Abstract
Thiamine deficiency (TD) is a model of neurodegeneration induced by mild impairment of oxidative metabolism. TD produces time-dependent glial activation, inflammation, oxidative stress, altered metabolism of amyloid precursor protein (APP), exacerbation of plaque formation from APP and finally selective neuron death in specific brain regions. The submedial thalamic nucleus (SmTN) is the most sensitive region to TD. Alteration in APP metabolism and nuclear translocation of carboxy-terminal fragments (CTF) of APP has been implicated in neuron death in other models of neurodegeneration. These experiments tested whether TD causes translocation of CTF into the nucleus of neurons in the SmTN that are destined to die after nine days of TD by examining overlapping immunoreactivity (IR) of antibody APP 369 with either Alz90, 6E10 or 4G8 epitopes in the nuclei of the neurons in the SmTN. TD caused the accumulation of the CTF of APP in nuclei of SmTN neurons within three days of TD. These changes did not occur in the cortex which is spared in TD. Western blot analysis of nuclear fractions revealed a (214%; p<0.152 and 61%; p<0.026) increase in CTF 15 and CTF 12 levels, respectively and corresponding decrease in Holo APP levels (34%; p<0.753) in TD SmTN compared to control. TD did not alter CTF 15 and 12 levels in cortex. These findings demonstrate that changes in APP metabolism occur in early stages of TD, and they may play an important role in TD-induced selective neuronal loss.
Keywords: Oxidative stress, Submedial thalamic nucleus, Neurodegeneration, Inflammation, Amyloid precursor protein
Introduction
Thiamine deficiency (TD) is a model of neurodegeneration induced by mild impairment of oxidative metabolism and enhanced oxidative stress. Thiamine dependent enzymes are diminished in multiple neurodegenerative diseases. The reductions have been particularly well documented for Alzheimer’s disease (AD) [1, 2]. In AD, the decline in thiamine-dependent enzymes is highly correlated to the decline in clinical dementia rating before the patients die [2, 3]. TD produces a highly reproducible time-dependent selective neuronal loss in specific brain regions. The earliest and most dramatic neuronal loss is in the sub-medial thalamic nucleus (SmTN) and occurs after nine days of TD [4]. Previous experiments identified markers of inflammation and oxidative stress in microglia [inducible nitric oxide synthase (iNOS), redox active iron, hemeoxygenase-1, CD-40, IL-1β, TNF-α], astrocytes (CD40L, IL-6) and endothelial cells [intracellular adhesion molecule-1 (ICAM-1), enothelial NOS (eNOS)] [5, 6] that precede neuronal loss. TD induced inflammation, stress, cell death and repair, and metabolic perturbation gene expression in thalamus and colliculus [7]. However, changes have not been demonstrated in neurons that could provide insight into why these neurons die.
Previous studies indicate that processing of amyloid precursor protein (APP) is altered in late stages of TD. In wild type mice, late stages of TD are characterized by the accumulation of clusters of dystrophic neurites of APP or amyloid precursor protein like proteins (APLP2) that are highly reminiscent of the morphology of amyloid plaques, except that the centers of lesions were formed from necrotic debris rather than from beta amyloid [8, 9]. Recent results demonstrate that in plaque competent mice expressing mutant human APP, TD exacerbates plaque formation [34]. Whether TD alters APP processing in early stages of TD has not been examined.
APP is a transmembrane protein that is abundant in neurons and may have a role in neuron death. Although its physiological function is unknown, experiments indicate roles for APP fragments in axonal transport [10], cell survival, gene transcription and cognitive function [11]. Increased amyloid precursor protein (APP) expression and intracellular accumulation of its toxic fragments have been associated with acute neuronal death processes. APP is cleaved by α, β, and γ secretases to generate amyloid-β-peptide (Aβ) and various carboxy terminal fragments of APP (CTF’s). Aβ is the primary component of the plaques that characterize Alzheimer’s disease (AD), and many studies demonstrate that it can be neurotoxic. Although the precise functions of the APP CTFs are unknown, they alter membrane currents and calcium homeostasis [12,13], stimulate mitogen activated protein kinase pathways, activate the transcription factor NF-kB and elevate iNOS [14]. The APP CTF’s accumulate in the nucleus and bind with Fe65 [15] and CP2 to alter transcription [14]. The translocation of CTF to the nucleus has also been implicated in neuronal death in vivo. C terminal fragments of APP translocate to the nucleus of the neurons destined to delayed degeneration following neurotoxic striatal lesions [16]. Genetic knockouts of APP attenuate microglial activation and enhance neuron survival in substantia nigra compacta after axotomy [17]. Nuclear APP fragments also play a role in the progressive loss of dopaminergic substantia nigra pars compacta neurons following unilateral medial forebrain bundle transaction [18]. Thus, multiple approaches suggest that nuclear translocation of C-terminal fragments of APP are important in cell death. Previous studies failed to identify markers in neurons that preceded the selective neuron loss. The current studies tested whether TD promotes alterations in APP processing that promote translocation of APP CTF to the nucleus to initiate neuronal death in early stages of TD.
Materials and Methods
Animals
Adult male C57BL/6 mice (6–8 weeks; 22–26 g) from Harlan Sprague Dawley, Inc (Indianapolis, IN, USA) were used in all experiments. The animals were housed with constant temperature (22 ± 2°C), humidity (50 ± 5 %) and illumination (12 h light/dark cycles). The Institutional Animal Care and Use Committee of Weill Medical College of Cornell University approved all procedures with the animals.
Induction of thiamine deficiency
Thiamine deficiency was induced in C57BL/6 male mice as described in our previous studies [4,6]. Experimental mice received a thiamine deficient diet (ICN Nutrition Biomedicals, Cleveland, OH) ad libitum, and daily intraperitoneal injections of the thiamine pyrophosphokinase inhibitor, pyrithiamine hydrobromide [19] (Sigma Chemical Co.; St. Louis, MO; 5 μg in 0.1 ml saline/10 g body weight) for 10 days. Control animals received a thiamine containing diet (ICN Nutrition Biomedicals, Cleveland, OH) ad libitum and daily intraperitoneal injections of saline (0.1ml/10 g).
Tissue preparation and Immunohistochemistry
Following treatment, the mice were administered with a lethal intraperitoneal dose of pentobarbitone sodium (200 mg/kg; i.p.; Abbott Laboratories, North Chicago, IL) and perfused via the ascending aorta with 50 ml of normal saline, followed by 100 ml of 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO) in 0.1 M phosphate buffer (pH 7.2) using a pump (Masterflex, Model 7518-00, Cole-Parmer Instrument Company, Barrington, IL) at 5ml/min. The brains were removed and post-fixed in 4% paraformaldehyde overnight, and then transferred to 30% sucrose (Sigma Chemical Co., St. Louis, MO) for at least an additional 24 hours. The brain block that contained the thalamus and cortex region was dissected on a Rodent Brain Matrix (ASI Instruments; Warren, MI) and sectioned (40 μm) with a sliding microtome (Microm Laborgerate GmbH, Welldorf, Germany). Sections were collected from the Bregma level −0.94 to −1.94 [35].
The staining protocol employed a modified ABC immunohistochemistry procedure [20]; [6]. Briefly, sections were washed with 0.1 M potassium phosphate buffered saline (PBS, pH 7.4) and incubated in 1% H2O2 for 30 min to quench the endogenous peroxidase. Then sections were treated with 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 15 min. Sections were washed with PBS and blocked with 2% bovine serum albumin (BSA) in PBS for 1 hr. Sections were incubated with mouse monoclonal anti-NeuN (Chemicon, Temecula, CA; 1:1000), in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO) overnight at room temperature. After rinsing in PBS, sections were incubated with biotinylated anti-mouse (Vector Laboratories Inc., Burlingame, CA; 1:200 in PBS containing 0.25% BSA) for one hr. Sections were then incubated in avidin-biotin-peroxidase complex for one hr (Vector Laboratories Inc., Burlingame, CA; 1:100 in PBS), rinsed in PBS and developed in 0.05% 3,3′-diaminobenzidine (DAB) (Vector Laboratories Inc., Burlingame, CA) and 0.003% H2O2 in PBS.
Double immunofluorescence was performed to demonstrate the localization of APP domains. Briefly, Sections were incubated with rabbit C-terminal Ab G369 (Gift from Dr. Sam Gandy, Thomas Jefferson University, Philadelphia, PA) with either mouse Ab Alz90 (RDI Research diagnostics, Concord, MA) or mouse Ab 6E10 or mouse Ab 4G8 (Chemicon, Temecula, CA) in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO) overnight at 4 °C. After rinsing in PBS, sections were incubated with Rhodamine Red conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) and Alexa 488- anti-mouse IgG (Molecular Probes, Eugene, OR) in PBS containing 0.25% BSA for one hour. Sections were rinsed, mounted on a super frost slides, air-dried and coverslipped with Cytoseal (Stephens Scientific, Kalamazoo, MI). To confirm the colocalization of CTF’s in nuclei, few sections were counterstained with DAPI (4′,6′diamidino-2-phenylindole)-containing Vectashield mountant (Vector Laboratories Inc., Burlingame, CA).
Quantitation of nuclear immunoreactivity
Processed brain sections were analyzed with a Zeiss LSM 510 META confocal scanning laser microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY, USA). For quantitative colocalization, eight fields in each section, and a total of three sections spanning from anterior to posterior SmTN per animal containing an average of 5–6 cells per field were imaged using a PlanApochromat 63× (NA = 1.4) oil immersion lens, yielding a total of 120 cells. Computer-aided quantification of nuclear co-localization was performed using the MetaMorph 6.1 software (Universal Imaging Corp, Downington, PA). Threshold was kept constant throughout the analysis. The analysis was performed in a blinded fashion.
Nuclear extraction and Western blotting
The SmTN and cortex region were micropunched as reported previously [6]. The SmTN is quite small (i.e., about one third the size of the substantia nigra). The punches from five or three brain samples were pooled from the SmTN or cortex and nuclear fractions were isolated using the Nu-CLEAR extraction kit (Sigma, ST. Louis, MO). Briefly, the brain tissue was suspended in 1X lysis buffer containing dithiothreitol (DTT) and protease inhibitor cocktail. These samples were vortexed for 5 seconds and placed over an electric rocker and gently rocked for 30 min and centrifuged for 5 min at 10,000 X g. The supernatant was saved for the cytosolic fraction. The crude nuclear pellet was resuspended in extraction buffer containing DTT and protease inhibitor. The tube was vortexed for 5 seconds and gently rocked for 30 min and centrifuged for 5 min at 20,000 X g. The supernatant was transferred to a clean tube and stored at −80 °C.
Protein concentrations of the lysates were determinedby a bicinchoninic colorimetric acid (BCA) assay (Pierce Chemical Company, Rockford, IL). A total of 10 μg of protein was mixed with SDS loading buffer, denatured at 100 °C, and loaded on a 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (Pierce Chemical Company, Rockford, IL). Membrane blots were blocked with 50% Odyssey blocking buffer, 50% Tris-buffered saline (TBS) overnight at 4°C. After blocking the membranes were incubated with primary antibodies, APP C-terminal specific polyclonal antibody G369 (1:1000), monoclonal antibody Alz90 (1:1000; Research diagnostics, Concord, MA) or β-actin (1:10,000; Sigma) for 120 min at room temperature. Subsequently, blots were washed with TBS plus 0.1% triton, and incubatedat room temperature for one hour with secondary Odyssey Goat anti-Rabbit IR Dye 680 antibody and Odyssey goat anti-Mouse IRDye 800 antibody (1:10,000; LICOR Biosciences, Lincoln, NB) and analyzed using the Odyssey infrared imaging program (LI-COR Biotechnology, Nebraska). Values were normalized to actin, which is an abundant cell protein that also exist in isolated nuclei [21].
Statistical Analysis
All the values were expressed as mean ± SEM. Statistical significance of group differences was tested by two tailed paired t test. P < 0.05 was considered significant. SPSS (SPSS Co., Chicago, IL) software was used for statistical analysis.
Results
TD induced neuronal loss in SmTN
TD induced loss of NeuN immunoreactivity was analyzed in control and TD brains. Similar to our previous studies, TD caused loss of NeuN immunoreactivity in the SmTN region compared to brains from mice on the normal thiamine-containing diet (Fig. 1). Our previous studies using Nissl staining indicate that neurons undergo dramatic morphological changes on TD 9 [4].
Fig. 1. TD induced neuronal loss in SmTN.

Mice were sacrificed after 9 days of TD. Brains were processed for NeuN immunoreactivity. Representative brain sections from control and TD brains through SmTN region stained for NeuN antibody. TD brains show clear neuronal loss in SmTN. In the figure, Mammilary tract and Submedial thalamus is labeled as “MT” and “SmTN”. Scale bar: 100 μm.
TD induced accumulation of APP CTFs in the nucleus
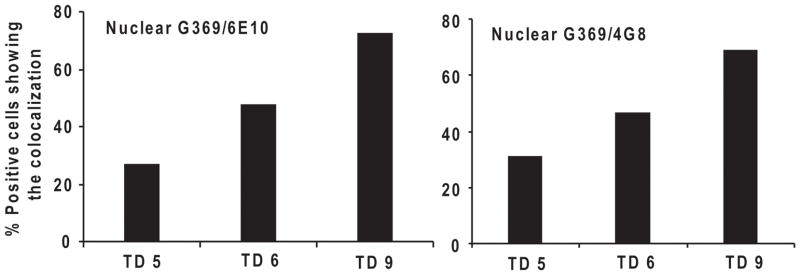
Whether TD induced the accumulation of nuclear APP CTFs was tested in the vulnerable SmTN, which has extensive TD-induced neuronal loss, and in non vulnerable cortex, which does not have TD-induced neuronal loss. The accumulation APP CTF’s immunoreactivity was tested by examining the co-localization of APP G369 antibody with either Alz90, 6E10 or 4G8 antibodies. Mice were made TD for 2, 3, 4, 5, 6 and 9 days. By day 3 of TD colocalization of immunoreactivity for APP C-terminal (G 369) and Alz90 epitopes was apparent in the nuclei of the vulnerable submedial thalamus (SmTN) neurons. Quantification of the colocalized APP CTF in SmTN nuclei revealed increases that were highly significant by day 4 (Fig. 2A, B). No changes occurred in the control SmTN or cortex (data not shown). The nuclear localization of the overlapping signals was confirmed by immunostaining with DAPI. Immuostaining revealed co-localization of APP36 and Alz90 and DAPI. This confirms the nuclear colocalization (Fig. 3). The co-localization of APP CTF with β amyloid domains was also studied using co-localization of 6E10 or 4G8 antibodies with G369 on days 5, 6 and 9. APP immunoreactivity co-localized with 6E10 or 4G8 epitopes in the nuclei of the SmTN neurons (Fig. 4).
Fig. 2. TD induced APP CTF translocation in SmTN.
Mice were sacrificed on days 2, 3, 4, 5, 6 and 9 of TD. Brains were fixed and processed for colocalization studies. (A) Representative brain sections from the control and TD-4 brains in SmTN region on day 4 were stained with G369 and Alz90 antibodies to the carboxy terminal fragments. The yellow color in the nucleus indicates the CTF positive cells colocalized for G369 (red) and Alz90 (green). Scale bars: 50 μm. Insets 20 μm. (B) Graph represents the CTF positive cells colocalized for G369 and Alz90 at 2, 3, 4 days of TD in SmTN. Ninety to one hundred neurons from three SmTN sections per mouse were scored at each time point. Data represent means ± SEM in each group. *P<0.05 compared with control group (n=5).
Fig. 3. Migration of APP CTF into the nucleus with TD.

Mice were sacrificed after four days of TD. Photomicrographs show the triple label staining to confirm the nuclear colocalization. Rabbit anti-G369 (Red), mouse anti-Alz90 (green) and DAPI (blue) were used. In the merged images, the arrows (white color) indicate the nuclear colocalization of CTF fragments. Scale bar: 20 μm.
Fig. 4. TD induced APP CTF translocation in SmTN.
Mice were sacrificed on days 5, 6 and 9 of TD. Brains were fixed and processed with G369 and 6E10 or 4G8 antibodies for colocalization studies. Graph represents the percentage positive cells colocalized for G369 and 6E10 or 4G8 antibodies at 5, 6, 9 days of TD in SmTN. Ninety to one hundred neurons from three SmTN sections per mouse were scored at each time point.
Nuclear localization of APP carboxy terminal fragments by Western blot analysis
To further test whether TD causes the translocation of the CTF to the nucleus in SmTN neurons, micropunches were obtained from the SmTN or cortex by methods that were reported previously [6]. A total of 6 samples from each region were prepared from 22 control or TD mice and analyzed by Western blot. Samples were probed with APP C-terminal specific G369 and Alz90 antibodies. G369 antibody revealed the full length APP (~95kDa) and CTF’s (15kDa and 12kDa) bands (Fig. 5A). Quantitation of the blots revealed a significant (61%; p<0.26; paired t test) increase in CTF 12 levels in TD SmTN (2.08 ± 0.56) compared to control SmTN (1.29 ± 0.41) (Fig. 5B). Although, TD increased CTF 15 levels in TD SmTN (1.95 ± 0.73) compared to control SmTN (0.62 ± 0.52) by 214%; p<0.665 and decreased the full length Holo APP levels in TD SmTN (0.32 ± 0.30) compared to control SmTN (0.47 ± 0.18) by 34%; p<0.753), the differences were not significant. On the other hand, TD did not induce significant changes in cortex in either full length APP or CTF protein levels (Fig. 5B). Western blot analysis of nuclear fractions with Alz 90 antibody did not reveal any significant increase in (~40 kDa) band in TD SmTN compared to controls (Fig. 5A, B).
Fig. 5. TD increased APP C-terminal fragments.
TD C57BL/6 mice were sacrificed after 4 days of treatment. Western blots were performed with nuclear fractions prepared from pooled micropunched samples from the SmTN or cortex from three control and three TD mice. Panel (A) shows representative Western blot probed with G369 antibody specific and Alz90 against APP holo protein and C-terminal fragments and actin, an internal control. The blots were visualized by LI-COR IR dyes and quantified by LI-COR imaging system. Panel (B) shows the densitometric analysis of APP holo protein and C-terminal fragments and Alz 90 band intensity. Data represent means ± SEM in each group. *P<0.05 compared with control group (n=6).
Discussion
Previous studies have identified several markers of inflammation and oxidative stress in multiple cell types (neurons, microglia, astrocytes and endothelial cells) that precede neuronal loss [5, 6, 22, 23, 24]. The present study is the first to demonstrate TD induced alteration in APP metabolism and nuclear translocation of CTF of APP occur in early stages of TD in vulnerable region SmTN. APP is a transmembrane protein. When proteolytically cleaved by α-, β- and γ-secretases, APP generates Aβ and carboxy terminal fragments of APP. In the amyloidogenic pathway, APP is cleaved by β-secretase (β-APP-site cleaving enzyme; BACE) to release an N-terminal, soluble APP-β (sAPP-β) fragment and amyloidogenic C-terminal fragment (CTF) known as β-CTF or C99. β-CTF is further cleaved by γ secretase to produce Aβ and amyloid intracellular domain (AICD) (CTF57 and CTF59). In the non-amyloidogenic pathway, APP is cleaved by α-secretase in the middle of the Aβ sequence to release N-terminal, soluble APP- α (sAPP- α) fragment and a retained non-amyloidogenic C-terminal fragment (CTF) known as α-CTF or C83 [25, 26, 27]. Also, caspases produce CTF31 [28]. Recently, APP cleaved product (56 kDa), termed as Abeta*56 was shown to involved in memory deficits [29]. These resultant amyloidogenic CTF’s are neurotoxic and linked to pathogenesis of AD [30, 31].
Oxidative stress and inflammation favor proteolysis via β-secretase pathway to generate neurotoxic C terminal fragments. Recent in vivo studies demonstrate that mitochondrial respiratory chain inhibitors (e.g., rotenone, 3-nitropropionic acid and sodium azide) and oxidants (e.g., including Fe3+ and Aβ fibrils) elevate BACE protein levels and increase β-CTF [32]. In addition, acute inhibition of energy metabolism with various pharmacological agents (insulin, 2-deoxyglucose, 3-nitropropionic acid and kainic acid) in C57BL/6 wild type and APP transgenic mice (Tg2576) elevates BACE-1 levels and activity, and increase levels of Aβ [33]. In wild type, non-plaque competent mice, TD induces the formation APP neuritic clusters by ten days of TD [8, 9]. In plaque competent mice bearing double mutant forms of human APP, TD alters the APP processing towards amyloidgenic pathway, increases the β-CTF levels and exacerbates amyloid plaque formation after 10 days of TD [34]. Changes in APP processing including translocation of CTF to the nucleus have not been tested previously at earlier stages of TD.
The translocation of CTF of APP to the nucleus has been implicated in neuronal death. Nuclei of the neurons in the rat substantia nigra pars reticulata (SNpr) targeted for delayed degeneration following neurotoxic striatal lesions show intense immunoreactivity for APP C-terminal and beta-amyloid domains but not for an N-terminal sequence [16]. Genetic knockout of APP attenuates microglial activation and enhances neuron survival in substantia nigra compacta after axotomy [17]. Nuclear APP fragments also play a role in the progressive loss of dopaminergic substantia nigra pars compacta neurons following unilateral medial forebrain bundle transaction. The data show a novel nuclear C-terminal fragment appeared coincident with SNpc neuron degeneration, and that APP deficiency provided significant neuroprotection in vivo [18].
The present studies demonstrate translocation of APP fragments to the nucleus in TD, and only in those neurons that are destined to a die several days later. TD induced colocalization of amyloidgenic CTF, β-amyloid and Alz90 domains of APP in the nuclei of SmTN neurons. Quantitation of the co-localized carboxy terminal fragments revealed a significant increase on day 4 in TD SmTN neurons compared to control. Western blot analysis of nuclear fractions from SmTN and cortex using antibody G369 revealed full-length holo-APP and bands corresponding to CTF’s. TD decreased the full length holoAPP levels, while increased the CTF 15 and CTF 12 levels by (214% and 61%) in vulnerable SmTN. Interestingly, TD did not cause any changes in the holo APP or CTF levels in cortex. Thus, the data consistently support the suggestion that the translocation of APP fragments to the nucleus initiate neuronal death in TD.
In the past, several mechanisms for TD-induced selective degeneration of SmTN neurons have been proposed. The present studies provide data that much earlier alteration in APP processing leads to translocation of APP CTF to the nucleus to initiate neuronal death. These results support the hypothesis that APP CTF products may play a major role in the progressive degeneration of SmTN neurons. These findings provide strong evidence for a substantial role of APP and cleaved products in TD-induced selective neuronal loss.
Acknowledgments
The authors thank Dr. Harriet Baker and Julian Moore for assistance with the pictures. This work was supported by NIH grants: AG14600, AG11921, AG10491, and AG14930
References
- 1.Gibson GE, Zhang H, Sheu KF, et al. Alpha-ketoglutarate dehydrogenase in Alzheimer brains bearing the APP670/671 mutation. Ann Neurol. 1998;44:676–681. doi: 10.1002/ana.410440414. [DOI] [PubMed] [Google Scholar]
- 2.Gibson GE, Haroutunian V, Zhang H, et al. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48:297–303. [PubMed] [Google Scholar]
- 3.Bubber P, Ke ZJ, Gibson GE. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem Int. 2004;45:1021–1028. doi: 10.1016/j.neuint.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE. Reversal of thiamine deficiency-induced neurodegeneration. J Neuropathol Exp Neurol. 2003;62:195–207. doi: 10.1093/jnen/62.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Calingasan NY, Chun WJ, Park LC, et al. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol. 1999;58:946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiol Dis. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vemuganti R, Kalluri H, Yi JH, et al. Gene expression changes in thalamus and inferior colliculus associated with inflammation, cellular stress, metabolism and structural damage in thiamine deficiency. Eur J Neurosci. 2006;23:1172–1188. doi: 10.1111/j.1460-9568.2006.04651.x. [DOI] [PubMed] [Google Scholar]
- 8.Calingasan NY, Gandy SE, Baker H, et al. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol. 1996;149:1063–1071. [PMC free article] [PubMed] [Google Scholar]
- 9.Calingasan NY, Gandy SE, Gibson GE. Thiamine deficiency alters APP but not presenilin-1 immunoreactivity in vulnerable brain regions. Neuroreport. 1997;8:2631–2634. doi: 10.1097/00001756-199707280-00041. [DOI] [PubMed] [Google Scholar]
- 10.Kamal A, Stokin GB, Yang Z, et al. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 11.Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 12.Fraser SP, Suh YH, Djamgoz MB. Ionic effects of the Alzheimer’s disease beta-amyloid precursor protein and its metabolic fragments. Trends Neurosci. 1997;20:67–72. doi: 10.1016/s0166-2236(96)10079-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Park CH, Cha SH, et al. Carboxyl-terminal fragment of Alzheimer’s APP destabilizes calcium homeostasis and renders neuronal cells vulnerable to excitotoxicity. Faseb J. 2000;14:1508–1517. doi: 10.1096/fj.14.11.1508. [DOI] [PubMed] [Google Scholar]
- 14.Chang KA, Suh YH. Pathophysiological roles of amyloidogenic carboxy-terminal fragments of the beta-amyloid precursor protein in Alzheimer’s disease. J Pharmacol Sci. 2005;97:461–471. doi: 10.1254/jphs.cr0050014. [DOI] [PubMed] [Google Scholar]
- 15.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 16.DeGiorgio LA, DeGiorgio N, Milner TA, et al. Neurotoxic APP C-terminal and beta-amyloid domains colocalize in the nuclei of substantia nigra pars reticulata neurons undergoing delayed degeneration. Brain Res. 2000;874:137–146. doi: 10.1016/s0006-8993(00)02545-2. [DOI] [PubMed] [Google Scholar]
- 17.DeGiorgio LA, Shimizu Y, Chun HS, et al. APP knockout attenuates microglial activation and enhances neuron survival in substantia nigra compacta after axotomy. Glia. 2002;38:174–178. doi: 10.1002/glia.10052. [DOI] [PubMed] [Google Scholar]
- 18.DeGiorgio LA, Shimizu Y, Chun HS, et al. Amyloid precursor protein gene disruption attenuates degeneration of substantia nigra compacta neurons following axotomy. Brain Res. 2002;938:38–44. doi: 10.1016/s0006-8993(02)02483-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu JY, Timm DE, Hurley TD. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J Biol Chem. 2006;281:6601–6607. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- 20.Calingasan NY, Huang PL, Chun HS, et al. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol. 2000;59:207–217. doi: 10.1093/jnen/59.3.207. [DOI] [PubMed] [Google Scholar]
- 21.Pederson T, Aebi U. Actin in the nucleus: what form and what for? J Struct Biol. 2002;140:3–9. doi: 10.1016/s1047-8477(02)00528-2. [DOI] [PubMed] [Google Scholar]
- 22.Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem Int. 2004;45:361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Ke ZJ, Calingasan NY, DeGiorgio LA, et al. CD40-CD40L interactions promote neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. Neurochem Int. 2005;47:204–215. doi: 10.1016/j.neuint.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Kruse M, Navarro D, Desjardins P, Butterworth RF. Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem Int. 2004;45:49–56. doi: 10.1016/j.neuint.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Steiner H, Duff K, Capell A, et al. A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- 26.De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 27.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 28.Gervais FG, Xu D, Robertson GS, et al. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 29.Lesne S, Koh MT, Kotilinek L, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 30.Nordstedt C, Gandy SE, Alafuzoff I, et al. Alzheimer beta/A4 amyloid precursor protein in human brain: aging-associated increases in holoprotein and in a proteolytic fragment. Proc Natl Acad Sci U S A. 1991;88:8910–8914. doi: 10.1073/pnas.88.20.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- 32.Xiong K, Cai H, Luo XG, et al. Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina. Exp Brain Res. 2007;181:435–446. doi: 10.1007/s00221-007-0943-y. [DOI] [PubMed] [Google Scholar]
- 33.Velliquette RA, O’Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer’s disease pathogenesis. J Neurosci. 2005;25:10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karuppagounder SS, Xu H, Shi Q, et al. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.12.013. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]