Abstract
The G2 DNA damage checkpoint inhibits Cdc2 and mitotic entry through the dual regulation of Wee1 and Cdc25 by the Chk1 effector kinase. Upregulation of Chk1 by mutation or overexpression bypasses the requirement for upstream regulators or DNA damage to promote a G2 cell cycle arrest. We screened in fission yeast for mutations that rendered cells resistant to overexpressed chk1+. We identified a mutation in tra1, which encodes one of two homologs of transformation/transcription domain-associated protein (TRRAP), an ATM/R-related pseudokinase that scaffolds several histone acetyltransferase (HAT) complexes. Inhibition of histone deacetylases reverts the resistance to overexpressed chk1+, suggesting this phenotype is due to a HAT activity, although expression of checkpoint and cell cycle genes is not greatly affected. Cells with mutant or deleted tra1 activate Chk1 normally and are checkpoint proficient. However, these cells are semi-wee even when overexpressing chk1+ and accumulate inactive Wee1 protein. The changed division response (Cdr) kinases Cdr1 and Cdr2 are negative regulators of Wee1, and we show that they are required for the Tra1-dependent alterations to Wee1 function. This identifies Tra1 as another component controlling the timing of entry into mitosis via Cdc2 activation.
THE control of the transition from G2 into mitosis is highly conserved and ancient in origin, being effectively an universal process in all eukaryotic cells (Nurse 1990). The key mitotic inducer is the mitotic cyclin-dependent kinase Cdc2, whose activity is controlled not only by binding to its cyclin partners, but also by a finely tuned and reversible inhibitory phosphorylation on tyrosine 15 (Y15) (Dunphy 1994). This phosphorylation is catalyzed by the Wee1 family of kinases, which maintains Cdc2 in its inactive state throughout interphase. For mitotic entry to occur, the Cdc25 family of phosphatases dephosphorylates Y15, rapidly activating Cdc2 to enable phosphorylation of proteins that promote mitosis.
The timing of Cdc2 activation is influenced by multiple checkpoint pathways that monitor the order and fidelity of cell cycle events, thus ensuring the readiness for chromosome segregation to proceed. Upon detection of DNA damage, the G2 DNA damage checkpoint delays entry into mitosis, enabling time for DNA repair prior to chromosome segregation (O'Connell et al. 2000; O'Connell and Cimprich 2005). Failure to establish this checkpoint results in catastrophic mitoses, where acentric chromosome fragments are lost and incompletely repaired chromosomes fail to segregate. This results in gross chromosomal rearrangements that can lead to cell death or, when less severe, tumorigenesis via activation of oncogenes and loss of tumor suppressors.
The effector kinase of the G2 DNA damage checkpoint, Chk1, elicits this delay through dual regulation of the Cdc25 phosphatases and Wee1 kinases that modulate Cdc2 activation (Raleigh and O'Connell 2000; O'Connell and Cimprich 2005). As with the core cell cycle machine, this checkpoint is also conserved from the fission yeast Schizosaccharomyces pombe to humans, and a detailed description of the molecular events leading to Chk1 activation has emerged from studies in multiple experimental systems (Kuntz and O'Connell 2009). The PI3-K-related ATM and ATR (ATM/R) protein kinases are targeted to sites of DNA damage that are processed into replication-protein-A–coated single-stranded DNA by binding their partners, the Mre11-Rad50-Nbs1 (MRN) complex and ATR-interacting protein (Rad26 in S. pombe), respectively (Falck et al. 2005). Independently, PCNA-related 9-1-1 complexes, composed of Rad9, Rad1, and Hus1, are loaded to sites of DNA damage by a replication factor C (RFC)-related complex where Rad17 replaces the large RFC1 component (Bermudez et al. 2003; Parrilla-Castellar et al. 2004). The assembly of these complexes and several ATM/R-catalyzed phosphorylation events recruits BRCT-domain mediator proteins (Canman 2003), which in turn recruit Chk1 to enable activating phosphorylation on residues in the C-terminal regulatory domain (Liu et al. 2000; Lopez-Girona et al. 2001b; Capasso et al. 2002; Gatei et al. 2003). The duration, rather than magnitude, of Chk1 activation is dependent on the extent of DNA damage (Latif et al. 2004), and the inactivation of Chk1 by dephosphorylation is necessary and sufficient for relief of the checkpoint-mediated arrest to allow mitotic entry (den Elzen et al. 2004; den Elzen and O'Connell 2004).
Precisely how phosphorylation activates Chk1 is not yet clear, although it may relieve in cis auto-inhibition of the N-terminal kinase domain by the C-terminal regulatory domain (Katsuragi and Sagata 2004). However, while deletion of the regulatory domain increases Chk1 activity in vitro (Chen et al. 2000), it is essential for Chk1 function in vivo (Kosoy and O'Connell 2008). Further, mutations in the C-terminal domain can either inactivate or super-activate Chk1 function in vivo (Wang and Dunphy 2000; Kosoy and O'Connell 2008; Palermo et al. 2008; Pereira et al. 2009), suggesting that it contributes more than an inhibitory function to the catalytic domain (Tapia-Alveal et al. 2009).
In S. pombe, Chk1 activated in G2 and then inactivated for mitotic entry is reactivated in the following cell cycle without apparent DNA damage and does not delay the progression of that cell cycle (den Elzen and O'Connell 2004; Harvey et al. 2004). Further, several DNA repair mutants cycle normally with active Chk1 (our unpublished observations), suggesting that additional signaling may be regulated by DNA damage that impacts on cell cycle progression. Indeed, we have shown that the changed division response (Cdr) kinases, Cdr1 and Cdr2, act as Chk1 antagonists through their negative regulation of Wee1 (Calonge and O'Connell 2006), and thus it is possible that other pathways controlling cell cycle progression await identification. Cdr1, and possibly Cdr2, directly inhibit Wee1 by phosphorylation (Coleman et al. 1993; Kanoh and Russell 1998). Recent observations implicate these kinases in the coordination of cell growth with division, where they are regulated within a spatial gradient controlled by another kinase, Pom1 (Martin and Berthelot-Grosjean 2009; Moseley et al. 2009). Notably, limited nutrition reduces the size at division, and Cdr1 and Cdr2 are particularly important in advancing cell cycle progression under these conditions. However, cdr1Δ and cdr2Δ cells are delayed in progression through G2 under normal exponential growth conditions (Feilotter et al. 1991; Breeding et al. 1998; Kanoh and Russell 1998), suggesting that the regulation of Wee1 by Cdr1 and Cdr2 may extend to other conditions and/or other stresses when there is no limitation to nutrition.
The overexpression of chk1+ is sufficient to cause a G2 cell cycle arrest without Chk1 C-terminal phosphorylation, DNA damage, or the upstream checkpoint components (Walworth et al. 1993; O'Connell et al. 1997; Lopez-Girona et al. 2001b). Here, we have taken a novel screening approach to search for genes that render cells resistant to overexpressed chk1+. The screen identified one of two S. pombe transformation/transcription domain-associated protein (TRRAP) homologs, Tra1, which is required for cell cycle arrest mediated by chk1+ overexpression, but is not required for Chk1 activation or G2 arrest following DNA damage. TRRAP proteins scaffold several histone acetyltransferase (HAT) complexes (Grant et al. 1998; Allard et al. 1999; Cai et al. 2003). They are proteins closely related to the ATM/R kinases, but lack critical residues required for ATP binding. TRRAP proteins therefore lack kinase activity and are thus referred to as pseudokinases (Boudeau et al. 2006). We show here that Tra1 is required for the positive regulation of Wee1 and appears to work in opposition to the Cdr1 and Cdr2 kinases. These data define another mode of Wee1 regulation important for integrating signals controlling the G2/M transition.
MATERIALS AND METHODS
General S. pombe methods:
All strains are derivatives of 972h− and 975h+. Standard media and methods were employed for strain construction, the propagation of cultures, the introduction of plasmids by transformation, and FACS analysis of DNA content (Moreno et al. 1991; Outwin et al. 2009). Survival assays on plates containing methyl methanesulfonate (MMS) used 10-fold serial dilutions of cultures starting at 1 × 106 cells/ml, and 5 μl of each dilution plated for 4 days at 30°. Microscopy was performed on a Nikon E800 microscope and images were captured on a Spot XE camera. Cell-length measurements were made using an eye-piece micrometer, using septated cells or, if cell cycle arrested, no length was recorded and these cultures were listed as “arrested.” For derepression of the nmt1 promoter (Basi et al. 1993; Maundrell 1993), exponential cultures growing in minimal media supplemented with 10 μg/ml thiamine were washed three times in thiamine-free medium and then grown for the indicated times. For nitrogen starvation assays, exponential cultures growing in supplemented EMM2 medium were extensively washed in nitrogen-free medium and reinoculated into EMM2 containing 100%, 10%, or 0% nitrogen and cultured for a further 16 hr before fixing in 70% ethanol and being processed for FACS analysis of DNA content.
Western blotting:
Whole-cell extracts for Western blotting were prepared in 8 m urea, 50 mm NaPO4, 10 mm Tris, pH 8.0, separated by SDS–PAGE and transferred to nitrocellulose in 10 mm N-cyclohexyl-3-aminopropanesulfonic acid, pH 11, 10% methanol. Overproduced Chk1 was detected with rabbit anti-Chk1 polyclonal antibodies (O'Connell et al. 1997). Endogenous expression of Chk1 (HA3), Wee1 (HA3), Cdc25 (Myc13), Cdr1 (Flag3), and Cdr2 (HA3) were detected using 12CA5 (HA, Roche), 9E10 (Myc, Santa Cruz Biotechnology), and M2 (Flag, Sigma) monoclonal antibodies. All epitope-tagged alleles were confirmed to retain wild-type function. Total Cdc2 was detected with anti-PSTAIRE antibodies (Santa Cruz Biotechnology), and Y15 phosphorylated Cdc2 was detected with phospho-specific antibodies (Cell Signaling Technologies). Actin was detected with HRP-coupled anti-actin antibodies (Santa Cruz Biotechnology). Band intensities were quantified by densitometry (BioRad GS-800 with Quantity One software).
Screen for Chk1-resistant mutants:
A 1.8-kb HindIII fragment containing the ura4+ gene was isolated and used to transform wild-type S. pombe to generate a library of stable and random Ura+ integrants via three successive rounds of selection for Ura+ and selection relief by growth in yeast extract plus supplements. The library was transformed with pREP41-chk1-E472D (Kosoy and O'Connell 2008), and Chk1-resistant colonies were selected on media lacking thiamine. The pREP41-chk1-E472D was then lost from 500 independent colonies by selection relief, and the plasmid was reintroduced by mating from a wild-type carrier strain. From this, 2 of the 500 colonies were shown to harbor chromosomal mutations leading to Chk1 resistance, with the remainder being due to plasmid-borne mutations. Of the two, one colony showed Chk1 resistance linked to the ura4+ marker. The resistance was also observed for pREP1-Chk1, which was used in subsequent assays.
Cloning of Tra1:
Using the Chk1-resistant strain from the screen above, Southern blot analysis with a Ura4 probe was performed with a panel of restriction enzymes, and the smallest fragment containing ura4+ was generated by digestion with MspI (2.4 kb). MspI-digested genomic DNA was then religated into circular molecules, and the ura4+ fragment was recovered by inverse PCR using the following primers within the ura4+ sequence: forward—GCGTTTTATGTCAGAAGGC; reverse—GAGGTTCTTGGTAGGACA. The PCR fragment was purified and the site of insertion determined by DNA sequencing, which was within chromosome 2 at the 3′ end of the tra1+ gene, truncating Tra1 at residue 3559 of 3699. This allele was denoted tra1-1. The entire tra1 ORF was also deleted and replaced with ura4+ by homologous recombination, creating tra1Δ.
Analysis of gene expression profiles:
cDNA was prepared from RNA acid extracted from exponentially growing wild-type and tra1-1 cells and labeled with Cy3 or Cy5 as previously described (Bimbo et al. 2005). This was hybridized onto oligonucleotide-based microarray slides covering all predicted genes, and signals were quantified as described (Bimbo et al. 2005). A full data set of relative expression ratios, derived from three independent experiments, is presented in Table S1.
Protein kinase assays:
Extracts were made in Chk1 IP buffer (Capasso et al. 2002), and Chk1 was immunoprecipitated with polyclonal anti-Chk1 antibodies (O'Connell et al. 1997). Following extensive washing in Chk1 IP buffer, the beads were washed into Chk1 assay buffer, and activity was determined on a peptide substrate as described (Harvey et al. 2004; Latif et al. 2004) and quantified by scintillation counting. For Cdc2 assays, extracts were made in histone-kinase buffer, and Cdc2 was captured by immunoprecipitation of the B-type cyclin Cdc13 using anti-Cdc13 monoclonal antibody (O'Connell et al. 1997). Cdc2 activity was determined using histone H1 as a substrate and quantified by Phosphorimager analysis of dried gels. In both cases, assays were performed in triplicate.
RESULTS
Tra1 is required for G2 arrest mediated by Chk1 overexpression:
Activation of Chk1 is essential for checkpoint arrest following DNA damage. chk1+ overexpression causes a G2 cell cycle arrest without the requirement for DNA damage or activating phosphorylation, enabling Chk1-mediated control of cell cycle progression to become uncoupled from other DNA-damage–regulated events. We hypothesized that other signaling pathways that control cell cycle progression may be regulated in concert with Chk1 in response to DNA damage. Previously, we had obtained evidence that this was the case for the negative regulators of Wee1, the Cdr1 and Cdr2 kinases (Calonge and O'Connell 2006). To search for additional components that modulate the G2/M transition relevant to Chk1 signaling, we undertook an insertional mutagenesis screen, using the ura4+ gene as the insertional mutagen, to identify genes required for cell cycle arrest caused by chk1+ overexpression (see materials and methods).
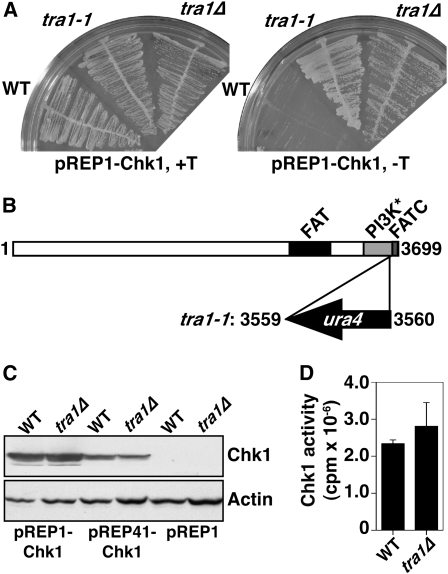
We identified one strain that was totally nonresponsive to chk1+ overexpression and detected the point of insertion by inverse PCR. This strain contained the ura4+ gene inserted near the 3′ end of a gene encoding one of two S. pombe TRRAP homologs, tra1 (Kanoh and Yanagida 2007), and this insertion allele was denoted tra1-1 (Figure 1, A and B). TRRAP proteins are large pseudokinases highly related to ATM, ATR, and DNA-PKcs (Boudeau et al. 2006). The insertion in tra1-1 truncates Tra1 at residue 3559 of 3699, deleting the C-terminal FATC domain, known to be critical in the function of the active kinases (Jiang et al. 2006). We deleted the entire open reading frame of tra1, replacing it with ura4+, but in all assays this allele (tra1Δ) phenocopied tra1-1, highlighting the importance of the FATC domain in Tra1. We confirmed that the resistance to chk1+ overexpression was not an artifact of loss of expression (Figure 1C) nor a lack of kinase activity (Figure 1D), and thus we concluded Tra1 is required for overexpressed chk1+ to elicit a cell cycle arrest.
Figure 1.—
Tra1 is required for cell cycle arrest by Chk1 overexpression. (A) The indicated strains containing pREP1-Chk1 were grown on media in the presence (promoter repressed) or absence (promoter derepressed) of thiamine for 4 days at 30°. (B) Tra1 is a 3699-amino-acid protein containing the FAT and FATC domains characteristic of the ATM and ATR kinases, but lacks ATP coordinating residues in the PI3K domain (labeled PI3K*) required for kinase activity. ura4 truncates Tra1 at residue 3559 and is expressed in the opposite direction. (C) Western blot analysis shows that expression levels of Chk1 (rabbit anti-Chk1) are unaffected in tra1Δ cells. Actin was used as a loading control. (D) Chk1 activity is also unaffected in tra1Δ cells. Data are mean ± SE; n = 3.
Inhibiting histone deacetylases blocks tra1Δ resistance to Chk1 overexpression:
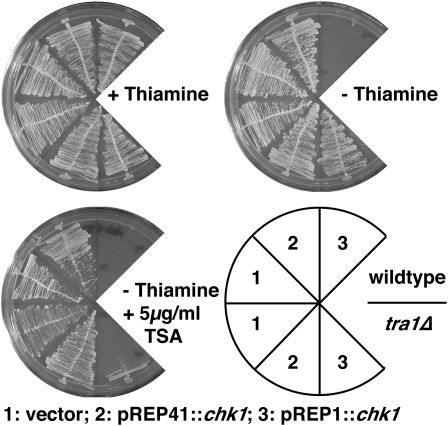
TRRAP proteins scaffold several HAT complexes. We therefore tested whether reducing histone deacetylase (HDAC) activity would reverse the resistance of tra1Δ cells to Chk1 overexpression. S. pombe cells have six HDACs representing the three classes of these enzymes encoded by hos2, clr6 (class I), clr3 (class II), and sir2, hst2, and hst4 (class III) (Ekwall 2005). Of these, only clr6 is essential, but this number of genes and redundancy of function makes genetic downregulation of total HDAC activities problematic. Therefore, to this end, we used the HDAC inhibitor trichostatin A (TSA) to dampen total HDAC activity. We measured growth with chk1+ expressed from the wild-type nmt1 promoter (pREP1) or from the attenuated nmt1 promoter (pREP41), which directs expression to ∼60-fold lower levels than wild type (Forsburg 1993). Expression of chk1+ from pREP41 causes a modest G2 cell delay (Calonge and O'Connell 2006) and was tolerated by both wild-type and tra1Δ cells. Colony formation in both strains under these conditions was not affected by addition of TSA (Figure 2). Expression of chk1+ from pREP1 is lethal to wild type but does not affect cell cycle progression in tra1Δ. However, addition of TSA to the medium restored pREP1∷chk1-mediated cell cycle arrest to tra1Δ cells (Figure 2 and Table 1). This is consistent with the resistance to chk1+ overexpression being due to a reduction in Tra1-dependent HAT activity, which is balanced by TSA-mediated HDAC inhibition.
Figure 2.—
The histone deacetylase inhibitor trichostatin A suppresses the Chk1 resistance of tra1Δ. Plates were incubated for 4 days at 30°.
TABLE 1.
Suppression of resistance to Chk1 overexpression of tra1Δ by HDAC mutants and TSA
| Genotype | Vector | nmt1∷chk1 |
|---|---|---|
| Wild type | 13.8 ± 1.0 | Arrested |
| Wild type + 5 μg/ml TSA | 14.7 ± 1.1 | Arrested |
| tra1Δ | 10.3 ± 1.1 | 12.4 ± 1.3 |
| tra1Δ + 5 μg/ml TSA | 13.5 ± 0.8 | Arrested |
| sir2Δ | 14.4 ± 1.3 | Arrested |
| sir2Δ tra1Δ | 10.7 ± 0.9 | 12.1 ± 2.0 |
| hos2Δ | 13.2 ± 1.2 | Arrested |
| hos2Δ tra1Δ | 13.6 ± 2.9 | 23.4 ± 4.4 |
| hst2Δ | 14.0 ± 1.2 | Arrested |
| hst2Δ tra1Δ | 12.6 ± 1.6 | 12.4 ± 2.2 |
| hst4Δ | 14.4 ± 1.0 | Arrested |
| hst4Δ tra1Δ | 16.6 ± 2.4 | 23.9 ± 6.0 |
| clr3Δ | 13.4 ± 0.8 | Arrested |
| clr3Δ tra1Δ | 12.1 ± 1.2 | Arrested |
| clr6-1 | 16.2 ± 1.5 | Arrested |
| clr6-1tra1Δ | 16.1 ± 2.7 | Arrested |
Numbers are cell length at division following 20 hr growth in the absence of thiamine at 30°, except for clr6-1, which is 30 hr at 25°. Cell cycle arrest was determined by nondividing highly elongated cells. Data are mean ± SD; n = 50.
We then asked whether inactivation of any of the individual HDAC genes could similarly suppress the resistance to chk1+ overexpression in tra1Δ cells (Table 1; supporting information, Figure S1). We employed null alleles of each gene except the essential clr6, for which we used clr6-1, a temperature-sensitive lethal mutation that is significantly compromised at 25° (Grewal et al. 1998). Deletion of sir2 or hst2, which encode members of the class III NAD-dependent family of HDACs, had no effect on the Chk1 resistance of tra1Δ cells. Deletion of the other class III HDAC, hst4, partially suppressed the Chk1 resistance of tra1Δ cells; colonies still formed, but the cells were delayed in cell cycle progression and thus are significantly elongated. Deletion of the class I HDAC gene hos2 conferred a similar partial suppression, whereas mutation of the other class I gene, clr6, or deletion of the class II HDAC gene clr3, completely suppressed the Chk1 resistance of tra1Δ cells. As these different HDACs have specificity for different genomic regions, and for different lysines on histones and nonhistone proteins (Ekwall 2005), these data suggest that a HAT deficiency in tra1Δ cells may reduce the acetylation at multiple residues and loci, and thus it is possible that phenotypes of tra1Δ cells are pleiotrophic in origin.
We also tested whether mutation in other nonessential components of HAT complexes conferred resistance to chk1+ overexpression. For this we utilized cells deleted for gcn5+ (Yamada et al. 2004) and mst2+ (Gomez et al. 2005), which encode components of the SAGA and NuA3 HATs, respectively. In both cases, these null mutants were sensitive to chk1+ overexpression (Figure S2), suggesting that tra1Δ affects either both classes of HAT complexes or the function on other subunits encoded by essential genes.
Finally, Tra1 has also been implicated as a member of the ASTRA complex (Shevchenko et al. 2008), which is essential for telomere maintenance. The deletion of each of the genes for the other members of this complex is lethal, presumably due to telomere erosion, and thus it is not possible to test if these genes are required for resistance to chk1+ overexpression. Nevertheless, we tested tel2Δ and tti1Δ heterozygous diploid strains for sensitivity to chk1+ overexpression, and both strains were wild type for this phenotype (Figure S3). Combined with the nonessential nature of tra1, the data suggest that the effects of tra1Δ are more likely via HAT function rather than telomere maintenance.
Gene expression profiles controlled by Tra1:
As the resistance to chk1+ overexpression appeared to be dependent on HAT activity, we presumed that this was due to altered gene expression affecting the response to Chk1. Global expression profiles were determined by microarray analysis, comparing wild type to tra1-1 cells, and a complete data set is presented in Table S1. Only 57 genes showed a ≥2-fold change in gene expression; 31 genes were downregulated (Table 2) and an additional 26 genes upregulated (Table 3). Interestingly, expression of tra1 itself was reduced 2.7-fold in tra1-1 cells (P = 0.005), suggesting that Tra1 controls its own expression.
TABLE 2.
Genes with ≥2-fold reduction in expression in tra1-1
| Gene | Protein | Fold change |
|---|---|---|
| SPBPB10D8.01 | Cysteine transporter (predicted) | −5.20 |
| obr1 | Ubiquitinated histone-like protein | −4.04 |
| gst2 | Glutathione S-transferase | −3.48 |
| abp2 | ARS binding protein | −3.07 |
| SPBPB10D8.04c | Membrane transporter (predicted) | −2.94 |
| SPAC869.05c | Sulfate transporter (predicted) | −2.77 |
| urg2 | Uracil phosphoribosyltransferase (predicted) | −2.74 |
| hsp16 | Heat-shock protein | −2.74 |
| SPBC428.11 | Homocysteine synthase | −2.72 |
| tra1 | TRAPP homolog | −2.70 |
| SPAC1039.02 | Phosphoprotein phosphatase (predicted) | −2.60 |
| SPAC869.02c | Nitric oxide dioxygenase (predicted) | −2.60 |
| SPCC70.08c | Methyltransferase (predicted) | −2.56 |
| cnp3 | CENP-C | −2.55 |
| SPBC1271.07c | N-acetyltransferase (predicted) | −2.49 |
| SPAC5H10.10 | NADPH dehydrogenase (predicted) | −2.46 |
| adg1 | Dequence orphan | −2.42 |
| vht1 | Vitamin H transporter | −2.41 |
| SPAC869.10c | Proline-specific permease (predicted) | −2.40 |
| plr1 | Pyridoxal reductase | −2.35 |
| SPBPB7E8.01 | Sequence orphan | −2.35 |
| SPCC569.05c | Spermidine family transporter (predicted) | −2.31 |
| mik1 | Mitotic inhibitor kinase | −2.24 |
| SPAC11D3.13 | ThiJ domain protein | −2.16 |
| SPAC11D3.01c | Conserved fungal protein | −2.15 |
| SPAC5H10.03 | Phosphoglycerate mutase family | −2.15 |
| SPBC359.03c | amino acid permease | −2.13 |
| urg1 | GTP cyclohydrolase II (predicted) | −2.12 |
| SPAC977.14c | Aldo/keto reductase | −2.09 |
| arg4 | Carbamoyl-phosphate synthase | −2.03 |
| SPCC569.07 | Aromatic aminotransferase (predicted) | −2.01 |
TABLE 3.
Genes with ≥2-fold increase in expression in tra1-1
| Gene | Protein | Fold change |
|---|---|---|
| isp6 | Vacuolar serine protease | +2.01 |
| SPAC5H10.04 | NADPH dehydrogenase (predicted) | +2.06 |
| ste4 | Adaptor protein | +2.07 |
| SPBC409.08 | Spermine family transporter (predicted) | +2.09 |
| SPBC1773.12 | Transcription factor (predicted) | +2.10 |
| SPBPB2B2.12c | UDP-glucose 4-epimerase | +2.10 |
| gas2 | 1,3-β-glucanosyltransferase Gas2 (predicted) | +2.13 |
| gpx1 | Glutathione peroxidase | +2.14 |
| itr2 | Myo-inositol transporter | +2.18 |
| SPBC8E4.01c | Inorganic phosphate transporter (predicted) | +2.26 |
| SPCC70.03c | Proline dehydrogenase (predicted) | +2.29 |
| mei2 | RNA-binding protein | +2.29 |
| SPAC23D3.12 | Inorganic phosphate transporter (predicted) | +2.47 |
| zym1 | Metallothionein | +2.59 |
| SPAC15E1.02c | DUF1761 family protein | +2.79 |
| SPAC186.07c | Hydroxyacid dehydrogenase (predicted) | +2.88 |
| mfm2 | M-factor precursor | +2.90 |
| ste11 | Transcription factor | +2.93 |
| SPBPB2B2.10c | Galactose-1-phosphate uridylyltransferase (predicted) | +3.48 |
| pho1 | Acid phosphatase | +4.26 |
| SPBC725.10 | tspO homolog | +4.79 |
| ght5 | Hexose transporter | +5.51 |
| hsp9 | Heat-shock protein | +6.00 |
| SPAC27D7.10c | But2 family protein | +7.10 |
| gpd3 | Glyceraldehyde 3-phosphate dehydrogenase | +8.45 |
| SPAC27D7.11c | But2 family protein | +8.73 |
Fold change is calculated compared to wild-type cells. Table S1 includes the full data set. Predicted protein functions are based on homology and assigned by GeneDB. None of these genes have been implicated in the progression of the mitotic cell cycle.
Inspection of the known or homology-based predicted functions for these genes identified only one gene that is implicated in Chk1 signaling, encoding the Wee1 kinase family member, Mik1. mik1+ expression was 2.24-fold lower in tra1-1 (P = 0.002), and although mik1+ expression is extremely low in cycling cells, it is upregulated during S-phase (Christensen et al. 2000), and this is essential for cell viability in the absence of Wee1 (Lundgren et al. 1991). Further, the deletion of mik1 has been reported to render cells resistant to Chk1 overexpression (Baber-Furnari et al. 2000; Rhind and Russell 2001), and thus we thought this a good candidate to explain the resistance of tra1 mutants to Chk1.
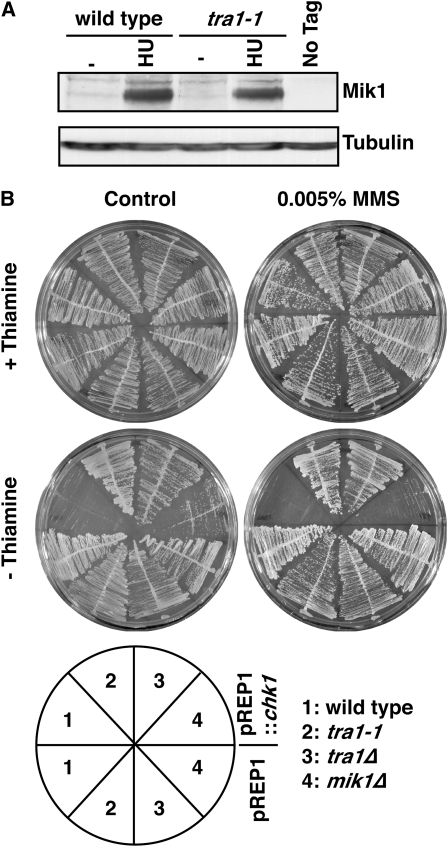
We therefore measured Mik1 protein levels in wild-type and tra1-1 cells, including conditions of replication arrest with the ribonucleotide reductase inhibitor hydroxyurea (HU) (Figure 3A). Mik1 levels were very difficult to detect in cycling cells, but were dramatically increased in HU-treated cells (Figure 3A). However, no significant difference was observed between wild type and tra1-1, and thus the observed reduction in mik1 mRNA levels in tra1-1 may represent subtle differences in S-phase progression (although no evidence for this is seen by FACS analysis) or may be an effect of the extremely low expression levels in cycling cells.
Figure 3.—
Chk1 resistance in tra1Δ is independent of Mik1. (A) Western blot of extracts from cells expressing Myc-tagged Mik1 or an untagged control (no tag) from untreated cells (−) or cells treated with 11 mm HU for 4 hr at 30°. Antitubulin antibodies were used as a loading control. (B) Cells harboring vector (pREP1) or pREP1-Chk1 were grown in the presence or absence of thiamine/MMS for 4 days at 30°. Note that mik1Δ cells are severely growth inhibited by Chk1 overexpression, and this is further exacerbated by MMS.
Next we tested the sensitivity of mik1Δ cells to chk1+ overexpression (Figure 3B). Growth of cells expressing chk1+ from pREP1 was severely impaired in mik1Δ cells, although it was slightly better than in wild-type cells. However, if cells were further sensitized to chk1+ overexpression through Chk1 activation by sublethal DNA damage (0.005% MMS), mik1Δ cells were completely growth inhibited, whereas the growth of tra1-1 and tra1Δ cells was unaffected. Thus, we cannot explain the complete resistance of tra1 mutant cells to chk1+ overexpression due to perturbations to Mik1.
Tra1 cells are defective in G2/M cell cycle control:
There are two possible explanations for the resistance of tra1-1 and tra1Δ cells to Chk1 overexpression. First, they could be specifically affected in some aspect of Chk1 function, although because expression and in vitro kinase activity are unaffected, this would be accounted for by an alternative explanation such as nuclear exclusion or the inability to interact with the substrate. Such a defect should cause a defect in the DNA damage checkpoint. Alternatively, tra1 mutants may have a defect in the regulation of mitotic entry downstream of Chk1. In this case, evidence for perturbation to negative regulation of cell cycle progression should exist, which in S. pombe is evident as division at a reduced cell size, the “wee” phenotype.
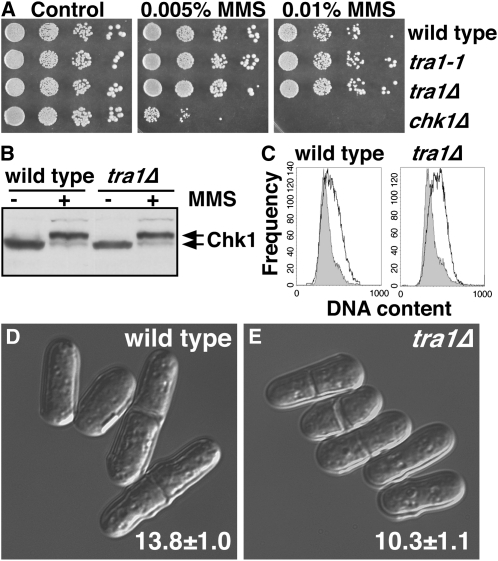
To assess the first possibility, we asked if Tra1 was required for Chk1-dependent cell cycle delay in response to DNA damage. Neither tra1-1 nor tra1Δ cells were sensitive to DNA damage or replication arrest, (Figure 4, A and C; Figure S4). tra1Δ and tra1-1 cells delayed cell cycle progression with wild-type kinetics following DNA damage and did not alter the sensitivity of multiple DNA-damage-sensitive mutants including chk1Δ (data not shown). Moreover, Tra1 was not required for Chk1 activation by phosphorylation, assayed as a mobility shift on Western blots (Figure 4B). Further, tra1Δ did not render cells resistant to the lethal effects of overexpression of mad2+, cds1+, or fin1+, which signal cell cycle delay/arrest by different mechanisms (data not shown). Therefore, as tra1Δ cells are responsive to Chk1 activated by DNA damage, the resistance to overexpressed chk1+ in the absence of DNA damage might be due to a defect in signaling downstream of Chk1 that enables progression into mitosis when chk1+ is overexpressed, but not when Chk1 is fully activated by the DNA damage checkpoint.
Figure 4.—
Tra1 is not required for checkpoint arrest, but is required for regulation of the G2/M transition. (A) Tra1 is not required for resistance to MMS. YES plates containing the indicated concentrations of MMS or no drug (control) were inoculated with spots of 10-fold serial dilutions of the indicated strains and were grown at 30° for 4 days. (B) Tra1 is not required for activating phosphorylation on Chk1 in the presence of MMS, showing that signaling through endogenous Chk1 is intact in tra1Δ cells. (C) FACS profiles of DNA content in cycling cells (shaded) or in MMS-treated cells (open). The cell cycle delay (cell elongation) in MMS broadens the profiles of these samples. The lengths of 50 exponentially growing (D) wild-type and (E) tra1Δ cells were determined by microscopy, and representative images are shown. Data are mean ± SD.
Microscopic observation of tra1Δ cells indicated that these cells have a semi-wee phenotype, dividing at only 10.3 μm, compared to the 13.8 μm of wild-type controls (Table 1 and Table 4; Figure 4, C and D). This semi-wee phenotype is less severe than a complete wee phenotype, where cells divide at ∼8 μm, but nevertheless is indicative of a shortened G2 period of the cell cycle in tra1Δ cells. Cells with wee or semi-wee phenotypes are checkpoint proficient but resistant to chk1+ overexpression (Walworth et al. 1993; O'Connell et al. 1997; Raleigh and O'Connell 2000; Calonge and O'Connell 2006), and with the normal response to DNA damage, we propose this is the reason for Chk1 resistance in tra1 mutants. Notably, both TSA and several of the HDAC mutants also suppressed the semi-wee phenotype of tra1Δ cells (Table 1).
TABLE 4.
cdr1 and cdr2 are required for resistance to chk1+ overexpression
| Genotype | Vector | nmt1∷chk1 |
|---|---|---|
| Wild type | 13.8 ± 1.0 | Arrested |
| tra1-1 | 11.1 ± 1.2 | 11.0 ± 1.0 |
| tra1Δ | 10.3 ± 1.1 | 12.4 ± 1.3 |
| cdr1Δ | 18.1 ± 0.9 | Arrested |
| cdr2Δ | 17.9 ± 1.1 | Arrested |
| tra1-1 cdr1Δ | 17.0 ± 1.4 | Arrested |
| tra1Δ cdr1Δ | 17.1 ± 1.4 | Arrested |
| tra1-1 cdr2Δ | 12.8 ± 1.2 | 17.2 ± 1.8 |
| tra1Δ cdr2Δ | 12.8 ± 1.1 | 18.3 ± 2.2 |
Numbers are cell length at division following 20 hr growth in the absence of thiamine. Cell cycle arrest was determined by nondividing highly elongated cells. Data are mean ± SD from three samples of 50 cells.
Altered regulation of Wee1 in tra1Δ cells:
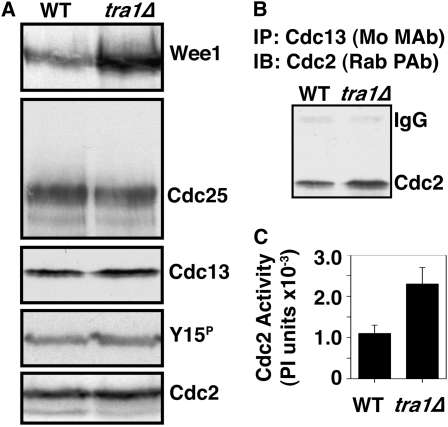
Chk1 signals cell cycle arrest by enforcing the inhibitory Y15 phosphorylation on Cdc2 (O'Connell et al. 1997; Rhind et al. 1997). This is achieved through the dual regulation of Wee1 and Cdc25 (Raleigh and O'Connell 2000). We therefore asked whether these proteins were altered in tra1Δ cells (Figure 5A). We observed that Cdc25 levels were unaffected in tra1Δ cells. Cdc25 activation is normally restricted to mitosis and is associated with a phosphorylation-dependent mobility shift (Moreno et al. 1990; Wolfe and Gould 2004), which we did not observe. Further, although chk1+ overexpression results in a net nuclear exclusion of Cdc25 (Lopez-Girona et al. 1999), this is not required for Chk1-dependent cell cycle arrest (Lopez-Girona et al. 2001a) and thus cannot explain the resistance of tra1Δ cells to chk1+ overexpression.
Figure 5.—
Wee1 levels accumulate in tra1Δ cells. (A) Western blotting shows that Wee1 protein accumulates in tra1Δ cells, but the levels of Cdc25, Cdc13, tyrosine-15 phosphorylated (Y15P), and total Cdc2 are not affected. Note that tra1Δ cells are semi-wee (Table 3) and that Y15P does not accumulate, indicating that the excess Wee1 in tra1Δ cells is not fully active. (B) Cdc13 was immunoprecipitated with a mouse monoclonal anti-Cdc13 antibody and that coprecipitating Cdc2 was detected by Western blotting with a rabbit polyclonal anti-Cdc2 antibody. The anti-rabbit secondary antibody weakly cross-reacts with the mouse IgG heavy chain. Immunoprecipitated Cdc13 comigrates with the IgG heavy chain, which precludes its detection by IP or Western blots, both mouse antibodies. (C) Cdc2 kinase activity in Cdc13 IPs is increased by approximately twofold in tra1Δ cells. Data are mean ± SE; n = 3.
Conversely, Wee1 protein levels were significantly upregulated in tra1Δ cells (approximately fivefold by densitometry). This was a surprising result, as wild-type cells are extremely sensitive to increased levels of Wee1. An enforced approximately fivefold increase in expression levels leads to a doubling of cell cycle duration, with cells dividing at 28 μm, compared to 14 μm for wild-type cells (Russell and Nurse 1987). As tra1Δ cells are semi-wee, the accumulated Wee1 protein cannot be fully active, suggesting that Tra1 is required for full Wee1 activity. Further, as Wee1 mRNA levels are unaffected in tra1-1 cells (Table S1), in this context Wee1 activity negatively correlates with Wee1 protein stability.
Consistent with semi-wee phenotype, exponentially growing tra1Δ cells had twofold higher Cdc2 kinase activity measured with IP of the major B-type cyclin, Cdc13 (Figure 5C). Although (inactive) Y15 phosphorylated Cdc2 levels were the same in wild-type and tra1Δ cells (Figure 5A), this inhibitory phosphorylation occurs only on Cdc2 molecules that are bound to a cyclin (Parker et al. 1991, 1992; Parker and Piwnica-Worms 1992), and consistently there was more Cdc2 in anti-Cdc13 IPs in tra1Δ than in wild-type cells (Figure 5B). Therefore, these complexes contain more dephosphorylated (active) Cdc2. The same phenomenon may account for unaltered Y15 phosphorylation levels in other “wee” mutants (Raleigh and O'Connell 2000). Cdc13 accumulates during G2 phase (Alfa et al. 1989), and despite the shortened G2 of the semi-wee tra1Δ cells, the steady-state levels of Cdc13 are unaffected (Figure 5A). Therefore, this increase in Cdc13–Cdc2 complexes could arise from more efficient complex formation in interphase, less efficient complex destruction in mitosis, or a combination of both these events.
Deletion of Cdr kinases suppresses cell cycle defects in tra1 mutants:
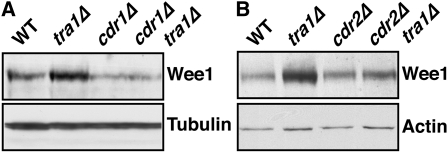
Cdr1 and Cdr2 are serine/threonine kinases that act as negative regulators of Wee1 (Coleman et al. 1993; Wu and Russell 1993; Breeding et al. 1998; Kanoh and Russell 1998). Consequently, cdr1Δ and cdr2Δ cells are delayed in cell cycle progression (Young and Fantes 1987). Dominant-negative alleles of cdr1, which interfere with both Cdr1 and Cdr2, render cells hyper-sensitive to chk1+ overexpression, whereas cdr1+ overexpression results in a wee phenotype and resistance to chk1+ overexpression (Calonge and O'Connell 2006). Given this relationship to the phenotypes of tra1Δ cells, we assayed whether Wee1 accumulation in tra1Δ was dependent on Cdr1 and/or Cdr2. Western blotting showed that this is indeed the case (Figure 6, A and B). In the case of cdr1Δ cells (wild type for tra1), Wee1 levels were also reduced compared to wild type, and yet cdr1Δ cells are delayed in cell cycle progression in a Wee1-dependent manner (Young and Fantes 1987). This is consistent with the negative correlation between Wee1 levels and activity and suggests that Cdr1 also regulates Wee1 levels in cycling cells.
Figure 6.—
Wee1 accumulation in tra1Δ cells is dependent on Cdr1 and Cdr2. (A and B) Western blot for HA-tagged Wee1 in the indicated strains grown to mid-logarithmic phase at 30°. Antitubulin and anti-actin were used as loading controls. Note that increased Wee1 levels are suppressed by cdr1Δ and cdr2Δ.
We next asked whether Cdr1 or Cdr2 affected the semi-wee and chk1+ overexpression resistance phenotypes of tra1 mutants. Deletion of cdr1 suppressed both phenotypes for tra1Δ and tra1-1, whereas deletion of cdr2 partially suppressed these phenotypes (Table 4). Therefore, these Wee1 regulators either directly or indirectly influence tra1 mutant phenotypes, which firmly establishes altered regulation of Wee1 as the root of these effects. Further, as upregulation of Cdc25 suppresses the cell cycle delay of cdr mutants (Kanoh and Russell 1998), it is unlikely that Cdc25 is altered in the tra1 mutants. However, as cdr2Δ fully rescues the elevated Wee1 levels, but only partially rescues the cellular phenotypes, these must be affected by more than just the amount of Wee1 protein.
The phosphorylation of Wee1 by Chk1 also stabilizes Wee1 (Raleigh and O'Connell 2000), and although this increases the cellular pool of Wee1 by approximately twofold, it does not alter the specific activity of the enzyme (O'Connell et al. 1997). We tested the effects of chk1+ overexpression in cells lacking Tra1 and/or Cdr1. As in wild-type cells, chk1+ overexpression increased Wee1 levels in both cdr1Δ and cdr1Δ tra1Δ cells, which are responsive to Chk1. However, in tra1Δ cells, Wee1 levels are already higher than in wild-type cells overexpressing chk1+ and did not significantly increase upon chk1+ overexpression (Figure S5); presumably chk1+ overexpression has no effect on Wee1 activity in tra1Δ cells, as they are nonresponsive to overexpressed chk1+, and thus the effects of lacking Tra1 on Wee1 are epistatic to those derived by Chk1-catalyzed phosphorylation.
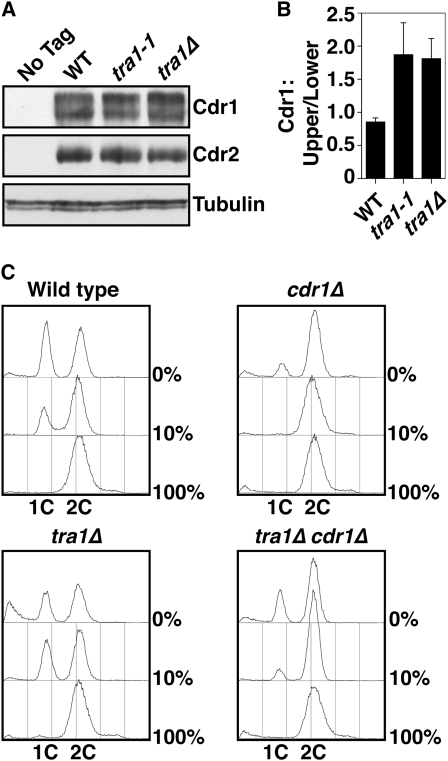
We next assayed if Cdr1 and/or Cdr2 were upregulated in tra1 mutant cells. Western blotting showed that the overall levels of each protein was unaffected by tra1-1 or tra1Δ (Figure 7, A and B). All published assays of Cdr1 and Cdr2 kinase activity in S. pombe have utilized recombinant (baculoviral) or overexpressed protein (Coleman et al. 1993; Wu and Russell 1993; Kanoh and Russell 1998). We attempted to assay the activities of endogenous (immunoprecipitated) Cdr1 and Cdr2 using Wee1 as a substrate and published conditions for the recombinant proteins, but did not find significant activity. We have extensively explored variations in assay conditions, including pH range, cation requirement, alternative substrates, and surfactants, and still are yet to find a robust in vitro activity. However, the autophosphorylation of Cdr1 is accompanied by a mobility shift visible on Western blots (Calonge and O'Connell 2006), and the upregulation of Cdr1 is itself sufficient to confer a wee phenotype (Calonge and O'Connell 2006). In both tra1-1 and tra1Δ cells, there was an ∼50–80% increase in phosphorylated (active) Cdr1 (Figure 7, A and B), suggesting that Cdr1 is indeed upregulated in the absence of Tra1.
Figure 7.—
Accumulation of active Cdr1 in tra1 mutants. (A) Western blot analysis of Cdr1 (Flag-tagged) and Cdr2 (HA-tagged) levels. Tubulin and actin are used as loading controls. (B) The ratio of the upper (phosphorylated) to lower (unphosphorylated) Cdr1 was determined by densitometry. Data are mean ± SD; n = 3. (C) tra1Δ cells have an enhanced nitrogen starvation response. FACS profiles of cells grown in 100%, 10%, and 0% nitrogen for 16 hr at 30°.
Under conditions of nitrogen starvation, wild-type S. pombe cells advance entry into mitosis at reduced cell size and arrest in the G1 phase of the cell cycle (Young and Fantes 1987). This is achieved, at least in part, by negative regulation of Wee1 by the Cdr kinases (Coleman et al. 1993; Parker et al. 1993; Wu and Russell 1993; Breeding et al. 1998; Kanoh and Russell 1998). Cells lacking Cdr1 are defective in this response and alternatively arrest in G2 phase. We assayed the response to nitrogen starvation in tra1Δ cells and observed an enhanced G1 arrest (1C DNA content) in 10% nitrogen and a combination of G1 arrest and dead cells (<1C DNA, although we do not suggest that this is apoptosis) in 0% nitrogen (Figure 7C). Both enhanced responses were largely suppressed by deletion of cdr1, although tra1Δ cdr1Δ cells had a residual starvation response not present in cdr1Δ.
Together, these observations suggest that Tra1 may, directly or indirectly, downregulate Cdr1 and, possibly, Cdr2 activity; thus, in the absence of Tra1, increased Cdr kinase activity results in a semi-wee phenotype, an enhanced nitrogen starvation response, and a resistance to chk1+ overexpression.
DISCUSSION
Orderly progression through the cell cycle is essential to maintain ploidy and stability of the genome. For the transition from G2 into mitosis, upstream checkpoint proteins signal the timing of mitotic entry. Among these are checkpoints to detect completion of DNA replication, the absence of genomic lesions, the doubling of cell mass, and the synthesis of macromolecules. Ultimately, these signals up- or downregulate the inhibitory Y15 phosphorylation of Cdc2, the universal switch for the transition from G2 into mitosis. Through controlling the kinases and phosphatases that phosphorylate and dephosphorylate Y15, these checkpoint-signaling pathways work together to ensure that mitosis is initiated only when it will result in two viable and identical daughters. Although most checkpoints halt cell cycle progression in response to an insult, osmotic stress and limited nutrition actually advance mitotic entry in S. pombe (Young and Fantes 1987; Shiozaki and Russell 1995). It is therefore likely that there must be coregulation of checkpoints such that cell cycle delay can occur in the face of other signals promoting entry into mitosis.
The DNA damage checkpoint, via its effector kinase Chk1, inhibits mitotic entry through direct regulation of Cdc25 and Wee1. In the case of Wee1, phosphorylation by Chk1 stabilizes this otherwise labile protein, increasing the total Wee1 activity in the cell (Raleigh and O'Connell 2000), but does not alter the specific activity of Wee1 (O'Connell et al. 1997). Under conditions of limited nutrition, the Cdr kinases Cdr1 and Cdr2 advance mitotic entry by negatively regulating Wee1, and in vitro they inhibit recombinant Wee1 by phosphorylation (Coleman et al. 1993; Wu and Russell 1993; Breeding et al. 1998; Kanoh and Russell 1998). We have previously shown that these Cdr kinases act as Chk1 antagonists. Blocking Cdr kinase activity with dominant-negative cdr1 alleles, which interfere with both Cdr1 and Cdr2 function, greatly sensitizes cellular sensitivity to overexpressed chk1+. Presumably Cdr-mediated inhibitory phosphorylation negates Chk1-mediated Wee1 stability (Calonge and O'Connell 2006). Here, we have identified a new positive regulator of Wee1, the TRRAP homolog Tra1. Cells lacking Tra1-mediated Wee1 regulation have a shortened G2 period of the cell cycle, resulting in a semi-wee phenotype. This renders cells resistant to the overexpression of chk1+.
tra1Δ cells accumulate Wee1 protein to levels approximately fivefold over wild-type cells without any change in wee1 mRNA. Wee1 homologs are short-lived PEST sequence proteins, which are subjected to ubiquitin-dependent proteolysis (Michael and Newport 1998; Watanabe et al. 2004, 2005). We were not able to demonstrate an increase in Wee1 half-life in tra1 mutants because a cycloheximide chase actually stabilizes Wee1 protein, which is thought to be a physiological response to protein synthesis rates (Suda et al. 2000), although it is not known whether Wee1 is active under these conditions. Further, the very low levels of Wee1 rendered 35S-methionine chase experiments below the level at which we could detect expression. Nevertheless, it remains likely that the increased levels of Wee1 in tra1Δ cells is due to a block in ubiquitin-dependent proteolysis.
However, tra1Δ cells are actually semi-wee (10–11 μm at division) and have increased Cdc2 activity. Therefore, the Wee1 molecules in these cells must have only residual activity; complete lack of activity would result in a full wee phenotype (division at ∼8 μm), while wild-type–specific activity for the increased levels of Wee1 would at least double the size at division (∼28 μm), as wild-type cells are very sensitive to increased Wee1 expression (Russell and Nurse 1987). We have not directly measured endogenous Wee1 activity in tra1Δ cells because we (and others) have managed this only with recombinant protein (O'Connell et al. 1997), which is still a very challenging assay.
How, then, does Tra1 affect the regulation of Wee1? As a component of HAT complexes, Tra1 presumably has an indirect effect on the regulation of Wee1 through altered gene expression, and this is consistent with the TSA- and HDAC mutation-mediated suppression of the Chk1 resistance. However, it is not clear from our expression profiling which genes may be having a direct effect or, indeed, whether this may be a complex and pleiotrophic effect of small changes to the expression of many genes. It is notable that S. pombe contains a second TRRAP homolog, Tra2 (SPAC1F5.11c), which, like the single TRRAP gene in Saccharomyces cerevisiae (TRA1), is essential for cell viability (Antony M. Carr, personal communication). Therefore, Tra2 may have a more profound effect on gene expression in S. pombe, and consistent with this, mass spectrometry analysis shows that Tra2 predominates in Tip60 HAT complexes in S. pombe (Shevchenko et al. 2008).
Most phenotypes of tra1Δ and tra1-1 are completely suppressed by cdr1Δ and partially suppressed by cdr2Δ. Therefore, signals resulting in reduced Wee1 activity in the tra1 mutants must involve increased signaling through the Cdr kinases. The accumulation of hyper-phosphorylated Cdr1 and the enhanced nitrogen starvation response in the tra1Δ mutants is consistent with this. However, further biochemical characterization will require the development of sensitive and quantitative assays for these kinases, which has yet to be achieved with the endogenous proteins. Further, the precise signals that control Cdr kinase activity are not known, and advances in this regard will inform further analysis of the gene expression profiles controlled by Tra1. We note that Cdr kinases are critical for starvation responses and that many of the genes downregulated by ≥2-fold in tra1-1 cells encode predicted transporter proteins and nutrient permeases. Therefore, the gene expression changes in tra1Δ may potentiate or mimic a starvation response that, via the Cdr kinases, regulates Wee1 and, hence, sensitivity to Chk1.
Importantly, we note that the mechanisms underlying Wee1 inhibition and Wee1 accumulation may not be the same. cdr1Δ only partially suppresses the enhanced starvation response of tra1Δ cells. Further, although cdr1 overexpression inhibits Wee1, this does not lead to Wee1 accumulation (Calonge and O'Connell 2006). These observations are consistent with Wee1 inactivation and stabilization being separately regulated events.
TRRAP homologs have also been shown to interact with the MRN complex (Robert et al. 2006) and with Tel2, an essential protein that also interacts with ATM, ATR, and DNA-PKcs (Kanoh and Yanagida 2007; Takai et al. 2007; Anderson et al. 2008) and is part of the ASTRA complex involved in telomere maintenance (Shevchenko et al. 2008). It cannot be ruled out that these and other yet to be uncovered molecular interactions also impact on the regulation of Wee1. Furthermore, acetylation of histones and other nonhistone proteins can have effects other than changes in gene expression, for example, in the establishment of epigenetically controlled chromosome segregation (Dunleavy et al. 2005; Pidoux and Allshire 2005), and these events could also affect Wee1 activity via stress signaling. We have assayed for the acetylation of immunoprecipitated Chk1, Wee1, and Cdr1 with anti-acetyl lysine antibodies, which we did not observe (not shown). Furthermore, we cannot detect a physical interaction between Tra1 and these proteins, although we treat these data with caution because a C-terminal epitope tag completely inactivates Tra1 and an N-terminal tag partially inactivates the protein (as measured by cell size and response to chk1+ overexpression). Therefore, additional biochemical tools need to be developed to pursue these studies.
Inhibitors of both Chk1 and HDACs have been developed and are in trial for use as anticancer agents. This work links these two biological processes and opens a window for investigation in areas where these types of therapeutics might be used together in targeted therapies. Like Chk1 and Wee1, TRRAP is highly conserved across species, and we anticipate that human TRRAP will impact on the Chk1-Wee1 pathway of Cdc2 regulation in humans that may prove to be useful in designing these therapeutic regimens.
Acknowledgments
We thank Kathy Gould, Michael Keogh, Eishi Nogouchi, Karl Ekwall, Shiv Grewal, and Lorraine Pillus for strains; the Genome Institute of Singapore microarray facility for manufacturing the S. pombe arrays; and Claudia Tapia-Alveal, Emily Outwin, Kirstin Bass, and Karen Kuntz for critical discussions. The microarray data have been deposited in the Gene Expression Omnibus database (accession no. GSE12674). This work was supported by grants from the National Institutes of Health/National Cancer Institute (CA100076 to M.O.C. and CA117927 to Z.R.), the GIS, and the Agency for Science, Technology and Research, Singapore (J.L.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.114769/DC1.
References
- Alfa, C. E., R. Booher, D. Beach and J. S. Hyams, 1989. Fission yeast cyclin: subcellular localisation and cell cycle regulation. J Cell Sci Suppl 12 9–19. [DOI] [PubMed] [Google Scholar]
- Allard, S., R. T. Utley, J. Savard, A. Clarke, P. Grant et al., 1999. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18 5108–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, C. M., D. Korkin, D. L. Smith, S. Makovets, J. J. Seidel et al., 2008. Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break. Genes Dev. 22 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber-Furnari, B. A., N. Rhind, M. N. Boddy, P. Shanahan, A. Lopez-Girona et al., 2000. Regulation of mitotic inhibitor mik1 helps to enforce the DNA damage checkpoint. Mol. Biol. Cell 11 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi, G., E. Schmid and K. Maundrell, 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123 131–136. [DOI] [PubMed] [Google Scholar]
- Bermudez, V. P., L. A. Lindsey-Boltz, A. J. Cesare, Y. Maniwa, J. D. Griffith et al., 2003. Loading of the human 9–1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc. Natl. Acad. Sci. USA 100 1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimbo, A., Y. Jia, S. L. Poh, R. K. Karuturi, N. den Elzen et al., 2005. Systematic deletion analysis of fission yeast protein kinases. Eukaryot. Cell 4 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau, J., D. Miranda-Saavedra, G. J. Barton and D. R. Alessi, 2006. Emerging roles of pseudokinases. Trends Cell Biol. 16 443–452. [DOI] [PubMed] [Google Scholar]
- Breeding, C. S., J. Hudson, M. K. Balasubramanian, S. M. Hemmingsen, P. G. Young et al., 1998. The cdr2(+) gene encodes a regulator of G2/M progression and cytokinesis in Schizosaccharomyces pombe. Mol. Biol. Cell 9 3399–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y., J. Jin, C. Tomomori-Sato, S. Sato, I. Sorokina et al., 2003. Identification of new subunits of the multiprotein mammalian TRRAP/TIP60-containing histone acetyltransferase complex. J. Biol. Chem. 278 42733–42736. [DOI] [PubMed] [Google Scholar]
- Calonge, T. M., and M. J. O'Connell, 2006. Antagonism of Chk1 signaling in the G2 DNA damage checkpoint by dominant alleles of Cdr1. Genetics 174 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman, C. E., 2003. Checkpoint mediators: relaying signals from DNA strand breaks. Curr. Biol. 13 R488–R490. [DOI] [PubMed] [Google Scholar]
- Capasso, H., C. Palermo, S. Wan, H. Rao, U. P. John et al., 2002. Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J. Cell Sci. 115 4555–4564. [DOI] [PubMed] [Google Scholar]
- Chen, P., C. Luo, Y. Deng, K. Ryan, J. Register et al., 2000. The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation. Cell 100 681–692. [DOI] [PubMed] [Google Scholar]
- Christensen, P. U., N. J. Bentley, R. G. Martinho, O. Nielsen and A. M. Carr, 2000. Mik1 levels accumulate in S phase and may mediate an intrinsic link between S phase and mitosis. Proc. Natl. Acad. Sci. USA 97 2579–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, T. R., Z. Tang and W. G. Dunphy, 1993. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell 72 919–929. [DOI] [PubMed] [Google Scholar]
- den Elzen, N. R., and M. J. O'Connell, 2004. Recovery from DNA damage checkpoint arrest by PP1-mediated inhibition of Chk1. EMBO J. 23 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen, N., A. Kosoy, H. Christopoulos and M. J. O'Connell, 2004. Resisting arrest: recovery from checkpoint arrest through dephosphorylation of Chk1 by PP1. Cell Cycle 3 529–533. [PubMed] [Google Scholar]
- Dunleavy, E., A. Pidoux and R. Allshire, 2005. Centromeric chromatin makes its mark. Trends Biochem. Sci. 30 172–175. [DOI] [PubMed] [Google Scholar]
- Dunphy, W. G., 1994. The decision to enter mitosis. Trends Cell Biol. 4 202–207. [DOI] [PubMed] [Google Scholar]
- Ekwall, K., 2005. Genome-wide analysis of HDAC function. Trends Genet. 21 608–615. [DOI] [PubMed] [Google Scholar]
- Falck, J., J. Coates and S. P. Jackson, 2005. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434 605–611. [DOI] [PubMed] [Google Scholar]
- Feilotter, H., P. Nurse and P. G. Young, 1991. Genetic and molecular analysis of cdr1/nim1 in Schizosaccharomyces pombe. Genetics 127 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg, S. L., 1993. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21 2955–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei, M., K. Sloper, C. Sorensen, R. Syljuasen, J. Falck et al., 2003. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 278 14806–14811. [DOI] [PubMed] [Google Scholar]
- Gomez, E. B., J. M. Espinosa and S. L. Forsburg, 2005. Schizosaccharomyces pombe mst2+ encodes a MYST family histone acetyltransferase that negatively regulates telomere silencing. Mol. Cell. Biol. 25 8887–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, P. A., D. Schieltz, M. G. Pray-Grant, J. R. Yates, III and J. L. Workman, 1998. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol. Cell 2 863–867. [DOI] [PubMed] [Google Scholar]
- Grewal, S. I., M. J. Bonaduce and A. J. Klar, 1998. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, S. H., D. M. Sheedy, A. R. Cuddihy and M. J. O'Connell, 2004. Coordination of DNA damage responses via the Smc5/Smc6 complex. Mol. Cell. Biol. 24 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., Y. Sun, S. Chen, K. Roy and B. D. Price, 2006. The FATC domains of PIKK proteins are functionally equivalent and participate in the Tip60-dependent activation of DNA-PKcs and ATM. J. Biol. Chem. 281 15741–15746. [DOI] [PubMed] [Google Scholar]
- Kanoh, J., and P. Russell, 1998. The protein kinase Cdr2, related to Nim1/Cdr1 mitotic inducer, regulates the onset of mitosis in fission yeast. Mol. Biol. Cell 9 3321–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh, J., and M. Yanagida, 2007. Tel2: A common partner of PIK-related kinases and a link between DNA checkpoint and nutritional response? Genes Cells 12 1301–1304. [DOI] [PubMed] [Google Scholar]
- Katsuragi, Y., and N. Sagata, 2004. Regulation of Chk1 kinase by autoinhibition and ATR-mediated phosphorylation. Mol. Biol. Cell 15 1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy, A., and M. J. O'Connell, 2008. Regulation of Chk1 by its C-terminal domain. Mol. Biol. Cell 19 4546–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz, K., and M. J. O'Connell, 2009. The G(2) DNA damage checkpoint: Could this ancient regulator be the achilles heel of cancer? Cancer Biol. Ther. 8 1433–1439. [DOI] [PubMed] [Google Scholar]
- Latif, C., N. R. Elzen and M. J. O'Connell, 2004. DNA damage checkpoint maintenance through sustained Chk1 activity. J. Cell Sci. 117 3489–3498. [DOI] [PubMed] [Google Scholar]
- Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez et al., 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona, A., B. Furnari, O. Mondesert and P. Russell, 1999. Nuclear localization of Cdc25 is regulated by DNA damage and a 14–3-3 protein. Nature 397 172–175. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona, A., J. Kanoh and P. Russell, 2001. a Nuclear exclusion of Cdc25 is not required for the DNA damage checkpoint in fission yeast. Curr. Biol. 11 50–54. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona, A., K. Tanaka, X. B. Chen, B. A. Baber, C. H. McGowan et al., 2001. b Serine-345 is required for Rad3-dependent phosphorylation and function of checkpoint kinase Chk1 in fission yeast. Proc. Natl. Acad. Sci. USA 98 11289–11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, K., N. Walworth, R. Booher, M. Dembski, M. Kirschner et al., 1991. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64 1111–1122. [DOI] [PubMed] [Google Scholar]
- Martin, S. G., and M. Berthelot-Grosjean, 2009. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature 459 852–856. [DOI] [PubMed] [Google Scholar]
- Maundrell, K., 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123 127–130. [DOI] [PubMed] [Google Scholar]
- Michael, W. M., and J. Newport, 1998. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science 282 1886–1889. [DOI] [PubMed] [Google Scholar]
- Moreno, S., P. Nurse and P. Russell, 1990. Regulation of mitosis by cyclic accumulation of p80cdc25 mitotic inducer in fission yeast. Nature 344 549–552. [DOI] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194 795–823. [DOI] [PubMed] [Google Scholar]
- Moseley, J. B., A. Mayeux, A. Paoletti and P. Nurse, 2009. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 459 857–860. [DOI] [PubMed] [Google Scholar]
- Nurse, P., 1990. Universal control mechanism regulating onset of M-phase. Nature 344 503–508. [DOI] [PubMed] [Google Scholar]
- O'Connell, M. J., and K. A. Cimprich, 2005. G2 damage checkpoints: What is the turn-on? J. Cell Sci. 118 1–6. [DOI] [PubMed] [Google Scholar]
- O'Connell, M. J., J. M. Raleigh, H. M. Verkade and P. Nurse, 1997. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 16 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, M. J., N. C. Walworth and A. M. Carr, 2000. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 10 296–303. [DOI] [PubMed] [Google Scholar]
- Outwin, E. A., A. Irmisch, J. M. Murray and M. J. O'Connell, 2009. Smc5-Smc6-dependent removal of cohesin from mitotic chromosomes. Mol. Cell. Biol. 29 4363–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo, C., J. C. Hope, G. A. Freyer, H. Rao and N. C. Walworth, 2008. Importance of a C-terminal conserved region of chk1 for checkpoint function. PLoS ONE 3: e1427. [DOI] [PMC free article] [PubMed]
- Parker, L. L., and H. Piwnica-Worms, 1992. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257 1955–1957. [DOI] [PubMed] [Google Scholar]
- Parker, L. L., S. Atherton-Fessler, M. S. Lee, S. Ogg, J. L. Falk et al., 1991. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 10 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. L., S. Atherton-Fessler and H. Piwnica-Worms, 1992. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. USA 89 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. L., S. A. Walter, P. G. Young and H. Piwnica-Worms, 1993. Phosphorylation and inactivation of the mitotic inhibitor wee1 by the nim1/cdr1 kinase. Nature 363 736–738. [DOI] [PubMed] [Google Scholar]
- Parrilla-Castellar, E. R., S. J. Arlander and L. Karnitz, 2004. Dial 9–1-1 for DNA damage: the Rad9-Hus1-Rad1 (9–1-1) clamp complex. DNA Repair (Amst.) 3 1009–1014. [DOI] [PubMed] [Google Scholar]
- Pereira, E., Y. Chen and Y. Sanchez, 2009. Conserved ATRMec1 phosphorylation-independent activation of Chk1 by single amino acid substitution in the GD domain. Cell Cycle 8 1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux, A. L., and R. C. Allshire, 2005. The role of heterochromatin in centromere function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh, J. M., and M. J. O'Connell, 2000. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci. 113 1727–1736. [DOI] [PubMed] [Google Scholar]
- Rhind, N., and P. Russell, 2001. Roles of the mitotic inhibitors Wee1 and Mik1 in the G(2) DNA damage and replication checkpoints. Mol. Cell. Biol. 21 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind, N., B. Furnari and P. Russell, 1997. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 11 504–511. [DOI] [PubMed] [Google Scholar]
- Robert, F., S. Hardy, Z. Nagy, C. Baldeyron, R. Murr et al., 2006. The transcriptional histone acetyltransferase cofactor TRRAP associates with the MRN repair complex and plays a role in DNA double-strand break repair. Mol. Cell. Biol. 26 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, P., and P. Nurse, 1987. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49 559–567. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., A. Roguev, D. Schaft, L. Buchanan, B. Habermann et al., 2008. Chromatin Central: towards the comparative proteome by accurate mapping of the yeast proteomic environment. Genome Biol 9: R167. [DOI] [PMC free article] [PubMed]
- Shiozaki, K., and P. Russell, 1995. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378 739–743. [DOI] [PubMed] [Google Scholar]
- Suda, M., S. Yamada, T. Toda, T. Miyakawa and D. Hirata, 2000. Regulation of Wee1 kinase in response to protein synthesis inhibition. FEBS Lett. 486 305–309. [DOI] [PubMed] [Google Scholar]
- Takai, H., R. C. Wang, K. K. Takai, H. Yang and T. de Lange, 2007. Tel2 regulates the stability of PI3K-related protein kinases. Cell 131 1248–1259. [DOI] [PubMed] [Google Scholar]
- Tapia-Alveal, C., T. M. Calonge and M. J. O'Connell, 2009. Regulation of Chk1. Cell Div. 4: 8. [DOI] [PMC free article] [PubMed]
- Walworth, N., S. Davey and D. Beach, 1993. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature 363 368–371. [DOI] [PubMed] [Google Scholar]
- Wang, S. X., and W. G. Dunphy, 2000. Activation of Xenopus Chk1 by mutagenesis of threonine-377. FEBS Lett. 487 277–281. [DOI] [PubMed] [Google Scholar]
- Watanabe, N., H. Arai, Y. Nishihara, M. Taniguchi, T. Hunter et al., 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA 101 4419–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., H. Arai, J. Iwasaki, M. Shiina, K. Ogata et al., 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA 102 11663–11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, B. A., and K. L. Gould, 2004. Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 23 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., and P. Russell, 1993. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature 363 738–741. [DOI] [PubMed] [Google Scholar]
- Yamada, T., K. Mizuno, K. Hirota, N. Kon, W. P. Wahls et al., 2004. Roles of histone acetylation and chromatin remodeling factor in a meiotic recombination hotspot. EMBO J. 23 1792–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P., and P. Fantes, 1987. Schizosaccharomyces pombe mutants affected in their division response to starvation. J. Cell Sci. 88 295–304. [DOI] [PubMed] [Google Scholar]