Abstract
Spitting cobras, which defend themselves by streaming venom towards the face and/or eyes of a predator, must be highly accurate because the venom they spit is only an effective deterrent if it lands on the predator's cornea. Several factors make this level of accuracy difficult to achieve; the target is moving, is frequently >1 m away from the snake and the venom stream is released in approximately 50 ms. In the present study we show that spitting cobras can accurately track the movements of a potentially threatening vertebrate, and by anticipating its subsequent (short-term) movements direct their venom to maximize the likelihood of striking the target's eye. Unlike other animals that project material, in spitting cobras the discharge orifice (the fang) is relatively fixed so directing the venom stream requires rapid movements of the entire head. The cobra's ability to track and anticipate the target's movement, and to perform rapid cephalic oscillations that coordinate with the target's movements suggest a level of neural processing that has not been attributed to snakes, or other reptiles, previously.
Keywords: defensive behavior, kinematics, sensory integration, snake
INTRODUCTION
Multiple species of Asiatic and African cobras have evolved the ability to ‘spit’ venom (Bogert, 1943). The venom, which is expelled via the same mechanism used to inject prey (Young et al., 2004), is preferentially directed at the face and/or eyes of a potential predator (Westhoff et al., 2005). If a small quantity of the venom lands on the corneal surface it will produce intense pain and possibly blindness, but contact with the skin, oral cavity or mucus membranes of the nasal passageway produces neither a local nor systemic reaction (Warell and Omerod, 1976). Thus, to be an effective deterrent the venom must be directed with enough accuracy to reach at least one eye of the target. Four factors make this level of precision particularly challenging: (1) the vertebrate predator targets are typically moving, not stationary; (2) the cobras frequently spit at targets that are over 1.5 m away (Rasmussen et al., 1995); (3) the spitting generally lasts less than 50 ms (Westhoff et al., 2005; Young et al., 2004; Young et al., 2009), so the cobras can not watch the stream in the air and correct accordingly; and (4) the exit orifice for the venom, the fang, is relatively fixed and not under fine muscular control (Bogert, 1943; Wüster and Thorpe, 1992).
A key component to the spitting behavior is rapid oscillation of the head about the neck in both the vertical and lateral planes (e.g. Westhoff et al., 2005; Young et al., 2004). These oscillations of the head, which ultimately become oscillations of the exit orifice of the fang, produce spatial dispersal patterns in the spat venom; these dispersal patterns have been interpreted as a means of optimizing the chance of successfully contacting the corneal surface with the spat venom (Westhoff et al., 2005; Young et al., 2009). Cobras modulate the range of these cephalic oscillations depending on the distance of the target; effectively ensuring that the venom dispersal pattern roughly corresponds to the size of the target's face (Berthé et al., 2009). Adding another layer of complexity to this behavior is the fact that the target itself is in motion – it is very difficult to induce cobras to spit at any stationary object – which would greatly reduce the spitting accuracy, unless the snake could effectively measure and accommodate for this motion.
Venom spitting in cobras is a projectile mechanism; once the bolus of venom is expelled from the fang tip the snake has no ability to direct or modulate the trajectory of the venom for increased accuracy. Similar projectile mechanisms have been investigated in other animals such as Archer fish (e.g. Timmermans, 2001; Schuster et al., 2006) and spitting spiders (Nentwig, 1985). These other animals using projectile mechanics increase their accuracy through visual tracking of their target; this allows the animal to effectively predict where the target will be in the near future and direct the projected matter to that spot (rather than where the moving target was at the time the bolus was discharged). The purpose of this study was to document if spitting cobras could track the movements of their targets and, if so, to determine how the tracking cues influenced the spitting behavior.
MATERIALS AND METHODS
Live animals
Long-term captive specimens of the red spitting cobra Naja pallida Boulenger 1896 [snout–vent length (SVL) 120–185 cm], the black-necked spitting cobra Naja nigricollis Reinhardt 1843 (SVL 140–165 cm) and the black-and-white spitting cobra Naja siamensis Laurenti 1768 (SVL 95 cm and 115 cm) were used in this study. These animals were maintained at 27–30°C, with a 12 h:12 h light:dark cycle, water ad libitium and a diet of pre-killed rodents. All experimental protocols were according to the Principles of Animal Care, National Institutes of Health (NIH) and conducted under University of Bonn approval 50.203.2-BN, issued July 2006.
Kinematics
Target movement was quantified using two dual-axis accelerometers [ADXL203E chips by Analog Devices (Norwood, MA, USA), integrated circuit boards custom built by Electro Mechanica (East Freetown, MA, USA)] mounted at 90 deg. to one another then rigidly mounted on the face shield worn by the target. Accelerometer signals were run through an A/D board (Power 1401, Cambridge Electronic Design, Cambridge, UK) then recorded (at 20 kHz) as three separate channels on a PC computer running Spike2 (Cambridge Electronic Design). Our previous experience with spitting cobras demonstrated to us that the snakes could be easily induced to spit at targets, or even inanimate objects (especially mirrors), if they exhibited discontinuous and rapid or ‘jerky’ movements. To maximize consistency, the last author served as a target for all trials. Since the object being moved (the last author's head) was a constant; we recorded acceleration data synchronized to 500 frames s−1 video sequences (see below) of movement against a known scale and used this data as a calibration to enable conversion of the kinematic data from acceleration to linear displacement.
The data acquisition system was programmed to send a 100 ms voltage pulse to a 5 mW LED red light laser (TIM 203, BLV Electronic, Aylesbury, Bucks, UK) once every second. The laser was positioned so that the beam flashed into a filming chamber 90 cm wide × 60 cm tall × 90 cm deep. The front end of this chamber was open, one side was formed by a solid glass plate and the top was formed by a mirror oriented at 45 deg. to the floor of the cage. The floor and back wall of the cage were covered with a 5 cm filming grid. Unrestrained adult specimens of N. pallida (N=4), N. nigricollis (N=5) and N. siamensis (N=2) were placed into the filming cage and induced to spit.
The movements of the cobras were recorded (at 500 frames s−1) using a high-speed digital video camera (Fastec Imaging, San Diego, CA, USA), and the distances between the cobra's fang and the target were recorded (at 30 frames s−1) using a standard video camera (TRV608, Sony, Tokyo, Japan). The pulses from the LED laser were used to synchronize the video and accelerometer data sets to a temporal resolution of 2 ms. The rotations of the cobra's head relative to the long axis of the neck were quantified in every frame, in both the vertical (lateral view) and horizontal (dorsal view) planes, using the angle measure tool in ImageJ (NIH, Bethesda, MD, USA).
To compare the displacements of the cobras' and targets' heads, the target's displacement sequence was offset to equal the reaction time of the cobra (see below). Once this was done, the pre-spit (defined as 60 ms prior to the first appearance of venom at the fang tip) and spit (defined as 60 ms beginning at the first appearance of venom at the fang tip) displacement sequences were compared in two ways. Initially the sequences were compared using a Pearson correlation test. Subsequently a constant multiplier was used to equate the means of the two displacement sequences (cobra and target), then polynomial algorithms were used to statistically (using a criterion of P=0.05) fit a single curve to both target and cobra displacement lines.
RESULTS
Behavioral trigger for spitting
Herein the onset of spitting is defined as the first appearance of venom at the fang of the cobra (this was easily visible on the high-speed video record). The two data sets (accelerometer and videographic) were aligned (using the laser pulse), then the accelerometer data from all three planes (side-to-side, up and down, and front and back) were examined for a 500 ms interval prior to the onset of spitting (Fig. 1). The timing and magnitude of the target's ‘head wiggles’ in each plane were quantified for each trial; quantification was performed several times (both by eye and by computer analysis, and by multiple authors) using several criteria for recognition of a head wiggle; in every case the temporal distribution of the head wiggles relative to the onset of spitting was non-random. Our final approach was to adjust the mean of the data trace to zero, rectify the trace, then define a head wiggle as a rapid acceleration and reversal of the head with a magnitude of acceleration greater than one standard deviation beyond the adjacent (baseline) movements of the target in that plane. These wiggles, which were found in all three planes of movement, can be fairly subtle in terms of duration and linear range (Fig. 1), the key component is the reversal of direction.
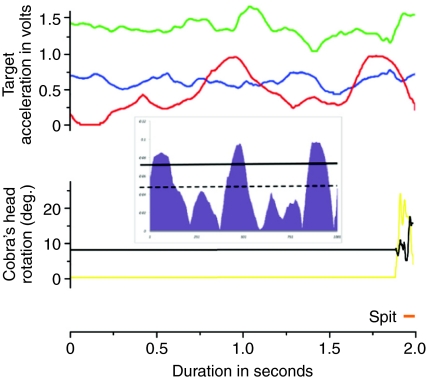
Fig. 1.
Raw data tracings of target acceleration (top three lines), angular displacement of the cobra's head (bottom two lines) and time of the spit (orange marker). Planes of movement for the target are coded as up–down (green), right–left (red) and front–back (blue). Planes of movement for the cobra are coded as vertical (black) and horizontal (yellow); note that the cobra remains stationary until just before venom expulsion. Inset shows right–left data trace from the target analyzed for head wiggles; the broken line is the mean (after adjustment and rectification) while the solid line is one standard deviation above the mean. Only reversals with magnitude above the solid line were counted as head wiggles. In this case, there is a clear head wiggle located ~200 ms prior to the onset of the spit.
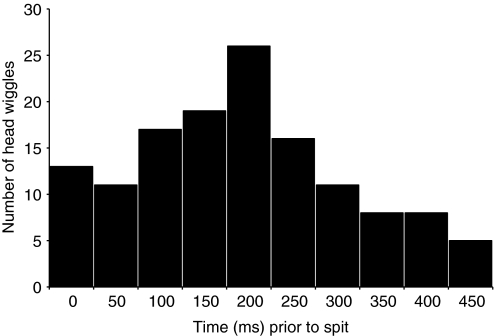
The distribution of the target's head wiggles relative to the onset of spitting were non-random (Fig. 2) and so clustered that their distribution was non-Gaussian (D'Agostino and Pearson test, χ2=23.80, P<0.001, N=86). The mean duration between the peak of the head wiggles and the onset of spitting was 208 ms (s.e.m.=7.8). A 25% percentile about the median of the temporal distribution of the head wiggles ranged from 175 ms to 231 ms (Fig. 2); the target made at least one head wiggle within this interval in every trial.
Fig. 2.
Frequency distribution of the target's head wiggles relative to the onset of spitting, note the highly skewed temporal distribution.
Rotational acceleration of cobra's head during spitting
Shortly (mean=65.2 ms; s.e.m.=4.6, N=43) prior to the onset of spitting the cobra began to rotate its head about the long axis of its neck (both horizontally and vertically). These rotations continued for the duration of venom expulsion (mean=40.3 ms, s.e.m.=1.4, N=47) and sometimes continued for a short time afterwards. Despite this relatively short duration, these movements were fast enough to propel the head through complex trajectories during this time (Fig. 3). Each spitting episode resulted in a spatial pattern of head movements, which showed no clear trend across multiple trials using the same cobra, intraspecifically or interspecifically.
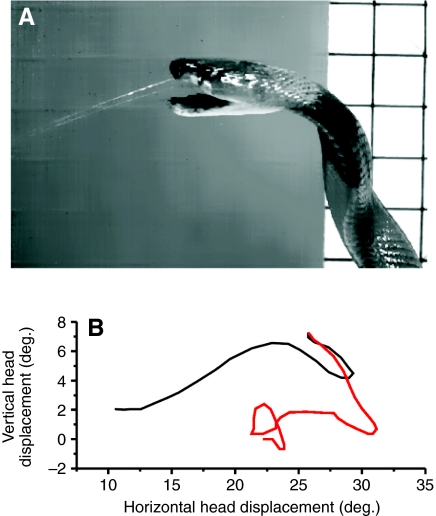
Fig. 3.
Movements of the cobra during venom spitting. (A) Isolated frame from the high-speed digital video camera; (B) angular displacement of the cobra's head in two dimensions during one 120 ms spitting trial (pre-spit movements are in black, spit movements are in red).
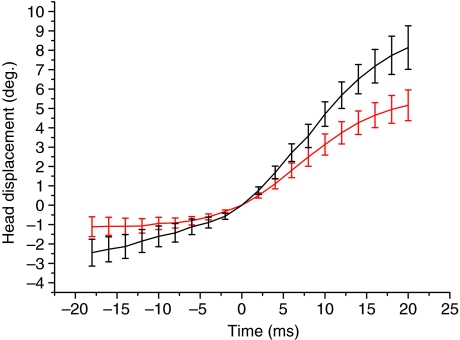
Plotting the displacement of the head in relation to the temporal pattern of venom expulsion demonstrates that the onset of spitting was typically concurrent, or nearly (±4 ms) so, with a change in the trajectory (35 out of 38 trials or 92%) of the cobra's head (Fig. 4). Additionally, in 33 out of 38 trials (or 87%), the angular velocities (in both the vertical and horizontal plane) during spitting were more than 2× the pre-spit angular velocities [analysis of variance (ANOVA) F=12.53, P<0.001, Fig. 5]. In addition to the initial trajectory change at the onset of spitting, the cobras always changed the direction of their head movements at least once (mean number of direction changes=2.6, s.e.m.=1.2) while the venom was being expelled (Figs 3, 4).
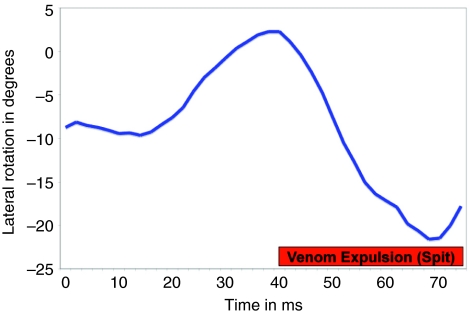
Fig. 4.
Trace of the angular displacement of a cobra's head in the vertical plane during venom spitting. Note that the onset of venom spitting is concurrent with a change in trajectory. During the 34 ms of this spit the head swept through an arc of over 25 deg. and changed direction twice (in this plane alone).
Fig. 5.
Mean (and s.e.m.) angular displacements of the cobra's head in the vertical (black) and horizontal (red) planes before and during spitting (acceleration at onset of spitting was adjusted to 0) showing the marked rotational acceleration of the head during spitting.
Target tracking
If the kinematic data from the target are offset to account for the 175–231 ms temporal delay between the target's head wiggle and the onset of spitting, the spatial congruence of the movements becomes evident (Fig. 6). A Pearson correlation test determined that both the vertical (R=0.407, P<0.0001) and horizontal (R=−0.102, P=0.002) pre-spit movements of the cobra were significantly correlated to the movements of the target. There was a high fidelity between the movements of the cobra and target. In 35 out of the 38 trials (or 92%) the cobra was moving in the same direction as the target (both vertically and horizontally). Furthermore, using polynomial algorithms and mathematically equating the means of the two tracks, a single curve was statistically (using a criterion of P=0.05) fit to both target and cobra pre-spit displacement lines (Fig. 7) in 13 out of the 38 trials (34%).
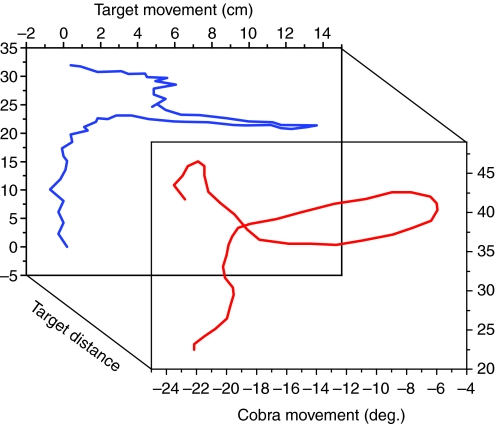
Fig. 6.
Target position (back plot) and angular displacement of the cobra's head (front plot) in two dimensions. Data are from the same trial but offset 180 ms to reflect the reaction time of the cobra. Note the agreement between the two displacements and the prominent direction change or ‘head wiggle’ on the part of the target.
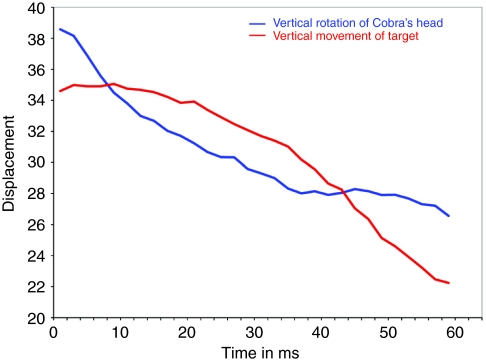
Fig. 7.
Example of the curve fitting showing the similarity in direction and slope of the two lines representing the (time synchronized) pre-spit vertical movements of the target (red) and the corresponding dorsal rotations of the cobra's head (blue).
The cephalic movements made by the cobra during spitting diverge from the target's movements more than was found leading up to the spit. The correlation coefficients between the target and cobra movements were lower during spitting (vertical R=0.148, P<0.0001; horizontal R=−0.078, P=0.016). Applying the same curve-fitting techniques revealed that in only 8 out of 38 (21%) of the trials could the displacement lines for the target and cobra be statistically explained by the same curve. Of the remaining 30 trials, 23 (or 77%) featured directional congruence between the cobra and the target.
DISCUSSION
The non-random distribution of the ‘head wiggles’ relative to the onset of spitting (Fig. 2), and the finding that a head wiggle was observed during every trial, is taken as evidence that this movement pattern on the part of the target serves as a behavioral trigger for venom spitting [see Jablonski (Jablonski, 1999) and Kibele (Kibele, 2006) for treatments of behavioral triggers]. From the perspective of efficient target tracking, it makes sense that the head wiggle would serve as a behavioral trigger. As defined herein, the head wiggle is characterized by a reversal of momentum (the head accelerates one way, stops, then accelerates in the other direction). At the onset of the new momentum it could be argued that the most likely course of future movements (at least over the short duration required for venom spitting) is in the same direction. Thus, the head wiggle both provides a clear ‘clue’ to future directions and represents a time of slower (at least initial) displacement of the head.
The target head wiggles occurred at a mean of 208 ms prior to the onset of spitting. This temporal duration is interpreted as the visual reaction time of the cobra [the time between the sensing of the visual cue (the head wiggle) and the motor response (venom expulsion)]. This visual reaction time is similar to what has been reported from humans (e.g. Burkhardt et al., 1987) but represents the first reported reaction time for a reptile.
At the present time we do not know what sensory cue(s?) initiates the target tracking. Whatever it is, the pre-spit tracking is marked by a fairly high (well above chance) congruence between the angular movements of the cobra's head and the linear movements of the target (Figs 6, 7). Tracking in this fashion gives cobras a distinct geometric advantage; even relatively large linear movements on the part of the target can be accommodated by rather modest angular movements of the cobra's head. During these experiments the mean distance between the cobra's fang and the target was 53 cm; at this distance a 10 cm lateral movement of the target's head could be accommodated by a 10 deg. rotation of the snake's head. The required angular displacement of the cobra's head will decrease with increasing target distance; Berthé et al. argued that spitting cobras modulate the extent of these cranial oscillations to accommodate target distance (Berthé et al., 2009).
When the cobra recognizes the behavioral trigger for spitting (the head wiggle of the target), the skeletal muscles associated with venom expulsion (Young et al., 2004) are activated, which will be followed shortly by the onset of spitting. The delay represents the time needed for the skeletal muscle to generate adequate force and for the venom to flow from the venom gland to the exit orifice of the fang. At the onset of spitting, the cobra is at a 200 ms disadvantage; unless the cobra compensates for its visual reaction time, it will be spitting at a space where the target was 200 ms earlier.
The compensation employed by spitting cobras is angular acceleration. The onset of spitting is marked by a distinct increase in the angular velocity of the head (Fig. 5). This burst of angular acceleration is coupled with a change in trajectory, corresponding to the change in trajectory of the head wiggle. This marks a distinct behavioral shift on the part of the cobra. Prior to the acceleration the cobra is tracking the movements of the target; the angular acceleration effectively eliminates the 200 ms visual reaction time delay (enabling the cobra to ‘catch up’ to the target) and forces the cobra to anticipate or predict the subsequent movements of the target. Not surprisingly, the match between the movements of the cobra and the target is greater when tracking than it is when anticipating (statistical curve fit in 34% and 21% of the trials, respectively). Interestingly, the cobras are able to match the direction of the target in 92% of the tracking and 82% of the anticipating segments; yet they could only statistically match both velocity and direction in 34% and 21% (tracking and anticipating, respectively) of the trials. It is not clear if this disparity reflects the different evaluative criteria applied to the two analyses or simply indicates that in cobras (as in other vertebrates) direction is easier to extract from visual cues than is velocity (e.g. Debruyn and Orban, 1988; Eckmeier and Bischof, 2008).
This behavioral sequence (target tracking, behavioral trigger, target anticipation) could possibly be accurate enough even if the cobras released the spat venom as a focused ‘point’ of venom. Instead, as we have detailed elsewhere (Young et al., 2009), the cobras always make oscillations of the head while the venom is being released (Figs 1, 3 and 4). These cephalic oscillations produce a spatial dispersal pattern in the spat venom. These oscillations are made while the spitting cobra is predicting or anticipating the movements of the target; the dispersal pattern is significantly correlated to the cephalic movements of the cobra (Young et al., 2009), which in turn are significantly related to the movements of the target. Thus, the movements of the target influence the ultimate dispersal pattern of the spat venom. The spat venom typically covers an area slightly greater than the interoccular distance (Berthé et al., 2009; Westhoff et al., 2005); this relative spread, when coupled with the relatively good fit between the movements of the cobra and the target during anticipation, greatly increases the likelihood that some of the expelled venom will contact the cornea. Cundall has described rapid cephalic movements during fang repositioning in vipers (Cundall, 2009); these movements are slightly slower than those reported herein and differ due to the relative kinetic independence of the viperid, compared to the elapid, fang and maxilla.
This study incorporated three species of spitting cobra: N. nigricollis, N. pallida and N. siamensis. This investigation was not designed as a study of interspecific behavior, nor does our data set support such an analysis; nevertheless, all three species exhibited the same three stages to venom spitting (target tracking, behavioral trigger, target anticipation), and there were no marked differences in the relative accuracy of target tracking or target anticipation among the three species. Naja siamensis is part of the Asiatic radiation of spitting cobras (e.g. Wüster and Thorpe, 1991) whereas N. nigricollis and N. pallida belong to the African radiation (e.g. Broadley, 1974). The recent analysis of Wüster et al. (Wüster et al., 2007) has confirmed the monophyly of the cobra radiation, and left open the possibility that venom spitting has evolved only once (despite the current geographic and taxonomic distribution of spitting cobras). The similar morphological features of these snakes, and the apparent shared behavioral components of venom spitting, strongly suggest that this highly specialized form of venom expulsion is a synapomorphy.
While neither sensory isolation nor neurophysiological experiments have been performed, spitting in cobras appears to be based solely on visual cues. Several lines of evidence support this, including: (1) spitting is very difficult to induce in low light (B.A.Y., personal observation); (2) cobras lack specialized infra-red receptors, and there is no clear link between tongue-flicking and spitting (suggesting that thermal and chemosensory cues are not playing key roles); (3) typically cobras will only spit at a moving object, and many visual responses are keyed to stimulus movement (e.g. Fleishman, 1992; Nicoletto, 1985; Vincent et al., 2005); and (4) other behavioral responses in these species have been shown to be visually mediated (e.g. Chiszar et al., 1987; Kardong et al., 1997). If vision is the dominant sensory modality used during venom spitting, it would suggest that both visual acuity and the integration of visual information with neuromuscular control is far greater in cobras than previously recognized.
Animals that project material at a moving target – whether that material be water (e.g. Rossel et al., 2002; Timmermans, 2001), silk (Nentwig, 1985), a tongue (e.g. Herrell et al., 2000) or venom – are able to achieve considerable accuracy. Westhoff et al. reported that spitting cobras had an accuracy of approximately 90% for a single spit (Westhoff et al., 2005); because these animals can spit multiple times in quick succession (Cascardi et al., 1999; Rasmussen et al., 1995), the accuracy could be even greater on a per-encounter basis. Functional studies of snakes have historically centered on the snake strike; yet venom spitting has a shorter duration, covers a greater distance and has a higher level of accuracy [see Kardong and Bels (Kardong and Bels, 1998) and Young et al. (Young et al., 2001) for analyses of the snake strike]. Accordingly, the case could be made that venom spitting represents one of best examples of sensory/motor integration in snakes.
ACKNOWLEDGEMENTS
This work was funded in part by Washburn University, the Kansas Academy of Sciences, the National Institutes of Health (through the Kansas Idea Network of Biomedical Excellence program) and the German Science Foundation (BL 242/15-1). We benefitted from the technical expertise available at Analog Devices and Electro Mechanica. Deposited in PMC for release after 12 months.
REFERENCES
- Berthé R. A., de Pury S., Bleckmann H., Westhoff G. (2009). Spitting cobras adjust their venom distribution to target distance. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 195, 753-757 [DOI] [PubMed] [Google Scholar]
- Bogert C. M. (1943). Dentitional phenomena in cobras and other elapids with notes on adaptive modifications of fangs. Bull. Am. Mus. Nat. Hist. 81, 285-360 [Google Scholar]
- Broadley D. (1974). A review of the cobras of the Naja nigricollis complex in Southwestern Africa. Cimbebasia Ser. A 2, 155-162 [Google Scholar]
- Burkhardt D., Gottesman J., Keenan R. (1987). Sensory latency and reaction time: dependence on contrast polarity and early linearity in human vision. J. Opt. Soc. Am. 4, 530-539 [DOI] [PubMed] [Google Scholar]
- Cascardi J., Young B., Husic D., Sherma J. (1999). Protein variation in the venom spat by the pink spitting cobra (Naja pallida) (Reptilia: Serpentes). Toxicon 37, 1271-1279 [DOI] [PubMed] [Google Scholar]
- Chiszar D., Radcliffe C. W., Boyer T., Behler J. L. (1987). Cover seeking behavior in red spitting cobras (Naja mossambica pallida): effects of tactile cues and darkness. Zoo Biol. 6, 161-167 [Google Scholar]
- Cundall D. (2009). Viper fangs: functional limitations of extreme teeth. Physiol. Biochem. Zool. 82, 63-79 [DOI] [PubMed] [Google Scholar]
- Debruyn B., Orban G. A. (1988). Human velocity and direction discrimination measured with random dot patterns. Vis. Res. 28, 1323-1335 [DOI] [PubMed] [Google Scholar]
- Eckmeier D., Bischof H. J. (2008). The optokinetic response in wild type and white zebra finches. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 194, 871-878 [DOI] [PubMed] [Google Scholar]
- Fleishman J. B. (1992). The influence of the sensory system and the environment on motion patterns in the visual displays of Anoline lizards and other vertebrates. Am. Nat. 139, S36-S61 [Google Scholar]
- Herrell A., Meyers J. J., Aerts P., Nishikawa K. C. (2000). The mechanics of prey prehension in Chameleons. J. Exp. Biol. 203, 3255-3263 [DOI] [PubMed] [Google Scholar]
- Jablonski P. (1999). A rare predator exploits prey escape behavior: the role of tail-fanning and plumage contrast in foraging of the painted redstart (Myioborus pictus). Behav. Ecol. 10, 7-14 [Google Scholar]
- Kardong K. V., Bels V. (1998). Rattlesnake strike behavior: kinematics. J. Exp. Biol. 201, 837-850 [DOI] [PubMed] [Google Scholar]
- Kardong K. V., Kiene T. L., Johnson E. (1997). Proximate factors influencing the predatory behavior of the Red spitting cobra, Naja mossambica pallida. J. Herpetol. 31, 66-71 [Google Scholar]
- Kibele A. (2006). Non-consciously controlled decision making for fast motor reactions in sports: a priming approach for motor responses to non-consciously perceived movement features. Psychol. Sport Exer. 7, 591-610 [Google Scholar]
- Nentwig W. (1985). Feeding ecology of the tropical spitting spider Scytodes longipes (Araneae, Scytodidae). Oecologia 65, 284-288 [DOI] [PubMed] [Google Scholar]
- Nicoletto P. F. (1985). The roles of vision and the chemical senses in predatory behavior of the skink, Scincella lateralis. J. Herpetol. 19, 487-491 [Google Scholar]
- Rasmussen S., Young B. A., Grimm H. (1995). On the spitting behaviour in cobras (Serpentes: Elapidae). J. Zool. 237, 27-35 [Google Scholar]
- Rossel S., Corlija J., Schuster S. (2002). Predicting three-dimensional target motion: how archer fish determine where to catch their dislodged prey. J. Exp. Biol. 205, 3321-3326 [DOI] [PubMed] [Google Scholar]
- Schuster S., Wöhl S., Griebsch M. (2006). Animal cognition: how archer fish learn to down moving targets. Curr. Biol. 16, 378-383 [DOI] [PubMed] [Google Scholar]
- Timmermans P. J. (2001). Prey catching in the archer fish: angles and probability of hitting an aerial target. Behav. Processes 55, 93-105 [DOI] [PubMed] [Google Scholar]
- Vincent S., Shine R., Brown G. (2005). Does foraging mode influence sensory modalities for prey detection in male and female filesnakes, Achrochordus arafurae? Anim. Behav. 70, 715-721 [Google Scholar]
- Warrell D. A., Ormerod L. D. (1976). Snake venom opthalmia and blindness caused by the spitting cobra (Naja nigricollis) in Nigeria. Am. J. Trop. Med. Hyg. 25, 525-529 [DOI] [PubMed] [Google Scholar]
- Westhoff G., Tzschätzsch K., Bleckmann H. (2005). Spitting behavior of two species of spitting cobras. J. Comp. Physiol A Neuroethol. Sens. Neural Behav. Physiol. 191, 873-881 [DOI] [PubMed] [Google Scholar]
- Wüster W., Thorpe R. S. (1991). Asiatic cobras: systematics and snakebite. Experientia 47, 205-209 [DOI] [PubMed] [Google Scholar]
- Wüster W., Thorpe R. S. (1992). Dentitional phenomena in cobras revisited: spitting and fang structure in the Asiatic species of Naja (Serpentes: Elapidae). Herpetologica 48, 424-434 [Google Scholar]
- Wüster W., Crookes S., Ineich I., Mané Y., Pook C., Trape J. F., Broadley D. G. (2007). The phylogeny of cobras inferred from mitochondrial DNA sequences: Evolution of venom spitting and the phylogeography of the African spitting cobras (Serpentes: Elapidae: Naja nigricollis complex). Mol. Phylogenet. Evol. 45, 437-453 [DOI] [PubMed] [Google Scholar]
- Young B. A., Phelan M., Jaggers J., Nejman N. (2001). Kinematic modulation of the strike of the western diamondback rattlesnakes (Crotalus atrox). Hamadryad 26, 316-349 [Google Scholar]
- Young B. A., Dunlap K., Koenig K., Singer M. (2004). The buccal buckle: the functional morphology of venom spitting in cobras. J. Exp. Biol. 207, 3483-3494 [DOI] [PubMed] [Google Scholar]
- Young B. A., Boetig M., Westhoff G. (2009). The functional bases of the spatial dispersal of venom during cobra “spitting”. Physiol. Biochem. Zool. 82, 80-89 [DOI] [PubMed] [Google Scholar]