sherman and S. L. Forsburg, 2004. S. pombe Cdc45/Sna41 requires MCM and Rad4/Cut5 for chromatin binding. Chromosoma 113 145–156. [DOI] [PubMed] [Google Scholar]
- Du, L. L., T. M. Nakamura, B. A. Moser and P. Russell, 2003. Retention but not recruitment of Crb2 at double-strand breaks requires Rad1 and Rad3 complexes. Mol. Cell. Biol. 23 6150–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker, B. P., and G. W. Brown, 2003. Cdc7 kinases (DDKs) and checkpoint responses: lessons from two yeasts. Mutat. Res. 532 21–27. [DOI] [PubMed] [Google Scholar]
- D'urso, G., B. Grallert and P. Nurse, 1995. DNA polymerase alpha, a component of the replication initiation complex, is essential for the checkpoint coupling S phase to mitosis in fission yeast. J. Cell Sci. 108 3109–3118. [DOI] [PubMed] [Google Scholar]
- Edwards, R. J., N. J. Bentley and A. M. Carr, 1999. A Rad3–Rad26 complex responds to DNA damage independently of other checkpoint proteins. Nat. Cell Biol. 1 393–398. [DOI] [PubMed] [Google Scholar]
- Farah, J. A., G. Cromie, W. W. Steiner and G. R. Smith, 2005. A novel recombination pathway initiated by the Mre11/Rad50/Nbs1 complex eliminates palindromes during meiosis in Schizosaccharomyces pombe. Genetics 169 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, J. A., G. A. Cromie and G. R. Smith, 2009. Ctp1 and Exonuclease 1, alternative nucleases regulated by the MRN complex, are required for efficient meiotic recombination. Proc. Natl. Acad. Sci. USA 106 9356–9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg, S. L., 2004. Eukaryotic MCM proteins: beyond replication initiation. Microbiol. Mol. Biol. Rev. 68 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss, E., 2001. Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae. Genetics 157 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton, J., A. Irmisch, C. M. Green, A. Neiss, M. Trickey et al., 2006. Postreplication repair and PCNA modification in Schizosaccharomyces pombe. Mol. Biol. Cell 17 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, A. D., J. Ou, S. Bueler and G. W. Brown, 2002. A conserved domain of Schizosaccharomyces pombe dfp1+ is uniquely required for chromosome stability following alkylation damage during S phase. Mol. Cell. Biol. 22 4477–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, E. B., V. T. Angeles and S. L. Forsburg, 2005. A screen for Schizosaccharomyces pombe mutants defective in rereplication identifies new alleles of rad4+, cut9+, and psf2+. Genetics 169 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert, B., and P. Nurse, 1996. The ORC1 homolog orp1 in fission yeast plays a key role in regulating onset of S phase. Genes Dev. 10 2644–2654. [DOI] [PubMed] [Google Scholar]
- Griffiths, D. J. F., N. C. Barbet, S. McCready, A. R. Lehmann and A. M. Carr, 1995. Fission yeast rad17: a homologue of budding yeast RAD24 that shares regions of sequence similarity with DNA polymerase accessory proteins. EMBO J. 14 5812–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J. C., and J. E. Haber, 2006. Surviving the breakup: the DNA damage checkpoint. Annu. Rev. Genet. 40 209–235. [DOI] [PubMed] [Google Scholar]
- Hayashi, M. T., T. S. Takahashi, T. Nakagawa, J. Nakayama and H. Masukata, 2009. The heterochromatin protein Swi6/HP1 activates replication origins at the pericentromeric region and silent mating-type locus. Nat. Cell Biol. 11 357–362. [DOI] [PubMed] [Google Scholar]
- Hope, J. C., S. M. Mense, M. Jalakas, J. Mitsumoto and G. A. Freyer, 2006. Rqh1 blocks recombination between sister chromatids during double strand break repair, independent of its helicase activity. Proc. Natl. Acad. Sci. USA 103 5875–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, L. H., H. Masai and A. Sugino, 1999. First the CDKs, now the DDKs. Trends Cell Biol. 9 249–252. [DOI] [PubMed] [Google Scholar]
- Kai, M., K. Furuya, F. Paderi, A. M. Carr and T. S. Wang, 2007. Rad3-dependent phosphorylation of the checkpoint clamp regulates repair-pathway choice. Nat. Cell Biol. 9 691–697. [DOI] [PubMed] [Google Scholar]
- Kai, M., and T. S. Wang, 2003. Checkpoint activation regulates mutagenic translesion synthesis. Genes Dev. 17 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaina, B., M. Christmann, S. Naumann and W. P. Roos, 2007. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst.) 6 1079–1099. [DOI] [PubMed] [Google Scholar]
- Kaina, B., K. Ochs, S. Grosch, G. Fritz, J. Lips et al., 2001. BER, MGMT, and MMR in defense against alkylation-induced genotoxicity and apoptosis. Prog. Nucleic Acids Res. Mol. Biol. 68 41–54. [DOI] [PubMed] [Google Scholar]
- Katou, Y., Y. Kanoh, M. Bando, H. Noguchi, H. Tanaka et al., 2003. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424 1078–1083. [DOI] [PubMed] [Google Scholar]
- Kaur, R., C. F. Kostrub and T. Enoch, 2001. Structure-function analysis of fission yeast hus1–rad1–rad9 checkpoint complex. Mol. Biol. Cell 12 3744–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsey, S. E., S. Montgomery, K. Labib and K. Linder, 2000. Chromatin binding of the fission yeast replication factor Mcm4 occurs during anaphase and requires ORC and Cdc18. EMBO J. 19 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, J. B., and J. D. Boeke, 1994. Efficient targeted integration at leu1–32 and ura4–294 in Schizosaccharomyces pombe. Genetics 136 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, T. J., G. S. Martin, S. L. Forsburg, R. J. Stephen, A. Russo et al., 1993. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell 74 371–382. [DOI] [PubMed] [Google Scholar]
- Kim, J. M., K. Nakao, K. Nakamura, I. Saito, M. Katsuki et al., 2002. Inactivation of Cdc7 kinase in mouse ES cells results in S-phase arrest and p53-dependent cell death. EMBO J. 21 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. M., M. Yamada and H. Masai, 2003. Functions of mammalian Cdc7 kinase in initiation/monitoring of DNA replication and development. Mutat. Res. 532 29–40. [DOI] [PubMed] [Google Scholar]
- Kostrub, C. F., K. Knudsen, S. Subramani and T. Enoch, 1998. Hus1p, a conserved fission yeast checkpoint protein, intereacts with Rad1p and is phosphorylated in response to DNA damage. EMBO J. 17 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings, G., and D. Bastia, 2004. swi1- and swi3-dependent and independent replication fork arrest at the ribosomal DNA of Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 101 14085–14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, S., B. Froget and A. M. Carr, 2007. Arrested replication fork processing: interplay between checkpoints and recombination. DNA Repair (Amst.) 6 1042–1061. [DOI] [PubMed] [Google Scholar]
- Liberi, G., G. Maffioletti, C. Lucca, I. Chiolo, A. Baryshnikova et al., 2005. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 19 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, H. D., D. J. F. Griffiths, R. J. Edwards, P. U. Christensen, J. M. Murray et al., 1998. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyes pombe. Genes Dev. 12 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby, M., J. H. Barlow, R. C. Burgess and R. Rothstein, 2004. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118 699–713. [DOI] [PubMed] [Google Scholar]
- Lisby, M., U. H. Mortensen and R. Rothstein, 2003. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat. Cell Biol. 5 572–577. [DOI] [PubMed] [Google Scholar]
- Lisby, M., R. Rothstein and U. H. Mortensen, 2001. Rad52 forms DNA repair and recombination centers during S phase. Proc. Natl. Acad. Sci. USA 98 8276–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, V. F., D. Bhaumik and T. S. F. Wang, 1999. Mutator phenotype induced by aberrant replication. Mol. Cell. Biol. 19 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, H. C., L. Wan, A. Rosebrock, B. Futcher and N. M. Hollingsworth, 2008. Cdc7-Dbf4 regulates NDT80 transcription as well as reductional segregation during budding yeast meiosis. Mol. Biol. Cell 19 4956–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti, M. A., S. Kumar, E. Hartsuiker, M. Maftah, A. M. Carr et al., 2002. A single unbranched S-phase DNA damage and replication fork blockage check point pathway. Proc. Natl. Acad. Sci. USA 99 7472–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai, H., T. Miyake and K.-I. Arai, 1995. hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae Cdc7, is required for chromosomal replication. EMBO J. 14 3094–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos, J., J. J. Lipp, A. Bogdanova, S. Guillot, E. Okaz et al., 2008. Dbf4-dependent Cdc7 kinase links DNA replication to the segregation of homologous chromosomes in meiosis I. Cell 135 662–678. [DOI] [PubMed] [Google Scholar]
- Matsumoto, S., K. Ogino, E. Noguchi, P. Russell and H. Masai, 2005. Hsk1–Dfp1/Him1, the Cdc7–Dbf4 kinase in Schizosaccharomyces pombe, associates with Swi1, a component of the replication fork protection complex. J. Biol. Chem. 280 42536–42542. [DOI] [PubMed] [Google Scholar]
- Meister, P., M. Poidevin, S. Francesconi, I. Tratner, P. Zarzov et al., 2003. Nuclear factories for signalling and repairing DNA double strand breaks in living fission yeast. Nucleic Acids Res. 31 5064–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, P., A. Taddei, L. Vernis, M. Poidevin, S. M. Gasser et al., 2005. Temporal separation of replication and recombination requires the intra-S checkpoint. J. Cell Biol. 168 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memisoglu, A., and L. Samson, 2000. Contribution of base excision repair, nucleotide excision repair, and DNA recombination to alkylation resistance of the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 182 2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194 795–823. [DOI] [PubMed] [Google Scholar]
- Muris, D. F. R., K. Vreeken, A. M. Carr, B. C. Broughton, A. R. Lehmann et al., 1993. Cloning the rad51 homolog of Schizosaccharomyces pombe. Nucleic Acids Res. 21 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. M., M. Tavassoli, R. Al-Harithy, K. S. Sheldrick, A. R. Lehman et al., 1994. Structural and functional conservation of the human homologue of the Schizosaccharomyces pombe rad2 gene, which is required for chromosome segregation and recovery from DNA damage. Mol. Cell. Biol. 14 4878–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture, S. K., C. E. Doneanu, C. S. Velu, N. I. Bailey and K. S. Srivenugopal, 2005. Proteomic analysis of human O6-methylguanine-DNA methyltransferase by affinity chromatography and tandem mass spectrometry. Biochem. Biophys. Res. Commun. 337 1176–1184. [DOI] [PubMed] [Google Scholar]
- Njagi, G. D., and B. J. Kilbey, 1982. a Cdc7–1: a temperature sensitive cell-cycle mutant which interferes with induced mutagenesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 186 478–481. [DOI] [PubMed] [Google Scholar]
- Njagi, G. D., and B. J. Kilbey, 1982. b Mutagenesis in cdc7 strains of yeast: the fate of premutational lesions induced by ultraviolet light. Mutat. Res. 105 313–318. [DOI] [PubMed] [Google Scholar]
- Noguchi, E., C. Noguchi, L. L. Du and P. Russell, 2003. Swi1 prevents replication fork collapse and controls checkpoint kinase Cds1. Mol. Cell. Biol. 23 7861–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, E., C. Noguchi, W. H. McDonald, J. R. Yates, 3rd and P. Russell, 2004. Swi1 and swi3 are components of a replication fork protection complex in fission yeast. Mol. Cell. Biol. 24 8342–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka, N., T. Kitajima, Y. Shihori, G. Xiao, M. Yamamoto et al., 2002. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4 89–93. [DOI] [PubMed] [Google Scholar]
- Osman, F., M. Adriance and S. McCready, 2000. The genetic control of spontaneous and UV-induced mitotic intrachromosomal recombination in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 38 113–125. [DOI] [PubMed] [Google Scholar]
- Osman, F., I. R. Tsaneva, M. C. Whitby and C. L. Doe, 2002. UV irradiation causes the loss of viable mitotic recombinants in Schizosaccharomyces pombe cells lacking the G2/M DNA damage checkpoint. Genetics 160 891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich, A. G., C. D. Armour and L. H. Hartwell, 1998. The Saccharomyces cerevisiae RAD9, RAD17, RAD24 and MEC3 genes are required for tolerating irreparable, ultraviolet-induced DNA damage. Genetics 150 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, R. J., E. Kassianidis, A. M. Slawin and D. Philp, 2006. Comparative analyses of a family of potential self-replicators: the subtle interplay between molecular structure and the efficacy of self-replication. Chemistry 12 6829–6840. [DOI] [PubMed] [Google Scholar]
- Pessoa-Brandao, L., and R. A. Sclafani, 2004. Cdc7/Dbf4 functions in the translesion synthesis branch of the RAD6 epistasis group in Saccharomyces cerevisiae. Genetics 167 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasanuma, H., K. Hirota, T. Fukuda, N. Kakusho, K. Kugou et al., 2008. Cdc7-dependent phosphorylation of Mer2 facilitates initiation of yeast meiotic recombination. Genes Dev. 22 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith, H. A., G W. Brownand S. L. Forsburg, 2000 Schizosaccharomyces pombe Hsk1p is a potential Cds1p target required for genome integrity. Mol. Cell. Biol. 20 7922–7932. [DOI] [PMC free article] [PubMed]
- Sommariva, E., T. K. Pellny, N. Karahan, S. Kumar, J. A. Huberman et al., 2005. Schizosaccharomyces pombe Swi1, Swi3, and Hsk1 are components of a novel S-phase response pathway to alkylation damage. Mol. Cell. Biol. 25 2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, E., C. R. Chapman, F. Al-Khodairy, A. M. Carr and T. Enoch, 1997. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16 2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, T., K. Ogino, E. Matsui, M. K. Cho, H. Kumagai et al., 1999. A fission yeast gene, him1(+)/dfp1(+), encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage. Mol. Cell. Biol. 19 5535–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, T., K. Ogino, K. Tatebayashi, H. Ikeda, K. Arai et al., 2001. Regulation of initiation of S phase, replication checkpoint signaling, and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast. Mol. Biol. Cell 12 1257–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K., and P. Russell, 2001. Mrc1 channels the DNA replication arrest signal to checkpoint kinase Cds1. Nat. Cell Biol. 3 966–972. [DOI] [PubMed] [Google Scholar]
- Tavassoli, M., M. Shayeghi, A. Nasim and F. Z. Watts, 1995. Cloning and characterisation of the Schizosaccharomyces pombe rad32 gene: a gene required for repair of double strand breaks and recombination. Nucleic Acids Res. 23 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama, M., K. Arai and H. Masai, 2001. Sna41goal, a novel mutation causing G1/S arrest in fission yeast, is defective in a CDC45 homolog and interacts genetically with polalpha. Mol. Genet. Genomics 265 1039–1049. [DOI] [PubMed] [Google Scholar]
- Ueno, M., T. Nakazaki, Y. Akamatsu, K. Watanabe, K. Tomita et al., 2003. Molecular characterization of the Schizosaccharomyces pombe nbs1+ gene involved in DNA repair and telomere maintenance. Mol. Cell. Biol. 23 6553–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkade, H. M., S. J. Bugg, H. D. Lindsay, A. M. Carr and M. J. O'Connell, 1999. Rad18 is required for DNA repair and checkpoint responses in fission yeast. Mol. Biol. Cell 10 2905–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkade, H. M., T. Teli, L. V. Laursen, J. M. Murray and M. J. O'Connell, 2001. A homologue of the Rad18 postreplication repair gene is required for DNA damage responses throughout the fission yeast cell cycle. Mol. Genet. Genomics 265 993–1003. [DOI] [PubMed] [Google Scholar]
- Wan, L., H. Niu, B. Futcher, C. Zhang, K. M. Shokat et al., 2008. Cdc28–Clb5 (CDK-S) and Cdc7–Dbf4 (DDK) collaborate to initiate meiotic recombination in yeast. Genes Dev. 22 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., P. M. Watt, E. J. Louis, R. H. Borts and I. D. Hickson, 1996. Pat1: a topoisomerase II-associated protein required for faithful chromosome transmission in Saccharomyces cerevisiase. Nucleic Acids Res. 24 4791–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, R. S., J. S. Williams and J. A. Tainer, 2007. Mre11–Rad50–Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell Biol. 85 509–520. [DOI] [PubMed] [Google Scholar]
- Wyatt, M. D., and D. L. Pittman, 2006. Methylating agents and DNA repair responses: methylated bases and sources of strand breaks. Chem. Res. Toxicol. 19 1580–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. J., M. Davenport and T. J. Kelly, 2006. Two-stage mechanism for activation of the DNA replication checkpoint kinase Cds1 in fission yeast. Genes Dev. 20 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T., K. I. Mizuno, K. Hirota, N. Kon, W. P. Wahls et al., 2004. Roles of histone acetylation and chromatin remodeling factor in a meiotic recombination hotspot. EMBO J. 23 1792–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L., and S. J. Elledge, 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300 1542–1548. [DOI] [PubMed] [Google Scholar]