Abstract
A century ago, Cajal noted striking similarities between the neural circuits that underlie vision in vertebrates and flies. Over the past few decades, structural and functional studies have provided strong support for Cajal’s view. In parallel, genetic studies have revealed some common molecular mechanisms controlling development of vertebrate and fly visual systems and suggested that they share a common evolutionary origin. Here, we review these shared features, focusing on the first several layers - retina, optic tectum (superior colliculus) and lateral geniculate nucleus in vertebrates, and retina, lamina and medulla in fly. We argue that vertebrate and fly visual circuits utilize common design principles, and that taking advantage of this phylogenetic conservation will speed progress in elucidating both functional strategies and developmental mechanisms, as has already occurred in other areas of neurobiology ranging from electrical signaling and synaptic plasticity to neurogenesis and axon guidance.
Almost as soon as it was recognized that neurons form connections with each other, it became evident that patterns of connectivity are stereotyped and complicated. Since that time, neuroscientists have tried to understand how these circuits arise and how, once formed, they underlie mental activities.
Although nearly every nook and cranny of the nervous system has been analyzed with these issues in mind, the visual system has been especially well studied. Several features have contributed to this focus. One is that, as highly visual animals, humans have an easy, intuitive understanding of what the visual system does. We can therefore more readily design stimuli to probe visual capabilities than, say, taste or smell. Like other sensory systems (but unlike, for example, memory systems), visual circuits have a discrete starting point, photoreceptors, that favors systematic analysis. In addition, the first several layers of the visual system are compact (unlike, for example, somatosensory or motor systems), physically accessible (unlike, for example, the ear) and laid out with a pleasing regularity that facilitates identification of cell types on the basis of their position (Figure 1).
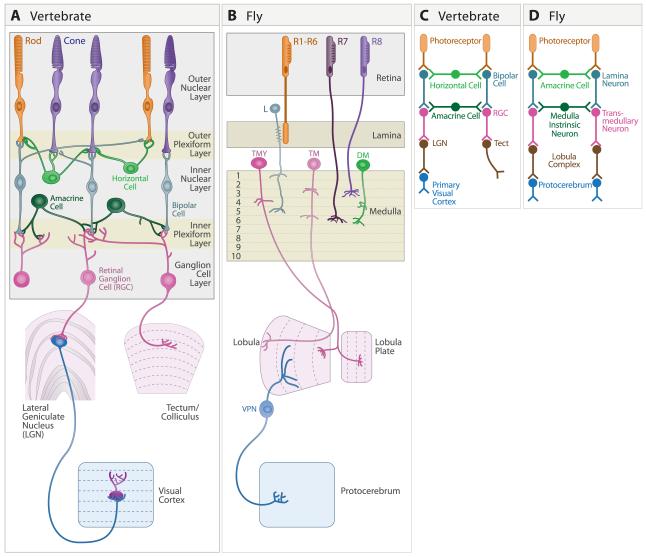
Figure 1. Structures underlying the first stages of visual processing.
A. Mammalian visual system, showing retina, dorsal lateral geniculate nucleus (LGN), superior colliculus (called optic tectum in lower vertebrates) and primary visual cortex (also called Area 17, striate cortex, or V1). Main retinal cell types are indicated.
B. Drosophila visual system, showing retina, lamina, medulla, and the lobula complex, which comprises the lobula and lobula plate. A few cell types are shown.
C,D. Similar steps in transfer of information through early stages of vertebrate and Drosophila visual systems.
The visual systems of vertebrates and insects share not only these technical advantages, but also many structural, functional and developmental features. Shared structural features include the arrangement of cells and their connections in parallel layers, a regular spacing of neurons within each layer, and radial connections that run perpendicular to the layers (Figure 2). Shared functional attributes include two modes of parallel processing: each small patch of the visual world is processed in multiple ways to extract salient visual features such as color, form and motion, and this processing is repeated for each portion of the visual field. The layers and radial arrays form the structural bases for feature detection and coverage of the visual field, respectively. The conservation of layered and radial arrangements over millions of years attests to their utility. Finally, recent studies of development have revealed that similar strategies and even some similar molecules are used to assemble the vertebrate and fly visual systems.
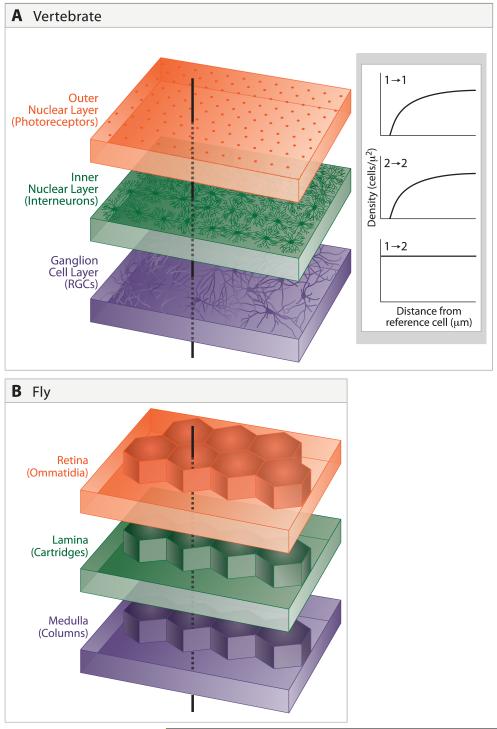
Figure 2. Laminar and radial arrangement of cells and connections.
A. Cells are arranged in mosaics in the outer and inner nuclear and ganglion cell layers of the vertebrate retina. Because of mosaic spacing, a line drawn perpendicular to the layers will intersect the dendritic field of at least one representative of each cell type. Inset shows distribution of cells within two amacrine mosaics, redrawn from Rockhill et al. (2000). For each cell type, spacing is non-random, with fewer near neighbors than would be expected by chance. However, mosaics are independent of each other, so distances between cells of types 1 and 2 are randomly distributed.
B. In Drosophila, spatial relationships among successive stages are strictly determined rather than probabilistic. Retinal ommatidia overly laminar cartridges, which in turn overly medullary columns.
Our goal in this review is to highlight these similarities, focusing on the first several structures –retina, optic tectum (superior colliculus) and lateral geniculate nucleus in vertebrates, and retina, lamina and medulla in fly. Our motivations are first, that conserved features are likely to be the most fundamental and second, that knowledge gained from each system will be useful to students of the other. In these regards, we echo Cajal, who first drew attention to these parallels a century ago (Figure 3). Our conviction is that recent advances in genetic and genomic technologies will make these parallels both more compelling and more useful over the next several years.
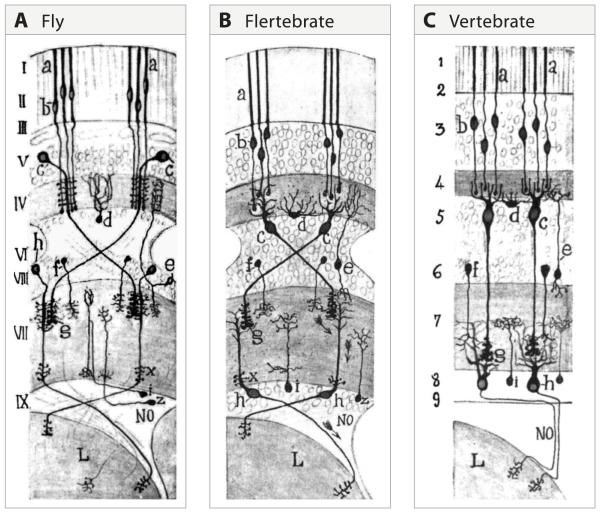
Figure 3. Cajal recognized the similarity of fly and vertebrate visual systems.
A. The retina (I – III), lamina (IV and V) and medulla (VI – VIII) and lobula region (L) of the fly visual system. The somas appear in their natural position. a, b. photoreceptor; c, lamina monopolar neuron; h, transmedullary neuron.
B. In this drawing of the insect visual system, Cajal “moved” the cell bodies to correspond to their positions in vertebrates, without changing the positions of their synaptic contacts. We refer to this as the “Flertebrate” arrangement. c, lamina monopolar neurons take on the appearance of bipolar neurons (see “c” in right panel); d, amacrine cells in the fly appear as horizontal cells (see “d” in right panel). h, transmedullary cells appear as retinal ganglion cells (see “h” in right panel).
C. Schematic of the main cell types in the vertebrate retina and their connections. The Arabic numerals indicate regions of the vertebrate retina that Cajal viewed as similar to the corresponding layers marked with Roman numerals in the left panel. (From Cajal and Sanchez, 1915; adapted from Meinertzhagen, 1993).
SYNAPTIC ORGANIZATION OF THE VERTEBRATE VISUAL SYSTEM
Overview
There are 6 principal cell types in the vertebrate retina: photoreceptors, projection neurons (retinal ganglion cells or RGCs), 3 types of interneurons (horizontal, bipolar and amacrine cells), and glial cells (Muller glia). These cells are arranged in 3 histologically distinct “nuclear” layers that contain cell bodies but no synapses separated by two “plexiform” layers that contain synapses but no cell bodies (Figure 1A,C). The cellular layers are, from outside in: the outer nuclear layer, containing photoreceptors; the inner nuclear layer, containing interneurons and Muller glia; and the ganglion cell layer, containing RGCs and some amacrines (Masland, 2001; Wassle, 2004). As we shall see, each of the main neuronal types can be subdivided into multiple subtypes, based on structural, physiological and, more recently, molecular criteria. Moreover, the plexiform layers can be divided into multiple sublaminae. However, the regular arrangement of the cells and synapses, and the ability to classify many subtypes into a few main types provides a helpful simplifying framework for analysis.
Although the distribution of cells within retinal layers is not perfectly regular as is the case in fly retina (see below), it is highly non-random. Cells of a particular subtype are spaced more evenly than would be expected by chance alone (Wassle and Riemann, 1978; DeVries and Baylor, 1997; Rockhill et al., 2000; Novelli et al., 2005; Eglen, 2006). Most often, regularity is measured as the reduced frequency of finding two cells of a single type near each other; the presence of an “exclusion zone” defines and characterizes the so-called “mosaic” arrangement (Figure 2A). As a consequence, a pin piercing the retina vertically will pass through the dendritic field of at least one cell of each subtype. Mosaics can be viewed as a means of ensuring that all regions of the visual world are sampled by each set of processors.
RGCs, the sole output neurons of the retina, send axons to more than 10 areas of the brain. In most vertebrates, the main target is the optic tectum, called superior colliculus in mammals. In higher mammals, such as cats and primates, the dorsal lateral geniculate nucleus (LGN) in the thalamus emerges as the major target. This difference reflects the fact that thalamic nuclei relay sensory information to the cerebral cortex, which is rudimentary in lower vertebrates and relatively small in lower mammals. In both of these structures, RGC axons arborize and terminate in specific layers. As in retina, each layer receives input from specific RGC subtypes.
From the LGN and tectum/colliculus, visual information travels further into the brain. Best studied is the projection from the LGN to the primary visual cortex, called V1, area 17 or striate cortex. From there, parallel streams fan out to numerous areas (Van Essen et al., 1992).
Outer retina
The outer plexiform layer is the simpler of the two synaptic regions in the vertebrate eye. It contains chemical and electrical synapses that link photoreceptors, horizontal cells and bipolar cells. Its main synaptic type is a multiple contact synapse made by unusually large photoreceptor nerve terminals onto postsynaptic processes of horizontal and bipolar cells (Wassle, 2008; Figure 4A).
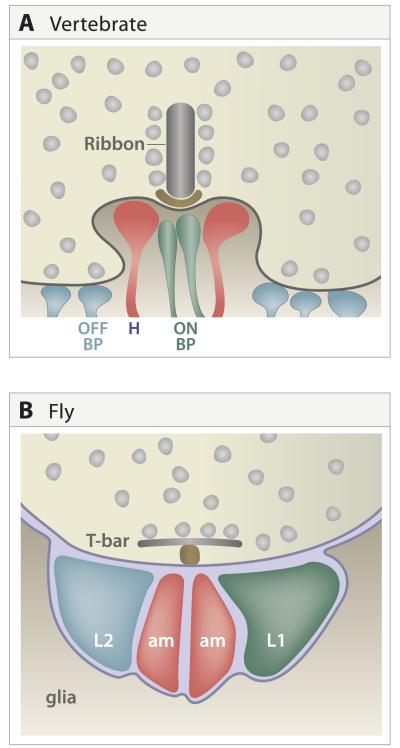
Figure 4. Multiple contact synapses.
A. Portion on a cone pedicle in the outer plexiform layer, showing its synapses with processes of multiple horizontal, ON bipolar and OFF bipolar cells. Ribbon, structure at the active zone of these synapses specialized for tonic transmitter release.
B. Tetrad in Drosophila lamina, showing synapses of photoreceptor axon on processes of L1, L2 and amacrine (am) cell dendrites. Like the vertebrate photoreceptor, that of Drosophila contains an unusually large presynaptic specialization, in this case a T-bar (T). Schematic is simplified to show all postsynaptic elements in the same plane; L1 and L2 are paired equatorially while the am elements are paired in polar positions. In some cases, one amacrine cell process is replaced with a process from L3.
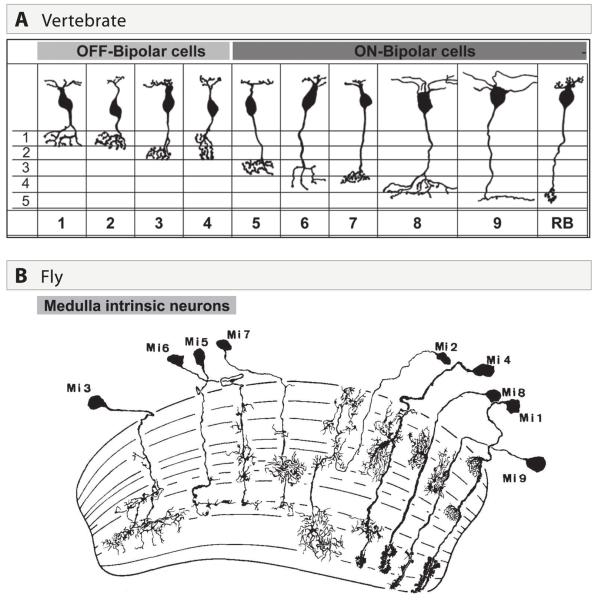
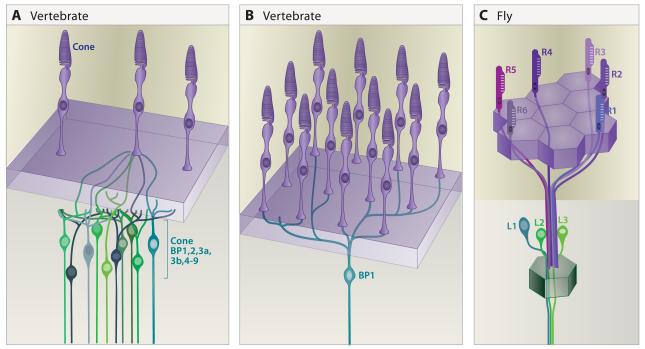
Complexity in this simple pattern arises from the existence of multiple subtypes of each main cell type (e.g., Fischer et al., 2007; Wassle, 2008; Li et al., 2009; Dacey, 2000). The two basic photoreceptor types are rods and cones, with cones further divided into subtypes (for example, two in mice, three in humans and five in chickens) on the basis of the visual pigment (opsin) they express. In a comprehensive analysis, Wassle et al. (2009) identified 11 bipolar subtypes in mice (Figure 5A). They go on to show that each cone photoreceptor contacts at least one member of each of 10 bipolar subtypes and that each cone bipolar cell receives input from ~10 cones (Figure 6A,B). Thus, divergence and convergence begin at the first synapse in the visual system.
Figure 5. Diversification of main neuronal types into multiple subtypes.
A. Comprehensive inventory of bipolar cell subtypes in mouse retina (from Wassle et al. 2009).
B. Comprehensive inventory of medulla intrinsic neuron subtypes in Drosophila (from Fischbach and Dittrich et al. 1989).
Figure 6. Divergent and convergent connections of photoreceptors.
A. A single mouse cone connects with at least one of each of the 10 cone bipolar cell types.
B. A single mouse cone bipolar (BP1 sketched here) receives input from at least 10 cones.
C. A single lamina neuron cartridge receives input from a single cell of each R1-R6 class, each in a different ommatidium. All six R cells synapse on the L1-L3 neurons associated with the cartridge.
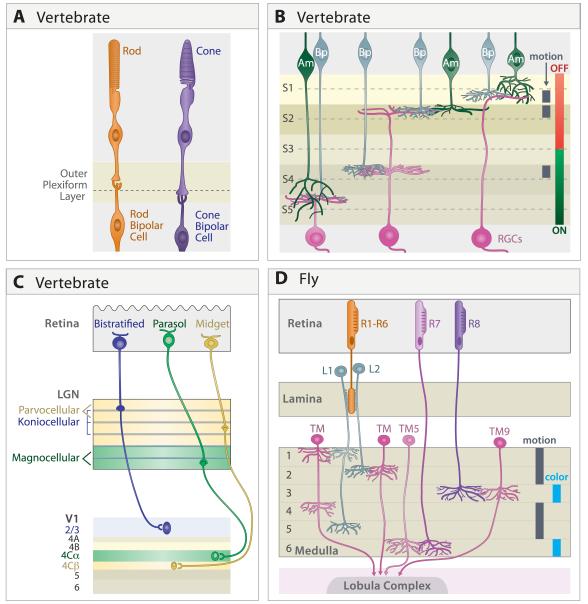
At least in mammals, rods and cones initiate two information-processing streams that remain partially segregated through multiple synapses. Rods mediate vision at low light intensities (so-called “scotopic vision”) but adapt or bleach at high light intensities; cones, which are less sensitive (mediating photopic vision) and more resistant to bleaching, generate the sensation of color (Dacey, 2000) (Figure 7A). The ability of cones to mediate color vision results from the existence of multiple cone types, each with a unique opsin and a distinct spectral sensitivity. In essence, the sensation of color arises from differential activation of cone types by light of particular wavelengths. Since rods are inactive at light levels that activate cones, they play little role in color vision; conversely, in dim light, when cones are inactive, objects generally seem gray. Rod and cone terminals, called spherules and pedicles, respectively, synapse on bipolars in distinct sublaminae within the outer plexiform layer (e.g., Li and DeVries, 2006; Dacey, 2000; Fischer et al., 2008; Tanabe et al., 2006; Wahlin et al., 2008). Bipolar cells are, with few exceptions, dedicated postsynaptic partners of either rods or cones. Although some rod-derived signals make their way to the cone pathway (Soucy et al., 1998; Wassle, 2004), the separate pathways for rod- and cone-derived signals maintain at least partial segregation of color-sensitive and –insensitive information through the inner nuclear layer.
Figure 7. Parallel processing of visual features in distinct laminae.
A. Rod and cone terminals occupy distinct sublaminae in the vertebrate outer plexiform layer, where they synapse on dendrites of rod and cone bipolars, respectively. In some species (for example, chicks) the cone sublamina is further subdivided.
B. Axons of ON bipolar cells form terminals in the inner part of the vertebrate inner plexiform layer, where they form synapses on ON RGCs, whose dendrites arborize in the same zone. Likewise, OFF bipolar axons and OFF RGC dendrites arborize and form synapses in an outer zone. Bipolar, amacrine and retinal ganglion cells are further subdivided into groups that form lamina-specified connections underlying sensitivity to other visual features; sublaminae specialized for processing directional motion in mouse are indicated.
C. In primates, distinct sets of RGCs form the magnocellular, parvocellular and koniocellular pathways. Although their functions are overlapping, they are sometimes viewed as being specialized for processing information about motion, form, and color, respectively. Synapses in these pathways, are segregated into distinct laminae in LGN and project to distinct targets in primary visual cortex.
D. In Drosophila, R1-R6 are critical for sensitivity to motion while R7 and R8 are required for color. These two classes of photoreceptors project directly (R7,8) or indirectly (R1-6, via the lamina) to separate layers in the medulla. Layers specialized to process information about color and motion are indicated. Only the distal 6 layers of the medulla are shown.
Inner retina
It is in the inner plexiform layer that laminar specificity is displayed in its fullest glory (Figures 5A, 7B). This layer contains synapses of amacrine, bipolar and retinal ganglion cells, arranged in a series of at least 10 narrow, parallel sublaminae, often divided into 5 sets (S1-S5 in Figures 5 and 7). Each sublamina contains processes of distinct cellular subtypes (Karten and Brecha, 1983; Wassle, 2004; Siegert et al., 2009). There are at least 20 types of RGCs (Volgyi et al.,2009) and 30 types of amacrine cells in mammalian retinae (e.g., MacNeil and Masland, 1998; MacNeil et al., 1999; Bin and Masland, 2008), along with the 11 types of bipolar cells mentioned above. The relationship between cellular diversity and sublamination was recognized by Cajal, whose pictures clearly show individual bipolar, amacrine and retinal ganglion cells with arbors confined to just one or a few sublaminae in the inner plexiform layer.
Of what significance are these subtypes and sublaminae? Insight came from two seminal discoveries. First, Kuffler (1953) established the principle that RGCs are not illumination detectors, but rather feature detectors. Specifically, he showed that RGCs respond poorly to diffuse, sustained illumination but briskly to borders between bright and dark regions. Some RGCs responded well to illumination of overlying photoreceptors when the bright spot was surrounded by a dark annulus; others responded best to dark spots surrounded by bright annuli. Kuffler called these ON- and OFF-center cells, respectively. Second, Kolb, Famiglietti and colleagues (Famiglietti and Kolb, 1976; Nelson et al., 1978) showed that Kuffler’s ON- and OFF-center cells had dendrites confined to the inner and outer halves of the inner plexiform layer, respectively (Figure 7B). This association provided a firm basis for Cajal’s basic tenet, that neuronal structure and function are closely interrelated. Perhaps most compelling is the observation that ON-OFF cells, which respond to both increases and decreases in light intensity, have bistratified arbors – one each in the inner and outer halves of the IPL.
The properties of ON and OFF RGC receptive field centers reflect the lamina-specified synapses they receive from bipolar cells. Two sets of bipolar cells receive inputs from the same photoreceptors, but respond in opposite ways, based on the glutamate receptor subtypes they express (Werblin and Dowling, 1969). Photoreceptors release glutamate in the dark; illumination hyperpolarizes the cell and reduces transmitter release. OFF bipolar cells bear ionotropic glutamate receptors that lead to depolarization by neurotransmitter; thus, they are inhibited (hyperpolarized) by light. In mammals, ON bipolar cells bear metabotropic glutamate receptors that lead to hyperpolarization by neurotransmitter, so they are excited (depolarized) by light (Slaughter and Miller, 1981); fish use other tricks to invert the sign of the ON response. The axons of the ON and OFF bipolar cells terminate in the ON and OFF sublaminae and selectively innervate dendrites of ON and OFF RGCs, respectively. As expected, ON-OFF RGCs receive inputs from both ON and OFF bipolars. Thus, processing by interneurons in the inner nuclear layer determines the visual features to which RGCs respond.
We now know that the division of the inner plexiform layer into ON and OFF zones is just the tip of the iceberg. Lamina-specified subsets of RGCs are tuned to respond preferentially to distinct visual features, such as motion or color (Nassi and Callaway, 2009; Roska and Werblin, 2001). The diversity and degree of specialization is exemplified by RGCs that respond preferentially to moving objects (Demb, 2007; Zhou and Lee, 2008). Pioneering work by Barlow and colleagues defined two general sets of direction-selective RGCs in rabbits – ON-OFF and ON. Remarkably, the ON-OFF cells came in four discrete “flavors,” responding to motion in four orthogonal directions, and the ON cells came in three discrete flavors, responding to motion in three directions at 120° angles (Barlow et al., 1964; Oyster and Barlow, 1967). More recently, an OFF RGC subtype has been identified that is selectively responsive to upward motion (Kim et al., 2008), and molecular markers have been identified for one ON, one OFF, and one ON-OFF direction-selective subtype (Kim et al., 2008; Yonehara et al., 2009; Huberman et al., 2009).
These receptive fields are built by inputs from the 10-11 subclasses of bipolars mentioned above and at least 30 amacrine subtypes, each with sublamina-specific projections. Distinct physiological signatures have already been characterized for several of them. In recent years, identification of molecular markers for lamina-specified subsets of pre- and postsynaptic partners in the IPL has facilitated their enumeration and categorization. These markers, alone or when used to direct expression of a reporter in transgenic mice, make possible new approaches to following the development of the cells, targeting them for recording, and tracing their connections (e.g., Badea et al., 2009; Kim et al., 2008; Siegert et al., 2009; Yonehara et al, 2009; Huberman et al, 2009; Kim et al., 2010).
Lateral geniculate nucleus and optic tectum
Unsurprisingly, given its role in relaying information to the visual cortex, the LGN is especially large in primates, and this expansion is associated with a striking laminar organization. The primate dLGN comprises six cell-rich laminae separated by narrow cell-poor zones. They are selectively innervated by three major groups of RGCs, which initiate processing streams of visual information that remain at least partially distinct through early stages of cortical processing (Figure 7C). One, comprising the 2 ventral-most laminae is called the magnocellular pathway; it is specialized for motion detection. Another set of RGCs innervates the 4 dorsal laminae, comprising the parvocellular pathway; it appears to subserve high acuity vision. Each pair of LGN sublaminae contains one innervated entirely by RGCs from the contralateral retina and one innervated by RGCs from the ipsilateral retina. The third visual pathway, called koniocellular, runs through the interlaminar leaflets and carries information about color (Hendry and Reif, 2000; Szmajda et al., 2008). Thus, the primate LGN contains 10 sublaminae, subdivided by modality and laterality, each innervated by distinct RGC subsets and each projecting to distinct cortical laminae.
Just as the LGN is well-laminated in higher mammals, where it passes information to the cortex, the optic tectum is most highly laminated in lower vertebrates, where it is a higher visual processing center. For example, RGCs terminate in at least 4 retinorecipient sublaminae in chickens, pigeons and zebrafish (Xiao and Baier, 2007; Karten and Brecha, 1983; Kuljis and Karten, 1988; Yamagata and Sanes, 1995, 2005; Yamagata et al., 2006). Unfortunately, physiological analysis of this structure has been limited, so little is known about the information carried by these parallel pathways. Until recently, it appeared that lamination of superior colliculus and LGN was rudimentary in rodents (Sachs and Schneider, 1984; Hofbauer and Drager, 1985). However, recent studies of transgenic mice in which distinct RGC subpopulations are marked reveal multiple sublaminar termination zones in both colliculus and dLGN (Huberman et al., 2009; Kim et al., 2010). Thus, the differences among species may be more apparent than real.
Primary visual cortex
The physical segregation of information processing streams extends from the lateral geniculate into the cortex (Figure 7C). Visual cortex, like other portions of the cerebral cortex, is conventionally divided into 6 main laminae, some of which are further subdivided into sublaminae. Most arbors and synapses of thamalocortical axons are confined to Layer 4. In primates, axons carrying information from the magnocellular and parvocellular streams terminate in layers IVCα and IVCβ respectively, forming overlapping retinotopic maps, much like those in the IPL and geniculate. The koniocellular stream is segregated in a different way: geniculate axons carrying this information project to spatially segregated areas called, sadly, “blobs.” From these projections, the streams project in partially separate (but highly interconnected) pathways to numerous other areas in what have been called the dorsal and ventral streams, which specialize in motion detection and object identification (and are therefore sometimes called “where” and “what” pathways), respectively (Nassi and Callaway, 2009).
SYNAPTIC ORGANIZATION OF THE FLY VISUAL SYSTEM
Overview
Cajal turned to the visual system of large flies with the hopes of finding a sensory system simpler than the vertebrate retina in which to deduce the flow of information from anatomy (Cajal, 1937). He was astonished to discover that the cellular diversity and complexity of the insect visual system rivals that of the vertebrate retina (Cajal and Sanchez, 1915). Fischbach and Dittrich (1989) later demonstrated that the smaller Drosophila visual system has a similar level of cellular diversity. In addition to the retina or compound eye, the Drosophila brain contains four principal regions devoted to visual information processing: lamina, medulla, lobula and lobula plate, the latter two sometimes referred to as the lobula complex (Figure 1B). The retina, lamina and medulla each contain precisely registered sets of ~750 units, called ommatidia, cartridges and columns, respectively (Figure 2B). Although visual systems of many insects have been studied, we focus on Drosophila as genetic tools make it particularly advantageous for developmental and functional analyses circuits.
Each retinal ommatidium is composed of 8 photoreceptor neurons or retinula (R) cells, along with supporting cells. There are three classes of R cells. The R1-R6 neurons express the same opsin (O’Tousa et al. 1985; Zuker et al. 1985) and are thought to provide an achromatic channel as they respond to a broad spectrum of wavelengths. The R7 and R8 neurons express UV- and blue-green-sensitive opsins, respectively, and provide chromatic information (Zuker et al. 1987; Montell et al 1987; Fryxell and Meyerowitz, 1987; Papatsenko et al. 1997; Chou et al. 1996). In a broad sense, R1-R6 neurons may be thought of as rods and R7 and R8 neurons as cones. All three classes of R cells are histaminergic (Hardie, 1987; Sarthy, 1991).
By contrast to vertebrate retina, there are no synapses within the fly retina. The R1-R6 neurons project axons to and form synapses within a structure directly beneath the retina called the lamina, a source of perpetual confusion in a system dominated by lamination. The R7 and R8 axons, along with lamina neurons, arborize in distinct layers within the medulla, where they synapse on interneurons and transmedullary neurons (Takemura etal, 2008). The transmedullary neurons then project into the lobula and lobula plate. Multiple pathways link the lobula complex to regions in the central brain (Otsuna and Ito, 2006; Strausfeld and Okamura, 2007) (Figure 1B,D).
The small diameter of lamina and medullary neurons presents a substantial barrier to conventional electrophysiological analysis. Homologous neurons of larger flies have been characterized (e.g. Douglass and Strausfeld, 1995, 1996; Gilbert et al. 1991) and recent studies report recordings from lamina neurons in Drosophila (e.g. Zheng et al. 2009; Nikolaev et al., 2009), but most efforts have focused on harnessing Drosophila genetics to selectively alter synaptic function in specific subsets of cells (Borst, 2009). Cell-type specific enhancer/promoter elements can be used to selectively manipulate the physiological properties of specific cells, and behavioral consequences can then be assessed. These methods are now providing insights into how neuronal circuits coordinate visually-evoked behaviors.
Lamina
The lamina contains five different monopolar neuron cell types (L1-L5), three classes of wide field neurons (including amacrine cells), and centrifugal fibers from the medulla (T1 cells) and lobula complex (C2 and C3 cells) (Meinertzhagen and Hanson, 1997). Each of the R1-6 axons synapses on L1-L3 neurons in a single cartridge. Each cartridge, in turn, receives inputs from one each of the R1-R6 photoreceptor cell types. Remarkably, however, these inputs do not arise from the ommatidium lying directly above it. Due to the orientation of the photoreceptors within an ommatidium, each R1-R6 neuron in a single ommatidium detects light from a different point of space. Conversely, due to the curvature of the eye, each R1-R6 neuron detects light from the same point in space as one cell in each of 5 neighboring ommatidia - i.e. an R1 in one ommatidium, an R2 in another and so on. The six R cells in six different ommatidia that “see” the same point in space project to a single cartridge (Figure 6C) (Vigier, 1909; Trujillo-Cenoz, 1965; Braitenberg, 1967). This arrangement, called neural superposition, increases the signal to noise ratio of phototransduction and, thereby, enhances visual sensitivity.
EM reconstruction studies have provided a detailed description of synaptic connections in the lamina (Meinertzhagen and O’Neill, 1991). In all cases, as in the vertebrate visual system, these are multiple contact synapses, with a single presynaptic terminal and between two and four postsynaptic elements (Figure 4B). The predominant synapses formed by photoreceptors are tetrads, single pre-synaptic R1-R6 terminals juxtaposing 4 postsynaptic elements. Two postsynaptic elements, from L1 and L2, are invariant with the remaining two derived from amacrine cells, L3 cells or glial cells.
To learn what information passes through the lamina, the activity of specific neurons can be selectively blocked using molecular genetic tricks and the effects assessed by measuring reflexive behavioral responses to pattern motion. Such analysis reveals that R1-R6 connections to the lamina initiate an achromatic input channel for processing information about motion, which is independent from the R7/R8 color channel (Yamaguchi et al. 2008). Further inactivation studies suggest that achromatic input from R1-R6 cells is segregated into multiple information streams for the general sensitivity to light and the specific motion computations that arise in higher order brain regions. For instance, inactivating a specific complement of lamina neurons results in complete loss of motion vision without comprising phototaxis behavior (Zhu et al. 2009).
Motion channels are further subdivided by lamina neurons (Rister et al., 2007; Katsov and Clandinin, 2008). Through selective inactivation of neurotransmitter release, or by selective rescue of function in animals lacking neurotransmitter receptor globally, different lamina neurons were shown to regulate responses to different stimuli (Rister et al., 2007). At high contrast motion stimuli, either L1 or L2 is sufficient to drive the optomotor response, while at low contrast both L1 and L2 are required. Interestingly, at medium contrast, roughly corresponding to those in natural habitats, L1 and L2 selectively mediate responses to motion in opposite directions (back-to-front for L1 and front-to-back for L2). This study also suggests that L3 contributes to orientation behavior while the amacrine/T1 pathway enhances the L1 pathway. Each pathway transmits information to discrete layers within the medulla neuropil. Whereas lamina neurons are not themselves motion selective, they show high-pass response characteristics that are suggested to pre-process visual inputs for higher order motion computations such as small target tracking (Wiederman et al. 2008). Like the feature detecting capabilities of RGCs of the vertebrate retina, fly lamina circuits may perform computations that provide additional “structure” to the signals relayed to higher processing centers.
Medulla
The medulla neuropil is divided into 10 layers, designated M1-M10 (Figure 1B, 5B, 7D). R7, R8 and L1-5 arborize and form synapses in one or a few of the outer six layers, M1-M6. Connections of R7 and R8 from a single ommatidium and lamina neurons from a single cartridge are largely restricted to a single medulla column, thus retaining strict retinotopic mapping of visual information in higher centers (Figure 2D). Postsynaptic neurons, such as medulla tangential neurons and interneurons, also arborize within discrete layers of the medulla, but some of their arbors extend beyond column boundaries to delineate receptive fields of large sizes. Connections of medulla interneurons remain confined to the medulla and promote processing within and between layers (Figure 5B). The two classes of tangential medulla neurons, by contrast, send axons to the lobula complex: Tm neurons to the lobula and TmY neurons to both the lobula and lobula plate. Catalogs assembled from Golgi staining and genetic marking suggest that there are minimally 12, 26, and 13 morphologically distinct medulla intrinsic neurons, TM, and TmY neurons, respectively (Fischbach and Dittrich, 1989; Morante and Desplan, 2008). In addition, there are multiple classes of wide field neurons (e.g 8 classes in the distal medulla) with extensive arborizations within specific layers in the medulla. Using electron microscopy, Takemura et al. (2008) have documented distinct patterns of layer-specific morphology and wiring specificity for each of at least 10 types of neurons within the distal 6 layers of the medulla neuropil. As in the lamina, all synapses are of the multiple contact variety, typically triads and tetrads. These studies reveal exquisite synaptic specificity within the column.
Of the four regions of the optic lobe, the medulla is functionally the most enigmatic. Recent behavioral analyses, however, have provided important insights into color processing (Gao et al., 2008). Using promoter analysis of the gene encoding a receptor for histamine, the photoreceptor neurotransmitter, four different histaminoceptive TM neurons were identified, each of which arborized in at least one medulla layer receiving input from R7 and R8. Several classes of centrifugal fibers and medulla intrinsic neurons were also tagged, including DM8 (distal medulla intrinsic neuron 8), which arborizes extensively and selectively within the R7 recipient layer. The axons of transmedullary neurons terminated within discrete layers of the lobula neuropil, arguing that these layers process chromatic information. Manipulation of histamine receptor function and synaptic activity revealed that DM8 neurons are both necessary and sufficient for mediating ultraviolet light sensitivity, probably via connections to TM5, which in turn transmits information to layer 5 in the lobula.
Together, these initial studies show that two main types of visual information, motion and spectral sensitivity, follow different paths through the early stages of the visual system and in the lobula complex.
Lobula Complex
Axons project from specific layers in the medulla to specific layers within the lobula and/or lobula plate of the lobula complex (Fischbach and Dittrich, 1989; Douglass and Strausfeld, 1996). Numerous cell types within these structures process different features of the visual world. Of these, the best characterized are motion-selective large field neurons tuned to horizontal and vertical motion (HS and VS cells, respectively) (Borst, 2009; Joesch et al. 2008; Raghu et al. 2007, 2009).
The HS and VS cells elaborate extensive dendritic trees. For instance, the dendrites of all 6 VS cells together cover the entire visual field. The receptive field of each VS cell spans 180 degrees along the elevation and on the order of 60 degrees along the azimuth. Dye coupling reveals that the dendritic fields not only overlap, but are linked through gap junctions. Physiological studies support a model in which motion across the retina activates L1 and L2 within sequential cartridges whose spatio-temporally correlated output is integrated via a “push/pull” combination of excitatory and inhibitory inputs into VS dendrites (Joesch et al., 2008). The identity of the cells that link motion sensitivity in L1 and L2 neurons to VS dendrites remain unknown.
In addition to the VS and HS neurons, many different visual projection neurons in the lobula complex project to glomerular structures within the protocerebrum (Otsuna and Ito, 2006; Strausfeld and Okamura, 2007). As neurites interlink these glomeruli at least in larger flies, further information processing is likely to occur between these elements prior to activating pre-motor descending pathways that regulate output behaviors.
In conclusion, a rich diversity of neuronal cell types and synaptic connections within the fly visual system has evolved to discriminate salient features of the visual world, including color and motion; process this information; and direct specific behaviors. New genetic methodologies provide a promising approach to unravel how these circuits function.
FLY AND VERTEBRATE VISUAL SYSTEMS COMPARED
We have highlighted numerous striking structural similarities between the vertebrate and fly visual systems. They include:
a small number of main neuronal types (5 in vertebrate retina, 6 in fly retina/lamina/medulla; Figures 1 and 3), divided into numerous subtypes (probably ~100 in each case; Figure 5);
multiple contact synapses with a single presynaptic terminal abutting multiple postsynaptic elements (Figure 4);
multiple cellular layers with regular arrangement of neurons in each layer (Figure 2);
orderly mapping of neuronal arrays at each level onto those at the next level, involving specific patterns of integration and convergence (Figures 2 and 6); and
segregation of synapses made by specific subtypes into parallel sublaminae within soma-free neuropil (inner and outer plexiform layers and medulla; Figure 7)
When these structural features are considered in light of visual function, several shared design principles emerge:
Lateral interactions and convergence mediate hierarchical processing
In both vertebrates and flies, photoreceptors transduce light into relatively simple electrical signals. These signals convey progressively more complex information as they proceed through a series of synaptic way stations: outer plexiform layer to inner plexiform layer to LGN to visual cortex in mammals; lamina to medulla to lobula to protocerebrum in flies (Figure 1). Complexity arises in large part through lateral interactions amongst parallel relays – for example horizontal and amacrine cells in vertebrate retina, lamina amacrine cells and medulla intrinsic neurons in flies. At the same time, the signal to noise ratio is optimized by having several photoreceptors connect to a single interneuron at the next stage – lamina neurons or bipolar neurons (Figure 6). As a consequence, cells only a few synapses away from the sensory neurons are both highly sensitive and selectively tuned to complex visual features, such as motion in a particular direction. Thus, the information that reaches the brain has already been heavily processed; in neither flies nor vertebrates is the eye in any sense a camera.
Repeated local modules tile the global visual field
Although the visual world is continuous, the visual system must divide it into discrete units for processing, and ensure that each unit is served by a complete set of feature detectors. Both visual systems must distribute cell subtypes so that each portion of the visual world is served by a complete set of feature detectors and can thus be analyzed in all relevant ways. In vertebrates a mosaic strategy accomplishes this while in flies each module is more rigidly constructed within a repeated crystal-like columnar structure (Figure 2).
Layered connections mediate parallel processing
Distinct functions are mediated by separate albeit linked pathways that act in parallel. Best studied in both systems are the largely separate streams for processing color and motion, two undeniably salient features (Figure 7). In both cases, comparisons of signals from photoreceptors with distinct excitation spectra underlie color vision– cones in vertebrates, R7 and R8 in fly. Likewise, in both cases, information about motion is largely color-independent – notably that conveyed by vertebrate rods and fly R1-6. Discrete layers in the IPL, LGN, medulla and lobula complex are devoted to further processing of these features.
A main structural basis for parallel processing is the organization of synaptic connections into parallel laminae. Afferent and efferent processes of each subtype confine their arbors, and therefore their synapses, to distinct strata, at least some of which are sandwiched together in somata-free neuropils – the inner and outer plexiform layer and the fly medulla. The specific connections formed in these laminae describe a set of pathways by which visual information is transformed in multiple ways and passed to the brain for integration and further processing. In short, lamina-specific connectivity underlies parallel processing both in a literal sense and as used in computer architecture (Figure 7).
DEVELOPMENT OF VISUAL CIRCUITRY
Given the anatomical and physiological similarities between the vertebrate and fly visual systems, one might imagine that they share a common evolutionary origin and thus common underlying genetic regulatory programs. Nevertheless, the differences in eye structures led to general acceptance, from Darwin through to the 1990s, of the view that the fly and vertebrate eyes arose independently, with their similarities reflecting convergent evolution. The discovery in the mid-1990s that vertebrate transcription factor Pax6 and its fly orthologue, eyeless, are crucial for eye development reawakened enthusiasm for the notion that visual systems share a common evolutionary origin (Quiring et al 1994; Halder et al. 1995; Gehring, 2004; Lamb, 2009).
Building on this foundation, further similarities in the assembly of the fly and vertebrate visual systems have been documented over the past decade. Here, we discuss mechanisms for diversification of neuronal subtypes, formation of modules (i.e. columns and mosaics) and the segregation of connections into discrete layers. As these are still early days and molecular information remains fragmentary, strong arguments cannot yet be made about which similarities reflect common origin and which result from convergence. Whatever their origin, however, we believe that the parallels are intriguing and instructive.
TRANSCRIPTIONAL PROGRAMS FOR SUBTYPE DIVERSIFICATION
Subsequent to discovery of the Pax6/eyeless parallel, further similarities were found in the transcriptional programs that regulate eye formation and specify its main cell types. They include vertebrate Six3 and fly So for early eye and optic lobe primordia; vertebrate Otx/Crx and fly otd for photoreceptor cell development; vertebrate Chx10 and fly Vsx for interneuronal (bipolar and transmedullary) development; vertebrate Brn3 and fly Acj6 in RGCs and lobula neurons; and various basic helix-loop-helix (bHLH) genes expressed widely in developing precursors and differentiating neurons (Pichaud and Desplan, 2002; Ohsawa and Kageyama, 2008; Erclik et al. 2008, 2009; Harada et al., 2007; Hennig et al., 2008; Agathocleous and Harris, 2009).
Much less is known about the transcriptional programs that regulate the diversification of the main cell types to generate multiple subtypes (e.g., Figure 5). Some progress has been made, however, in learning how photoreceptors diversify (Morante et al., 2007). In flies, all of the R1-R8 photoreceptor types are generated from common pluripotent pool of cells through local cellular interactions (Ready et al. 1976; Reinke and Zipursky, 1988). These interactions play crucial roles in modulating the transcriptional regulatory circuitry specifying the fate and position of each cell within the ommatidial unit. These pathways have been reviewed in detail previously (Zipursky and Rubin, 1994; Freeman, 1997; Nagaraj and Banerjee, 2004; Doroquez and Rebay, 2006). Here, we describe more recent studies examining specific intrinsic determinants of photoreceptor subtype specificity.
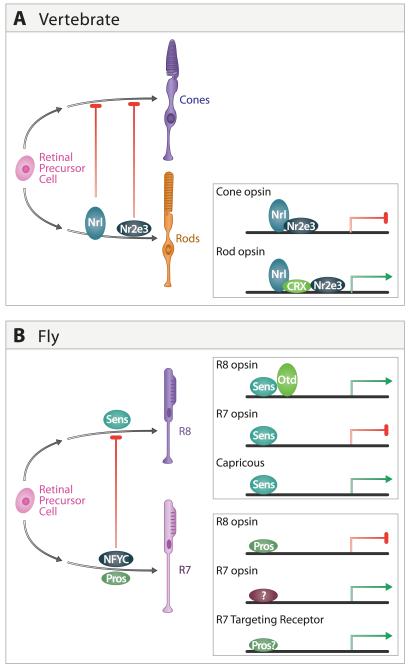
In flies, there is an intimate relationship between the transcription pathways regulating development of R7 and R8, two photoreceptor subtypes that share common features of development, mediate a chromatic pathway, and form connections in the medulla (Figure 8B). For instance, the transcription factor NFYC plays a crucial role in regulating the development of R7 neurons by actively repressing an R8 pathway of differentiation. The initial steps in R7 development are normal in NFYC mutants, but expression of the R8 opsin is de-repressed and mutant neurons terminate within the R8-recipient layer in the medulla (Morey et al. 2008). NF-YC executes this function by preventing R7 from expressing an R8-specific transcription factor, Senseless. In normal R8 cells, Senseless directly activates transcription of R8 opsins, as a part of a protein complex requiring the Otd transcription factor, which represses the expression of R7 opsins and controls the expression of cell surface molecules that regulate R8 target layer specificity (Xie et al. 2007; Morey et al. 2008). Another transcription factor, Prospero, acts through a parallel pathway to actively repress R8 opsin expression in R7, and prevent R7 from targeting to the M3 layer (Kauffmann et al, 1996; Xie et al, 2007; Miller et al, 2008; Morey et al. 2008). Thus, programs of photoreceptor subclass diversification involve the active repression of alternative pathways giving rise to closely related subtypes.
Figure 8. Transcriptional control of photoreceptor diversification.
A. Regulation of photoreceptor subtype identity in the mouse retina. Nrl and Nr2e3 promote rod development and repress cone cell programs in rods. These transcription factors also regulate photoreceptor subtype-specific expression of opsins in rods and are likely to regulate other genes controlling rod and cone cell development (adapted from Onishi et al. 2009).
B. Regulation of photoreceptor subtype in fly retina. NFYC represses R8 development in R7 by preventing Senseless expression. Senseless regulates R8 and R7 subtype-specific opsin expression and has also been implicated in expression of Capricious and other genes regulating R8 targeting. Prospero also regulates opsin expression and acts in parallel with other transcription factors to regulate R7 targeting. R7 and R8 subtypes can be further divided by the opsins they express (see text).
A similar developmental relationship exists among classes of photoreceptors in the vertebrate eye (Hennig et al, 2008; Onishi et al., 2009; Figure 8A). Although lineage relations remain incompletely defined (Cayouette et al., 2006), rods and cones appear to be generated from a common set of precursors and recent genetic studies suggests that development of rods occurs, at least in part, by repressing an alternative cone cell fate. For instance, loss of Nrl (neural retina leucine zipper protein) leads to loss of rods and increased cones (Nishiguchi et al., 2004; Mears et al. 2001), while widespread expression of Nrl generates an all rod retina (Oh et al. 2007), presumably by converting cones to rods. In rods, Nr2e3 acts downstream of both Nrl and Crx, the mouse homologue of Otd, to activate rod opsin and repress expression of cone opsins (Cheng et al. 2004; Peng et al. 2005). An accessory factor, Pias3, acts to repress cone and activate rod cell pathways (Onishi et al. 2009). Interestingly, Senseless in fly and Nrl and Nr2e3 in mouse both act in conjunction with members of the Otx transcription factor family to regulate photoreceptor subtype-specific expression (Xie et al. 2007; Hennig et al. 2008), suggesting evolutionary conservation in the molecular mechanisms regulating photoreceptor cell specialization.
A close relationship also appears to exist between the molecular control of different cone cell opsins in the mouse (S and M opsins; Hennig et al. 2008). Two members of the steroid hormone receptor family, Rxrγ (retinoic acid receptor family) and Trβ2 (thyroid hormone receptor family), regulate cone opsins. Based on genetic and biochemical studies, it has been hypothesized that in the mouse retina Rxrγ/Trβ2 heterodimers repress S-opsins whileTrβ2 homodimers activate M-opsins (Roberts et al. 2006).
While distinct retinal cell types can arise through determinative mechanisms (e.g. R8 induces R7 differentiation), they can also arise through stochastic processes. R7 neurons express either Rh3 or Rh4 opsin genes but not both; similarly R8 expresses either Rh5 or Rh6 (Chou et al, 1999; Morante et al. 2007). The choice is controlled by the stochastic expression of the transcription factor Spineless in a fraction of R7s (Wernet et al. 2006). Spineless promotes Rh4 expression and prevents Rh3 expression in R7, and represses a signal produced in R7 needed to induce Rh5 and inhibit Rh6 expression expression in the neighboring R8 cell. As such, in each ommatidium R7 and R8 exhibit paired expression of Rh3 and Rh5 or Rh4 and Rh6. The mutually exclusive expression of red and green opsins in the human retina also relies on a stochastic mechanism, albeit a very differentone. Red and green opsin genes are tandemly arranged on the X-chromosome and transcription of both genes is controlled by a single locus control region. A single such region can promote expression from only one gene at a time, thereby activating expression of red and green opsins in a mutually exclusive fashion (Nathans et al. 1989; Wang et al. 1992). As each cone expresses genes from only a single X-chromosome, mutually exclusive expression of red and green opsin is insured.
Together, these studies suggest that transcriptional switches among a limited number of alternative cellular states may provide a mechanism for the diversification of neuronal subtypes. The mechanisms controlling these switches remain poorly understood, but elegant studies by Jessell and others on specification of neuronal subtypes in the vertebrate spinal cord provide a conceptual basis for thinking about the problem (Briscoe and Novitch, 2008; Dalla Torre di Sanguinetto et al. 2008). Here, numerous transcriptional regulators have been identified that act in combinatorial and cross-regulatory fashions to specify interneurons and motor neurons from common progenitors, and then diversify each major class into numerous subtypes. Moreover, in a few cases, links have been made between transcriptional programs that specify cell types and the downstream genes that specify their connectivity (Kania and Jessell, 2003). We anticipate that in both vertebrates and flies, broadly similar transcriptional programs will diversify major cell types into subtypes and specify the “hard-wired” portions of their dendritic morphologies, synaptic specificities and physiological properties.
FORMATION AND CONNECTION OF MOSAICS AND COLUMNS
Formation of mosaics in vertebrates
As described above, cells in the vertebrate retina are arranged in mosaics, characterized by more regular spacing than would be expected by chance alone. The mechanisms by which mosaics arise must account for two key features. First, in nearly all cases, each mosaic is independent of others (Rockhill et al., 2000; Figure 2A), indicating a prime role for highly cell type-specific interactions. Consistent with this idea, manipulations that lead to loss of one cell type generally fail to disrupt mosaic formation by other cell types. Second, mosaics are present soon after neurons have reached proper layers and begun to make connections, although their regularity may increase at later stages (Novelli et al., 2005). Thus, mosaics are likely to arise through activity- and experience-independent processes.
Three sets of interactions have been proposed that may promote mosaic formation, and there is some evidence in favor of each of them. One is that regularity arises from control of neurogenesis by lateral inhibitory interactions. Indeed, cells of particular types emit signals that prevent nearby progenitors or neuroblasts from adopting the same fate (Waid and McLoon, 2001) and some of these signals may depend on Notch-mediated lateral interactions, as also occurs in Drosophila retina (Silva et al., 2003; Jadhav et al., 2006). Second, neurons might migrate laterally to maximize their distances from each other. Indeed, although new-born retinal neurons migrate in a primarily radial direction from the ventricular zone to the ganglion cell or inner nuclear layer, there is also some tangential dispersion within layers. Repellent homotypic interactions among neurons could transform an initially random pattern into one in which somata of a single type are regularly spaced. These interactions could be mediated by soluble factors or by contact among processes (Huckfeldt et al., 2009). Finally, retinal neurons, like vertebrate neurons generally, are over-produced, with the excess being eliminated by naturally occurring cell death (Pequignot et al., 2003). Indeed, selective elimination of near neighbors appears to increase regularity of spacing in some retinal populations (Raven et al., 2003; Resta et al., 2005).
Thus, lateral interactions that generate mosaics could influence generation, migration and death of neurons. Unfortunately, neither experimental nor modeling (Eglen, 2006) approaches have provided a satisfactory explanation of the relative importance of the various factors. Once molecules have been identified that mediate homotypic inhibitory interactions, it will be possible to use gain- and loss-of-function approaches to more deeply analyze the developmental mechanisms.
In considering how mosaics form, it is important to distinguish this process from a related phenomenon, tiling. Tiling, a prominent feature of many sensory systems, refers to the non-overlapping coverage of territory by dendritic or axonal arbors of neighboring cells. Typically, processes of adjacent neurons meet at their tips. In that case, the homotypic interactions that mediate tiling could also play a role in mosaic distribution of somata. Indeed, tiling is apparent in several retinal cell types, which are said to have a “coverage factor” of 1 - that is, each region of a layer is occupied by processes of just a single cell of the neuronal subtype in question. More often, however, the diameter of the dendritic arbor is greater than the distance between somata; dendrites therefore overlap, and the coverage factor is >1. These subtypes exhibit mosaic arrangements but not tiling, demonstrating that the latter is not the cause of the former.
Formation of columns in flies
While the vertebrate eye forms from a single primordium, the equivalent regions of the fly visual system (retina, lamina and medulla) are derived from two primordia: the retina arises from an epithelial sheet called the eye imaginal disc, while the lamina and outer medulla are derived from neuroblasts localized within the optic lobe of the brain (Meinertzhagen and Hanson, 1993). These two structures are joined by a tube, called the optic stalk.
Emergence of the columnar arrangement of the Drosophila visual system begins with the assembly of retinal ommatidia, a process controlled by local interactions among neighboring cells. The first ommatidia form in the posterior region of the imaginal disc and this is followed by wave of ommatidial assembly across the disc from posterior to anterior. As photoreceptor cells differentiate, they send axons down the optic stalk and into the brain.
As each R cell axon bundle enters the brain it becomes associated with a small group of lamina primordial cells (Meinertzhagen and Hanson, 1993). R cell axons promote the final division of these cells through the release of Hedgehog (Huang and Kunes, 1996, 1998; Umetsu et al., 2006) and induce their differentiation by secreting an EGF-like protein (Huang et al., 1998). These signals induce L1-L5 in the lamina cartridge that will lie directly beneath them in the adult eye. The lamina axons then extend along the R7 and R8 axons from the ommatidium above them and into incipient medulla columns. Thus, the precise matching of ommatidia to cartridges to columns is a consequence of local interactions between cells, between axons and cells, and among axons. The terminals and branches of L1-L5, R7 and R8 remain restricted to a single column and thus tile the medulla neuropil.
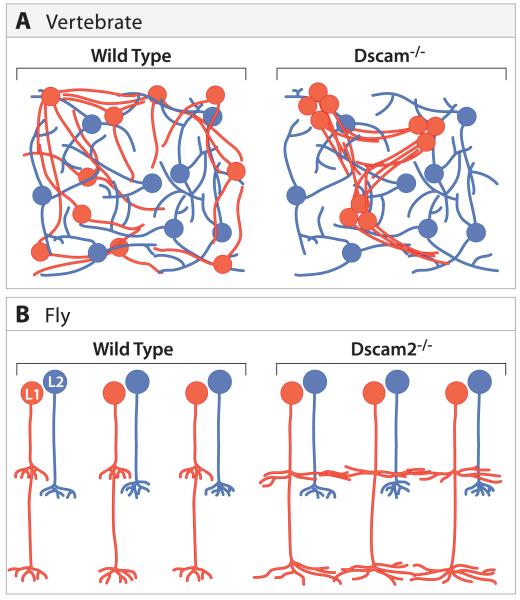
Dscam and repellent lateral interactions
Mosaic formation and tiling appear to depend on homotypic repellent interactions. Recently, Dscam proteins have emerged as candidate mediators of these interactions in both vertebrates and flies. Dscams are large immunoglobulin superfamily molecules that mediate homophilic adhesion; there are 4 Dscam genes in flies and 2 in mice and chicks. Fly Dscam1 exhibits extraordinary diversity as a result of alternative splicing, but the other 3 fly and 2 vertebrate Dscam genes are quite ordinary (Hattori et al., 2008).
In flies, mutant analysis indicates that Dscam2 mediates tiling between neighboring L1 neurons in the medulla (Figure 9B). These defects are highly specific as Dscam2 mutant R7, R8 and L2 neurons tile normally in layers M6, M3 and M2, respectively (Millard et al., 2007). Taken together, these data support a simple model in which Dscam2 on the surface of adjacent L1 neurons promotes tiling through a homophilic repulsive interaction.
Figure 9. Dscam proteins mediates homotypic repellent interactions required for tiling of visual interneurons.
A. Dscam is expressed in several amacrine cell subsets in mouse retina. In mutants lacking DscamSCAM, processes of these cells fasciculate and, in consequence, their mosaic arrangement is perturbed. Other amacrine subtypes, which did not express Dscam, are unaffected.
B. Dscam2 is required selectively in L1 neurons for tiling in the medulla. Terminals of L1 neurons lacking Dscam2 extend into neighboring cartridges. Tiling of L2 axon terminals is normal in Dscam2 mutants.
Recent studies in the mouse retina raise the interesting possibility that Dscam’s function in promoting repulsive interactions is evolutionarily conserved (Figure 9A). Mouse Dscam plays an important role in controlling the arrangement of neurites of two classes of amacrine cells found in different layers. Loss of Dscam leads to a marked disruption in these patterns, with neurites of a single cell, and of cells in a single class, forming large fascicles (Fuerst et al., 2008). Loss of Dscam-Like-1, the other vertebrate Dscam, leads to similar effects on other populations (Fuerst et al., 2009). These results suggest that DscamSCAM antagonizes adhesion between processes of the same cell, as well as interactions between processes of different cells of the same class, and may do so through homophilic repulsive interactions. These defects in fasciculation lead secondarily to disruption in mosaic spacing, but genes involved directly in mosaic formation remain to be identified.
Forming vertical connections among layers
To transfer an orderly map of visual space from the eye to the brain, each structure projects to the next processing station in visuotopic register –for example, retina to LGN to cortex in vertebrates, medulla to lobula and beyond in flies. While nothing is known about how the maps between the medulla and lobula form, the task of transferring a more-or-less continuous retinal map to the LGN and tectum/colliculus has been studied in great detail. In brief, Sperry (1963) initially conceived of a set of graded labels on retinal axons that would guide the axons to retinotopically appropriate sites in the tectum, and perhaps also bias synapse formation in favor of appropriately placed postsynaptic partners. Bonhoeffer and colleages devised elegant assays that allowed them to elucidate the cell biological basis of this guidance, and ultimately to isolation of the first “gradient molecule” ephrinA5 by Bonhoeffer and, independently by Flanagan and colleagues (Drescher et al., 1995; Cheng et al., 1995; Loschinger et al., 2000). The current view is that map-making depends critically on graded distributions of Eph kinases on retinal axons and of their ligands, the ephrins, in the tectum. Similar Eph-ephrin interactions may transfer the maps to the cortex and beyond. This area has been reviewed recently and will not be covered further here (McLaughlin and O’Leary, 2005; Huberman et al., 2008; Pasquale, 2008).
SELF-ASSEMBLY OF LAMINAE
The laminar organization of visual circuits has facilitated studies of their development. First, because synapses of a particular type are confined to a narrow plane, synaptic specificity can be assessed visually. This situation is vastly different from that in, say, the cerebral cortex, where synapses of a given type are widely dispersed in three dimensions. Second, because synapses of many different types form in closely apposed laminae, synaptic specificity can be analyzed separate from the related but distinct phenomena of axon guidance and neuronal migration. And third, because neuronal subtypes process information in different ways, their lamina-specific arbors directly relate neuronal structure to neuronal function. Accordingly, knowledge about the development of laminar specificity provides insight into the ontogeny of neural processing.
Just as there are structural similarities between laminar patterns in the vertebrate IPL and fly medulla, there are similarities in the formation of these structures. Most recently, common features have emerged from single cell studies in flies and vertebrates. In flies these studies are made possible by the MARCM technique and cell specific markers (Lee and Luo, 1999). In zebrafish and mice, the growing tool-box of cell-type specific GFP markers permits identification of cell subclasses and analysis of their development (Luo et al., 2008). In zebrafish, it is even possible to follow cells throughout development using live cell imaging techniques (Mumm et al., 2006), a capability largely unavailable for flies and mice.
Development of synaptic layers in the IPL
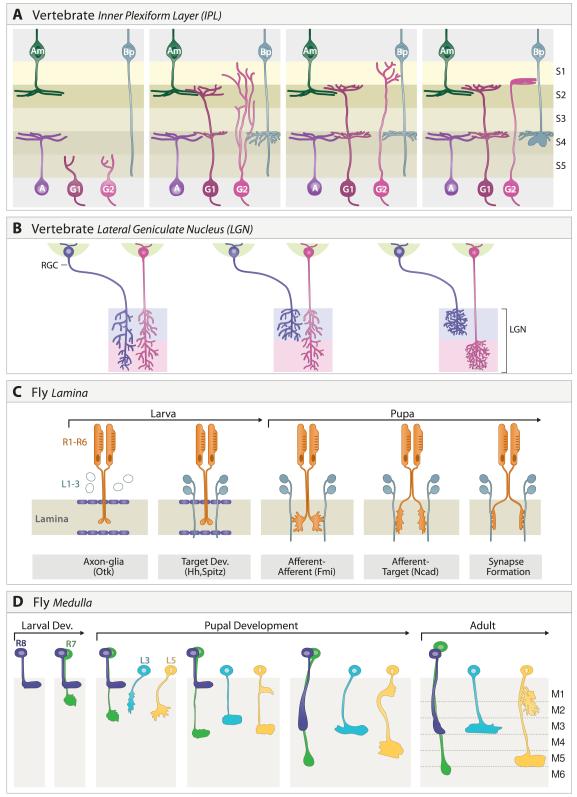
The IPL contains neuropil but no neuronal somata or potential guidepost cells. It forms as processes of interneurons and RGCs grow between the layer of early-born RGC somata and an initially diffuse layer of neuroblasts and postmitotic interneurons (Figure 10A) (Agathocleous and Harris, 2009). Accordingly, its formation involves self-assembly rather than recognition of a preexisting scaffold by incoming axons or dendrites. In this respect, the IPL differs from other laminated structures such as the cortex or tectum, in which incoming processes encounter and presumably recognize somata or processes that are already laminated (Sanes and Yamagata, 2009).
Figure 10. Formation of lamina-specific connections.
A. Inner plexiform layer. Processes of many amacrine cells ramify from the outset in appropriate sublaminae. Development of RGC dendrites exhibits subtype-specific variations: arbors of some types are initially diffuse then remodel, other are lamina-restricted from an early stage, and still others develop in discrete steps. The first two are shown here. Bipolar cells are born and extend axons late; their processes initially span multiple sublaminae, then become lamina-restricted.
B. Lateral geniculate nucleus. Axons from the ipsi- and contralateral eyes initially arborize broadly. Activity-dependent processes promote refinement to appropriate target laminae.
C. Fly lamina. The growth cones of R1-6 axons from the same ommatidium initially terminate in a tight cluster in a temporary layer bounded by glia. Interactions between growth cones promote extension away from one another in defined orientations where each projects to a different set of lamina neurons in surrounding cartridges and forms synapses with them. Some of the molecules implicated in discrete steps are indicated.
D. Fly medulla. The axons of R7, R8, L3 and L5 exhibit cell type-specific behaviors as they form lamina-specific terminals in the medulla.
Among the first processes to enter the IPL are those of early-born amacrine cells. Our clearest view of these events come from studies in zebrafish by Wong and colleagues, who were able to image GFP-labeled processes in live embryos. Amacrines target proper lamina from the outset (Godinho et al., 2005), even when RGCs have been genetically eliminated (Kay et al., 2004), suggesting that they do so by interactions with each other. An inference from this pattern is that amacrine processes may provide a scaffold on which RGC and bipolar processes laminate. One particular amacrine type, called starburst amacrines, are born and laminate very early (Stacy and Wong, 2003; Voinescu et al., 2009) and may play an especially important role. Consistent with this idea, dendrites of an RGC subset that receives input from starbursts become lamina-restricted earlier than other subsets studied to date (Kim et al., 2010). Attempts to test this idea by early ablation of amacrines have so far given disappointing results (Reese et al., 2001), but the manipulations may have occurred after RGC dendrites had already received their instructions.
Although RGC are the first-born retinal neurons, they extend dendritic processes into the IPL along with or even after those of amacrine cells. Arriving in the IPL, RGC dendrites therefore encounter processes of early-born amacrine subsets. The extent to which RGC dendrites arborize in a lamina-specific fashion from the outset has been unclear. Early studies in mammals suggested that RGC dendrites initially spanned multiple sublaminae and were then refined (Bodnarenko et al., 1995). Refinements appeared to be activity-dependent in that they are perturbed by blockade of excitatory or inhibitory neurotransmission, by dark-rearing, or by interference with neurotrophin signaling (Bodnarenko et al., 1995, 1999; Tian and Copenhagen, 2001, 2003; Xu and Tian, 2007). In zebrafish, on the other hand, at least some RGC dendrites arborize in appropriate laminae from the outset (Mumm et al., 2006). The resolution to this complexity may be that dendritic behavior is subtype-specific. Recent analysis in mice with transgenically marked RGC subsets has revealed one subset in which initially diffuse dendrites refine to a singly sublamina during development, other subsets in which arbors appear to be lamina-specified as soon as they can be visualized, and still others in which bistratified dendrites arborize in one lamina initially and the other only after a delay (Kim et al., 2010).
Last to enter the IPL are processes of bipolar neurons. In zebrafish and mice, bipolars initially extend processes that span the entire depth of the developing IPL and form small extensions in multiple laminae. This is followed by profuse but selective branching, resulting eventually in restriction of terminals to specific sublaminae (Morgan et al., 2006; Schroeter et al., 2006). In mice, interference with synaptic transmission alters the numbers of synapses that the bipolars form, but has no detectable effect on their laminar restriction (Kerschensteiner et al., 2009). Thus, bipolars seem to engage in limited rearrangement as they find proper lamina within which to arborize.
Development of synaptic layers in the lateral geniculate nucleus
In mammals, the best-studied target of retinal axons from the perspective of laminar specificity is the LGN. In particular, the LGN has been a test-bed for models of how correlated synaptic activity might influence synaptic specificity.
In the adult, axons arising from the ipsilateral and contralateral eyes are confined to distinct, alternating laminae within the LGN. Initially, however, individual retinal axons arising from either eye span laminae in which ipsilateral and contralateral axons will eventually terminate (Rakic, 1976). Specificity arises as axons both eliminate branches in inappropriate laminae and enlarge their arbors in appropriate laminae (Shatz, 1996)(Figure 10B).
Several studies indicated that this rearrangement requires a competitive interaction between RGCs from the two eyes, and that the competition is based on electrical activity in the RGCs. For example, segregation fails to occur if one eye is removed or if RGC activity is inhibited pharmacologically (Sretavan et al. 1988). Oddly, segregation occurs before photoreceptors form and the circuit becomes light responsive. It turns out, however, that RGCs are spontaneously active, with waves of activity passing across the retina (Galli and Maffei, 1988; Maffei and Galli-Resta, 1990; Meister et al., 1991). Thus, neighboring RGCs are much more likely to fire in synchrony with each other than with RGCs in the other eye. This pattern could underlie a Hebbian mechanism in which “neurons that fire together wire together”. Indeed, interference with the correlated waves of spontaneous intraretinal activity blocks segregation of RGC axons to single LGN laminae (Penn et al., 1998; Feller, 2009). Moreover, there is excellent evidence that another, related rearrangement, sharpening of the retinotopic map, requires correlated activity (Brickley et al., 1998; Yates et al., 2004).
Despite strong evidence for this view, it remains somewhat controversial, with some data supporting the idea that activity per se is required for segregation but that its precise pattern is not instructive (Huberman et al, 2003; Sun et al., 2008; Chalupa, 2009). The issue is further complicated by recent studies revealing that visual experience at a late stage is capable of remodeling axonal arbors (Hooks and Chen, 2007). Moreover, it seems likely that molecular recognition is involved in the stereotyped matching of ipsilateral and contralateral axons to particular laminae. Nonetheless, there is little doubt that activity in some form is critical for formation of RGC arbors in the LGN.
Development of synaptic layers in the lamina
Genetic studies have provided insights into the specific steps and, in some cases, the molecular mechanisms that underlie formation of connections between R1-R6 photoreceptors and neurons in the lamina (Figure 10C). As noted above, R cell axons provide anterograde signals that drive lamina neuron precursor proliferation and differentiation. An early step in axon ingrowth is regulated by glial cells, which flank the distal and proximal boundaries of the incipient lamina neuropil. These glia serve as transient targets that provide a yet-to-be identified stop signal preferentially recognized by R1-R6 growth cones (Poeck et al., 2001). In parallel, cells in the developing lamina secrete the wingless family member, Wnt4, which serves as an attractant for ventral R cell axons (Sato et al. 2006). Thus, signals produced both by afferents and targets play an important role at early steps in lamina assembly.
An unexpected result of developmental and genetic studies is that afferent/afferent interactions play a crucial role in synaptic specificity in the lamina (Clandinin and Zipursky, 2000). Selective removal of one discrete subset of R1-R6 neurons but not another disrupted the targeting of the remaining R1-R6 neurons. Initially, the axons of R1-R6 neurons from the same ommatidium form a tight fascicle with their growth cones terminating in a clustered arrangement associated with the five lamina neurons that form one cartridge. R1-R6 growth cones then defasciculate, with each targeting to a different lamina cartridge. As described earlier, a consequence of this rearrangement is that each cartridge receives innervation from 6 different R cells that “see” the same point in space (Figure 6C). As in earlier stages, afferent interactions do not act in isolation; axon-target interactions function in parallel to promote proper connectivity (Prakash et al., 2005).
Together these findings argue that lamina circuits emerge through a sequence of local interactions among processes of different cells types, not only afferents and targets as expected, but also between discrete subsets of afferents themselves.
Development of synaptic layers in the medulla
The neuropil of the fly medulla, like that of the vertebrate IPL, forms through a process of accretion or self-assembly, with the layered structure emerging through a multistep process (Figure 10D). At an early stage, R7, R8 and L1-L5 growth cones enter the developing medulla in a defined sequence and stack upon one another in a precise fashion that does not correlate strictly with their order of entry into columns (Nern et al, 2008; Petrovic and Hummel, 2008). Growth cones overlap extensively at this time, and their filopodial processes extend the entire depth of the incipient target layers, M1-M6. Subsequently, some growth cones swap positions and interstitial branches form at discrete sites. For instance, the R8 growth cone lies distal to the L2 growth cone early, but proximal to it later in development. Later still, L5 branches extend along the L2 growth cone into the incipient M2 layer.
Targeting to different layers occurs in a cell-type rather than a layer-specific fashion. Indeed, different neurons project to the same layer at different times (Figure 10D) and via different molecular mechanisms. For instance, R8 initially terminates distal to M1, then extends later to M3 (Ting et al, 2005). By contrast, L3 targets to the M3 layer from the outset (Nern et al, 2008).
Some of these recognition events are mediated by bidirectional signaling among processes of R cells and their targets. As mentioned above, early studies underscored the importance of anterograde signals from R cells that control lamina precursor proliferation and differentiation. More recent studies have shown that R7 and R8 neurons also express the secreted protein Jelly Belly, which is detected by the ALK receptor tyrosine kinase on processes of lamina and medulla neurons (Bazigou et al. 2007). ALK activation leads to changes in expression of cell recognition molecules in the immunoglobulin and cadherin superfamilies, which in turn may influence R cell growth cones. These studies argue that synaptic specificity relies on a complex interplay between processes in the neuropil and changes in gene expression in response to intercellular signals.
R7, R8 and L1-L5 axons target not only to specific layers but also to single columns. Diverse mechanisms restrict these projections, including autocrine suppression of growth cone motility (R7) (Ting et al. 2007), repulsive interactions between cells of the same class in adjacent columns (Millard et al. 2007; Ferguson et al., 2009), and adhesive interactions between different classes of cells in the same column (Nern et al 2008). As genetic tools become available for analyzing additional classes of neurons that synapse in the medulla, a more complete picture of the cellular dynamics leading to circuit assembly will emerge.
HOMOPHILIC ADHESION AND LAMINAR RECOGNITION
The cellular studies reviewed so far show that synaptic connections in the crowded confines of sublaminated structures are highly specific, that they form in a precise sequence of steps, and that many of these steps occur prior to and independent of visual experience. These features lead naturally to the hypothesis that synaptic specificity arises in large part from short-range interactions mediated by recognition molecules on the surfaces of retinal axons and their postsynaptic targets. Over the past decade, a few candidate mediators of these interactions have been identified in both flies and vertebrates. Several belong to the two major classes of proteins that have been implicated in cell-cell recognition generally, the cadherin and immunoglobulin (Ig) superfamilies (Shapiro et al. 2007; Takeichi 2007). More recently, a third family of recognition molecules, the leucine-rich repeat proteins, has received increasing attention (Kurusu et al., 2008; Linhoff et al., 2009). In many of these cases, the interactions appear to be homophilic. Here we describe several examples of cell recognition molecules that regulate laminar specificity in the IPL and medulla.
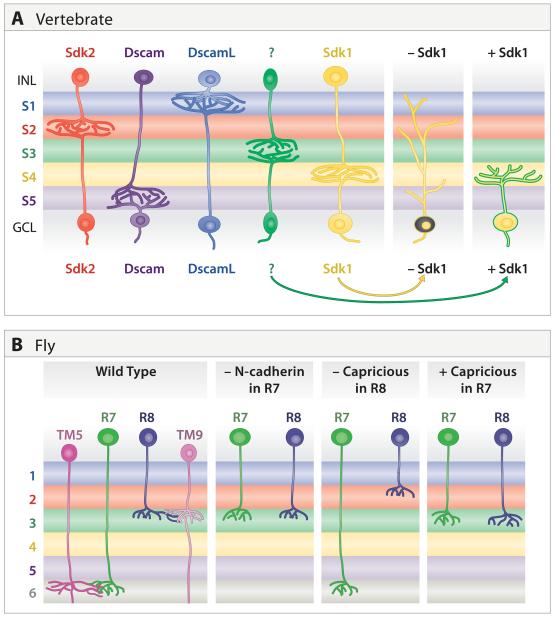
Immunoglobulin superfamily: Dscams and Sidekicks in the Chick IPL
A simple and appealing model for synaptic specificity would be one in which pre- and postsynaptic partners both expressed a single homophilic adhesion molecule. A mechanism of this type appears to underlie some aspects of lamina-specific connectivity in chick retina. Four closely related Ig superfamily members – Dscam, DscamL, Sidekick-1 and Sidekick-2 – are expressed by non-overlapping subsets of bipolar, amacrine and retinal ganglion cells that form synapses in distinct IPL sublaminae (Figure 11A). Each of the four proteins is concentrated within the appropriate sublaminae and each mediates homophilic adhesion. Loss- and gain-of-function studies in vivo indicated that these Ig superfamily members participate in determining the IPL sublaminae in which synaptic partners arborize and connect. Ectopic expression of any one of them in a small group of cells redirects their processes to the sublamina in which that IgSF is most prominent. Conversely, when expression of any of the four is decreased, by means of interfering RNAs, the processes wander beyond their appropriate sublaminae (Yamagata et al., 2002; Yamagata and Sanes, 2008). Synaptic localization and function of at least Sidekick appears to require its association with a scaffolding protein called MAGI that also binds other synaptic adhesion molecule; Sidekicks and Dscams might thereby recruit a multimolecular synaptogenic complex to appropriate contact sites (Yamagata and Sanes, 2010). Together, these results suggest the existence of an “Ig superfamily code” for laminar specificity in retina. Clearly this code has yet to be fully broken, however: only about half of the cells with processes in the IPL express any one of the four Sidekicks and Dscams, so other molecules, yet to be identified, must explain the behavior of the other half. Moreover, it is likely that each Sidekick and Dscam is expressed by multiple amacrine and RGC subtypes as defined morphologically, so other molecules must subdivide them and account for their subtype-specific behaviors. Taken together with the studies on mouse retina described above, these results suggest that Dscams may have both attractive and repulsive functions in retinal development, depending on the cell type, context, and possibly species in which they are expressed.
Figure 11. Homophilic interactions promote lamina-specific arborization and synapse formation.
A. In chick retina, non-overlapping subsets of interneurons and RGCs express one of four related immunoglobulin superfamily molecules: Sidekick 1, Sidekick 2, Dscam, and DscamL. Most of the pre- and postsynaptic cells expressing the same gene arborize in a distinct subset of inner plexiform sublaminae. Loss and gain of function studies support the idea that these genes promote lamina-specific synapse formation; as an example, sketches show results from manipulating Sidekick 1 levels in RGCs. Recognition molecules regulating the targeting of other subtypes (green) have not been identified.
B. In fly, N-cadherin and Capricious affect lamina-specific targeting of R7 and R8 terminals to appropriate medullar laminae. It is not known whether lamina-specific targeting requires expression of N-cadherin on TM5 and Capricious on TM9.
Cadherin superfamily: N-cadherin regulates R7 targeting in medulla
Classical cadherins have been widely assumed to play an important role in mediating the cellular recognition events underlying the formation of specific patterns of synaptic connectivity (Takeichi, 2007). It has been difficult to critically assess classical cadherin function in vertebrates due to redundancy on one hand and the early lethality associated with loss of classical cadherin function on the other. In contrast, subtle genetic manipulations in the fly visual system have provided strong evidence that N-cadherin plays distinct roles at multiple steps of circuit assembly (Figure 11B) (Lee et al, 2001; Nern et al., 2008).
Selective removal of N-cadherin from R7 leads to a highly specific phenotype: mutant R7 cells terminate in the M3 rather than the M6 layer, thus converting R7 to R8 targeting specificity. This specificity is surprising, given the widespread expression of N-cadherin in the developing medulla neuropil. As misexpression of N-cadherin in R8 does not re-direct R8 growth cones to M6, N-cadherin is necessary but not sufficient to determine R7 targeting specificity (Lee et al., 2001; Prakash et al., 2005; Ting et al., 2005).
Selective loss of function of N-cadherin in other cell types causes defects different from those seen in R7 neurons. For instance, removal of N-cadherin from L3 leads to a mistargeting phenotype opposite to that seen in R7; L3 neurons lacking N-cadherin no longer terminate in M3 but rather target to M6, the layer within which wild type R7s terminate (Nern et al., 2008). L2 neuron targeting requires N-cadherin in a cell non-autonomous fashion and L5 neurons require N-cadherin to extend interstitial branches from the M1 into the M2 layer. The precise spatiotemporal regulation of N-cadherin levels is surely critical for this diversity of function. An additional explanation is that N-cadherin may cooperate with different cell surface proteins to mediate distinct developmental steps. Consistent with this idea, mutations in the receptor tyrosine phosphatases Lar and Ptp69D have phenotypes similar to N-cadherin mutations in R7 but are not required in lamina neurons (Nern et al. 2008).
Cadherins play additional roles in other regions of both fly and vertebrate visual systems. In addition to its roles in medulla, fly N-cadherin is involved in targeting R1-R6 axons to the lamina (Lee et al., 2001). A protocadherin, flamingo, is required for R1-R6 axons to select the appropriate cartridge with in the lamina (Lee et al., 2003; Chen and Clandinin, 2008). Several classical cadherins are expressed in specific neuronal subsets in vertebrate retina and its central targets (Redies et al., 1998; Miskevich et al., 1998; Yamagata et al., 2006), suggesting the existence of a “cadherin code” for of retina-brain connectivity analogous to the intraretinal “Ig superfamily code” mentioned above. Supportining this idea, interference with N-cadherin mediates layer-specific targeting of chick RGC axons to the optic tectum (Inoue and Sanes, 1997). Finally, a cluster of 22 γ-protocadherins regulates survival and some physiological properties of neurons in the developing mouse retina (Lefebvre et al. 2008). Thus, cadherin superfamily members may play multiple roles in development of the visual system.
Leucine rich repeat proteins: Capricious plays an instructive role in R8 targeting
Three cell surface proteins have been identified which are necessary for R8 but dispensable for R7 targeting. They are Flamingo, Capricious and Golden Goal (Shinza-Kameda et al., 2006; Lee et al., 2003; Chen and Clandinin, 2008; Tomasi et al., 2008). Among these Capricious, a leucine-rich repeat protein, is particularly interesting as it is the only one that is sufficient to promote targeting to the M3 layer (Figure 11B). Capricious is expressed in R8 and in the region of the developing medulla neuropil to which R8 growth cones target (i.e. the incipient M3 layer), but is not expressed in R7 or in the layer to which R7 targets. This pattern raised the intriguing possibility that Capricious acts in an instructive fashion via homophilic adhesion to allow R8 to select the appropriate target layer. In support of this view, misexpression of Capricious in R7 results in targeting to the M3 layer (Shinza-Kameda et al., 2006). While it has been proposed that that this is due to a direct interaction between Capricious on both growth cones and target neurons this has not yet been rigorously assessed. Indeed, recent genetic studies demonstrated that while Capricious is required for olfactory targeting, it is likely that it functions non-homophilically (Hong et al., 2009). Nevertheless, these data indicate that Capricious acts in an instructive fashion to control targeting specificity within M3. As the chick homolog of Capricious, Lrrn, has been shown recently to regulate nerve muscle connectivity (Andreae et al. 2009), it will be particularly interesting to learn whether Lrrn functions in regulating targeting specificity in the IPL.
FLY AND VERTEBRATE VISUAL SYSTEM DEVELOPMENT COMPARED
Recent studies of the cellular and molecular mechanisms required for building visual systems have revealed numerous parallels between flies and vertebrates (Figures 8-11). Emerging themes include the following:
Neuron subclass diversity relies on modulation of common genetic pathways
Fly and vertebrate visual systems are both characterized by a remarkable degree of cellular specialization, mediated by an extraordinary diversification of basic cell types into subtypes. There are, for instance, some 26 different Tm neurons in the medulla and over 30 different types of amacrine cells in the mammalian retina. As diversification of different subclasses of photoreceptor neurons in the fly and the mouse rely on variations on common genetic programs, it is attractive to speculate that similar relationships may exist among programs regulating other related subclasses. Elucidating the genetic programs regulating the development of these closely related cell types may provide insights into the logic of cellular diversification and lead to the identification of genes regulating assembly of specific visual system circuits.
Repellent interactions space modules to ensure uniform coverage of the visual field
Flies and vertebrates both face the problem of ensuring that neurons are deployed through the visual field, so that there are no holes in coverage. They accomplish this goal, however, in different ways. In Drosophila, the retina is divided into ~750 ommatidia, and these modules pattern cartridges in the lamina, columns in the medulla and, perhaps, corresponding groupings in the lobula complex. In contrast, vertebrates use probabilistic methods to form mosaics of cell types, so each voxel in the retina corresponds only approximately to its neighbors in cell type composition. The projection of the retinal field onto subsequent stages -colliculus, LGN and cortex- is likewise an analog rather than a digital map, with graded distributions of molecules like ephrins generating a coarse correspondence and activity-dependent mechanisms sharpening the registration. Despite the different logic used by vertebrates and flies to form maps, however, repellent interactions play prominent roles in both.
Homotypic interactions mediate both adhesion and repulsion
Short-range, contact-dependent interactions appear to play the predominant role in assembling the vertebrate IPL and fly medulla. Both homotypic and heterotypic interactions are clearly involved but many of the molecules identified to date are homophilic. While homophilic binding has been traditionally viewed as adhesive in nature, it can also serve to elicit repulsion. Contact-dependent transient repulsion can prevent growth cones or branches from extending into a specific domain, redirect their processes, or initiate divergent process extension. Longer-lasting adhesion may promote growth of processes along specific surfaces, and the most stable adhesive contacts may promote synapse formation. As with traditional guidance receptors, it seems likely that homophilic binding of a single cell recognition molecule will have the capacity to promote different outputs depending upon its cellular context.
How many such homotypic molecules will be needed? Classic experiments have shown that cells can sort out from one another based on both the nature and the levels of the cell recognition proteins they express (Steinberg, 2007). Multiple proteins, like Sidekicks and Dscams, may act combinatorially to specify connectivity. However, cell-type specific modulation of a single protein may allow the same protein to mediate synaptic choices of different neurons at different times and places. For example, precise control of its levels, expression and subcellular localization appears to underlie the ability of N-cadherin to regulate many different developmental steps in the fly lamina and medulla.
CONCLUSION
Sermons commonly begin with a passage from a holy work; they proceed to develop the theme, then return to the text at the end. Like ministers, priests and rabbis, we end where we began, with Cajal’s Epistles to the Neuroscientists. A spate of new results has highlighted the structural similarities between the fly and vertebrate visual systems to which Cajal first drew attention (Figure 3). Moreover, a convergence of approaches is strengthening the case for functional and developmental similarities as well as structural ones. Most important, the advent of genetic, genomic and imaging technologies makes it possible to leverage knowledge obtained from one system to gain insights into the other.
Cajal argued that fly and vertebrate visual systems were essentially identical in key respects (Cajal and Sanchez, 1915). “If from the visual organ of the insect,” he wrote, “we discount the crucial fact of the dislocation of the soma…then the analogy between the visual apparatus [of the vertebrates and insects] converts almost in identity.” In one of the most remarkable drawings from that early era, he made his point by translocating the fly somata to a vertebrate position without altering the neuropil (Figure 3B). He noted that there were fundamental differences between the optical systems of flies and vertebrates, but argued that as regards “nervous organization … the essential plan was maintained with small variations and re-touches of adaptations.”
The prevailing view of naturalists at the time was that, despite their similarities, these structures could not share a common evolutionary origin. Today, we are more ready to accept that Cajal’s “singular structural concordances” across phyla imply evolution from a common urbilaterian ancestor. Such ancient and deeply conserved functions argue for fundamental molecular principles underlying the development of visual circuits, analogous to mechanisms regulating patterning of the embryonic axes.
ACKNOWLEDGEMENTS
We thank Renate Hellmiss for drawing the figures, Mark Frye, Markus Meister, Rachel Wong and Masahito Yamagata for helpful comments, and Eddy De Robertis for translating sections of Cajal’s studies from the Spanish. Work on visual system development in JRS’ lab is supported by the NIH. SLZ is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Andreae LC, Lumsden A, Gilthorpe JD. Chick Lrrn2, a novel downstream effector of Hoxb1 and Shh, functions in the selective targeting of rhombomere 4 motor neurons. Neural Dev. 2009;4:27. doi: 10.1186/1749-8104-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Hill RM, Levick WR. Retinal Ganglion Cells Responding Selectively to Direction and Speed of Image Motion in the Rabbit. J Physiol. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995;15:7037–7045. doi: 10.1523/JNEUROSCI.15-11-07037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A. Drosophila’s view on insect vision. Curr Biol. 2009;19:R36–47. doi: 10.1016/j.cub.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res. 1967;3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Dawes EA, Keating MJ, Grant S. Synchronizing retinal activity in both eyes disrupts binocular map development in the optic tectum. J Neurosci. 1998;18:1491–1504. doi: 10.1523/JNEUROSCI.18-04-01491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Novitch BG. Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos Trans R Soc Lond B Biol Sci. 2008;363:57–70. doi: 10.1098/rstb.2006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Cajal SR. Recollections of My Life. The MIT Press; Cambridge, MA, London, England: 1937. [Google Scholar]
- Cajal SR, Sanchez D. Contribucion al conocimiento de los centros nerviosos del los insectos. Trab. Lab. Invest. Biol. 1915;13:1–167. [Google Scholar]
- Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends Neurosci. 2006;29:563–570. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chalupa LM. Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:25. doi: 10.1186/1749-8104-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Gunhan E. Development of On and Off retinal pathways and retinogeniculate projections. Prog Retin Eye Res. 2004;23:31–51. doi: 10.1016/j.preteyeres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Chen PL, Clandinin TR. The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron. 2008;58:26–33. doi: 10.1016/j.neuron.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- Cheng H,J, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–81. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126:607–16. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- Dalla Torre di Sanguinetto SA, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr Opin Neurobiol. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Devries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. J Neurophysiol. 1997;78:2048–2060. doi: 10.1152/jn.1997.78.4.2048. [DOI] [PubMed] [Google Scholar]
- Doroquez DB, Rebay I. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross-talk. Crit Rev Biochem Mol Biol. 2006;41:339–385. doi: 10.1080/10409230600914344. [DOI] [PubMed] [Google Scholar]
- Douglass JK, Strausfeld NJ. Visual motion detection circuits in flies: peripheral motion computation by identified small-field retinotopic neurons. J Neurosci. 1995;15:5596–5611. doi: 10.1523/JNEUROSCI.15-08-05596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass JK, Strausfeld NJ. Visual motion-detection circuits in flies: parallel direction- and non-direction-sensitive pathways between the medulla and lobula plate. J Neurosci. 1996;16:4551–4562. doi: 10.1523/JNEUROSCI.16-15-04551.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–70. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Eglen SJ. Development of regular cellular spacing in the retina: theoretical models. Math Med Biol. 2006;23:79–99. doi: 10.1093/imammb/dql003. [DOI] [PubMed] [Google Scholar]
- Erclik T, Hartenstein V, Lipshitz HD, McInnes RR. Conserved role of the Vsx genes supports a monophyletic origin for bilaterian visual systems. Curr Biol. 2008;18:1278–1287. doi: 10.1016/j.cub.2008.07.076. [DOI] [PubMed] [Google Scholar]
- Erclik T, Hartenstein V, McInnes RR, Lipshitz HD. Eye evolution at high resolution: the neuron as a unit of homology. Dev Biol. 2009;332:70–79. doi: 10.1016/j.ydbio.2009.05.565. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Jr., Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K, Long H, Cameron S, Chang WT, Rao Y. The conserved Ig superfamily member turtle mediates axonal tiling in Drosophila. J Neurosci. 2009;29:14151–14159. doi: 10.1523/JNEUROSCI.2497-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach KF, Dittrich APM. The optic lobe of Drosophila Melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 1989;258:441–475. [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. J Comp Neurol. 2007;500:1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- Fryxell KJ, Meyerowitz EM. An opsin gene that is expressed only in the R7 photoreceptor cell of Drosophila. Embo J. 1987;6:443–451. doi: 10.1002/j.1460-2075.1987.tb04774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–97. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Gao S, Takemura SY, Ting CY, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong ST, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. Historical perspective on the development and evolution of eyes and photoreceptors. Int J Dev Biol. 2004;48:707–717. doi: 10.1387/ijdb.041900wg. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Penisten DK, DeVoe RD. Discrimination of visual motion from flicker by identified neurons in the medulla of the fleshfly Sarcophaga bullata. J Comp Physiol A. 1991;168:653–673. doi: 10.1007/BF00224356. [DOI] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Hardie RC. Electrophysiological analysis of the fly retina. I: Comparative properties of R1-R6 and R7 and 8. J. Comp. Physiol. 1979;129:19–33. [Google Scholar]
- Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol A. 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Inta D, Monyer H, Wassle H. Expression analysis of green fluorescent protein in retinal neurons of four transgenic mouse lines. Neuroscience. 2009;160:126–139. doi: 10.1016/j.neuroscience.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Hennig AK, Peng GH, Chen S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 2008;1192:114–133. doi: 10.1016/j.brainres.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer A, Drager UC. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J Comp Neurol. 1985;234:465–474. doi: 10.1002/cne.902340405. [DOI] [PubMed] [Google Scholar]
- Hong W, Zhu H, Potter CJ, Barsh G, Kurusu M, Zinn K, Luo L. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat Neurosci. 2009;12:1542–50. doi: 10.1038/nn.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Signals transmitted along retinal axons in Drosophila: Hedgehog signal reception and the cell circuitry of lamina cartridge assembly. Development. 1998;125:3753–3764. doi: 10.1242/dev.125.19.3753. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shilo BZ, Kunes S. A retinal axon fascicle uses spitz, an EGF receptor ligand, to construct a synaptic cartridge in the brain of Drosophila. Cell. 1998;95:693–703. doi: 10.1016/s0092-8674(00)81639-6. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wang GY, Liets LC, Collins OA, Chapman B, Chalupa LM. Eye-specific retinogeniculate segregation independent of normal neuronal activity. Science. 2003;300:994–998. doi: 10.1126/science.1080694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckfeldt RM, Schubert T, Morgan JL, Godinho L, Di Cristo G, Huang ZJ, Wong RO. Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat Neurosci. 2009;12:35–43. doi: 10.1038/nn.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Joesch M, Plett J, Borst A, Reiff DF. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr Biol. 2008;18:368–374. doi: 10.1016/j.cub.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–96. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Brecha N. Localization of neuroactive substances in the vertebrate retina: evidence for lamination in the inner plexiform layer. Vision Res. 1983;23:1197–1205. doi: 10.1016/0042-6989(83)90033-0. [DOI] [PubMed] [Google Scholar]
- Katsov AY, Clandinin TR. Motion processing streams in Drosophila are behaviorally specialized. Neuron. 2008;59:322–335. doi: 10.1016/j.neuron.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann RC, Li S, Gallagher PA, Zhang J, Carthew RW. Ras1 signaling and transcriptional competence in the R7 cell of Drosophila. Genes Dev. 1996;10:2167–2178. doi: 10.1101/gad.10.17.2167. [DOI] [PubMed] [Google Scholar]
- Kay JN, Roeser T, Mumm JS, Godinho L, Mrejeru A, Wong RO, Baier H. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development. 2004;131:1331–1342. doi: 10.1242/dev.01040. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kim I-J, Zhang Y, Meister M, Sanes JR. Laminar Restriction of Retinal Ganglion Cell Dendrites and Axons: Subtype-Specific Developmental Patterns Revealed With Transgenic Markers. J. Neuroscience. 2010 doi: 10.1523/JNEUROSCI.4779-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MD, Jan LY, Jan YN. The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 2006;20:2806–19. doi: 10.1101/gad.1459706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Karten HJ. Neuroactive peptides as markers of retinal ganglion cell populations that differ in anatomical organization and function. Vis Neurosci. 1988;1:73–81. doi: 10.1017/s0952523800001024. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;59:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD. Evolution of vertebrate retinal photoreception. Philos Trans R Soc Lond B Biol Sci. 2009;364:2911–24. doi: 10.1098/rstb.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Lee RC, Clandinin TR, Lee CH, Chen PL, Meinertzhagen IA, Zipursky SL. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. 2003;6:557–563. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–4151. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nat Neurosci. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Li YN, Matsui JI, Dowling JE. Specificity of the horizontal cell-photoreceptor connections in the zebrafish (Danio rerio) retina. J Comp Neurol. 2009;516:442–453. doi: 10.1002/cne.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Masland RH. Populations of wide-field amacrine cells in the mouse retina. J Comp Neurol. 2006;499:797–809. doi: 10.1002/cne.21126. [DOI] [PubMed] [Google Scholar]
- Loschinger J, Weth F, Bonhoeffer F. Reading of concentration gradients by axonal growth cones. Philos Trans R Soc Lond B Biol Sci. 2000;355:971–982. doi: 10.1098/rstb.2000.0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Maffei L, Galli-Resta L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc Natl Acad Sci U S A. 1990;87:2861–2864. doi: 10.1073/pnas.87.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA. The synaptic populations of the fly’s optic neuropil and their dynamic regulation: Parallels with the vertebrate retina. Progress in Retinal Research. 1993;12:13–39. [Google Scholar]
- Meinertzhagen IA, Hanson TE. The development of the optic lobe. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila Melanogaster. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. pp. 1363–1941. [Google Scholar]
- Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Seymour H, King C, Herman TG. Loss of seven-up from Drosophila R1/R6 photoreceptors reveals a stochastic fate choice that is normally biased by Notch. Development. 2008;135:707–715. doi: 10.1242/dev.016386. [DOI] [PubMed] [Google Scholar]
- Miskevich F, Zhu Y, Ranscht B, Sanes JR. Expression of multiple cadherins and catenins in the chick optic tectum. Mol Cell Neurosci. 1998;12:240–255. doi: 10.1006/mcne.1998.0718. [DOI] [PubMed] [Google Scholar]
- Montell C, Jones K, Zuker C, Rubin G. A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. J Neurosci. 1987;7:1558–1566. doi: 10.1523/JNEUROSCI.07-05-01558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante J, Desplan C, Celik A. Generating patterned arrays of photoreceptors. Curr Opin Genet Dev. 2007;17:314–319. doi: 10.1016/j.gde.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Baier H, Wong RO. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U. The little R cell that could. Int J Dev Biol. 2004;48:755–760. doi: 10.1387/ijdb.041881rn. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J, Davenport CM, Maumenee IH, Lewis RA, Hejtmancik JF, Litt M, Lovrien E, Weleber R, Bachynski B, Zwas F, Klingaman R, Fishman G. Molecular Genetics of Human Blue Cone Monochromacy. Science. 1989;245:831–838. doi: 10.1126/science.2788922. [DOI] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr., Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Nern A, Zhu Y, Zipursky SL. Local N-cadherin interactions mediate distinct steps in the targeting of lamina neurons. Neuron. 2008;58:34–41. doi: 10.1016/j.neuron.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin LM, Taylor MR, Baier H. Hardwiring of fine synaptic layers in the zebrafish visual pathway. Neural Dev. 2008;3:36. doi: 10.1186/1749-8104-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol D, Meinertzhagen IA. An analysis of the number and composition of the synaptic populations formed by photoreceptors of the fly. J Comp Neurol. 1982;207:29–44. doi: 10.1002/cne.902070104. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, Zheng L, Wardill TJ, O’Kane CJ, de Polavieja GG, Juusola M. Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: II mechanisms. PLoS One. 2009;4:e4306. doi: 10.1371/journal.pone.0004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi KM, Friedman JS, Sandberg MA, Swaroop A, Berson EL, Dryja TP. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc Natl Acad Sci U S A. 2004;101:17819–17824. doi: 10.1073/pnas.0408183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli E, Resta V, Galli-Resta L. Mechanisms controlling the formation of retinal mosaics. Prog Brain Res. 2005;147:141–153. doi: 10.1016/S0079-6123(04)47011-3. [DOI] [PubMed] [Google Scholar]
- Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci U S A. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Onishi A, Peng GH, Hsu C, Alexis U, Chen S, Blackshaw S. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron. 2009;61:234–246. doi: 10.1016/j.neuron.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol. 2006;497:928–958. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science. 1967;155:841–842. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124:1665–1673. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Peng GH, Ahmad O, Ahmad F, Liu J, Chen S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet. 2005;14:747–764. doi: 10.1093/hmg/ddi070. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Pequignot MO, Provost AC, Salle S, Taupin P, Sainton KM, Marchant D, Martinou JC, Ameisen JC, Jais JP, Abitbol M. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003;228:231–238. doi: 10.1002/dvdy.10376. [DOI] [PubMed] [Google Scholar]
- Petrovic M, Hummel T. Temporal identity in axonal target layer recognition. Nature. 2008;456:800–803. doi: 10.1038/nature07407. [DOI] [PubMed] [Google Scholar]
- Pichaud F, Desplan C. Pax genes and eye organogenesis. Curr Opin Genet Dev. 2002;12:430–434. doi: 10.1016/s0959-437x(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Poeck B, Fischer S, Gunning D, Zipursky SL, Salecker I. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. 2001;29:99–113. doi: 10.1016/s0896-6273(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Raghu SV, Joesch M, Borst A, Reiff DF. Synaptic organization of lobula plate tangential cells in Drosophila: gamma-aminobutyric acid receptors and chemical release sites. J Comp Neurol. 2007;502:598–610. doi: 10.1002/cne.21319. [DOI] [PubMed] [Google Scholar]
- Raghu SV, Joesch M, Sigrist SJ, Borst A, Reiff DF. Synaptic organization of lobula plate tangential cells in Drosophila: Dalpha7 cholinergic receptors. J Neurogenet. 2009;23:200–209. doi: 10.1080/01677060802471684. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Raven MA, Eglen SJ, Ohab JJ, Reese BE. Determinants of the exclusion zone in dopaminergic amacrine cell mosaics. J Comp Neurol. 2003;461:123–136. doi: 10.1002/cne.10693. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Giannotti KA, Johnson PT. Development of cholinergic amacrine cell stratification in the ferret retina and the effects of early excitotoxic ablation. Vis Neurosci. 2001;18:559–570. doi: 10.1017/s0952523801184063. [DOI] [PubMed] [Google Scholar]
- Reinke R, Zipursky SL. Cell-cell interaction in the Drosophila retina: the bride of sevenless gene is required in photoreceptor cell R8 for R7 cell development. Cell. 1988;55:321–330. doi: 10.1016/0092-8674(88)90055-4. [DOI] [PubMed] [Google Scholar]
- Resta V, Novelli E, Di Virgilio F, Galli-Resta L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development. 2005;132:2873–2882. doi: 10.1242/dev.01855. [DOI] [PubMed] [Google Scholar]
- Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proc Natl Acad Sci U S A. 2000;97:2303–2307. doi: 10.1073/pnas.030413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Sachs GM, Schneider GE. The morphology of optic tract axons arborizing in the superior colliculus of the hamster. J Comp Neurol. 1984;230:155–167. doi: 10.1002/cne.902300202. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Sarthy PV. Histamine: a neurotransmitter candidate for Drosophila photoreceptors. J Neurochem. 1991;57:1757–1768. doi: 10.1111/j.1471-4159.1991.tb06378.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci. 2006;9:67–75. doi: 10.1038/nn1604. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Wong RO, Gregg RG. In vivo development of retinal ON-bipolar cell axonal terminals visualized in nyx::MYFP transgenic zebrafish. Vis Neurosci. 2006;23:833–843. doi: 10.1017/S0952523806230219. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Emergence of order in visual system development. Proc Natl Acad Sci U S A. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron. 2006;49:205–213. doi: 10.1016/j.neuron.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Siegert S, Scherf BG, Del Punta K, Didkovsky N, Heintz N, Roska B. Genetic address book for retinal cell types. Nat Neurosci. 2009;12:1197–1204. doi: 10.1038/nn.2370. [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the Orderly Growth of Nerve Fiber Patterns and Connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988;336:468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Wong RO. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456:154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Okamura JY. Visual system of calliphorid flies: organization of optic glomeruli and their lobula complex efferents. J Comp Neurol. 2007;500:166–188. doi: 10.1002/cne.21196. [DOI] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmajda BA, Grunert U, Martin PR. Retinal ganglion cell inputs to the koniocellular pathway. J Comp Neurol. 2008;510:251–268. doi: 10.1002/cne.21783. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Takemura SY, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Takahashi Y, Sato Y, Kawakami K, Takeichi M, Nakagawa S. Cadherin is required for dendritic morphogenesis and synaptic terminal organization of retinal horizontal cells. Development. 2006;133:4085–4096. doi: 10.1242/dev.02566. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O’Connor MB, Zipursky SL, Lee CH. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, Yonekura S, Chung P, Hsu SN, Robertson HM, Chiba A, Lee CH. Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development. 2005;132:953–963. doi: 10.1242/dev.01661. [DOI] [PubMed] [Google Scholar]
- Tomasi T, Hakeda-Suzuki S, Ohler S, Schleiffer A, Suzuki T. The transmembrane protein Golden goal regulates R8 photoreceptor axon-axon and axon-target interactions. Neuron. 2008;57:691–704. doi: 10.1016/j.neuron.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Trujillo-Cenoz O. Some aspects of the structural organization of the intermediate retina of dipterans. J Ultrastruct Res. 1965;13:1–33. doi: 10.1016/s0022-5320(65)80086-7. [DOI] [PubMed] [Google Scholar]
- Umetsu D, Murakami S, Sato M, Tabata T. The highly ordered assembly of retinal axons and their synaptic partners is regulated by Hedgehog/Single-minded in the Drosophila visual system. Development. 2006;133:791–800. doi: 10.1242/dev.02253. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Vigier P. Mécanisme de la synthèse des impressions lumineuses recuillies par les yeux composé des Diptères. C.R. Acad. Sci. 1909;148:1221–1223. [Google Scholar]
- Voinescu PE, Kay JN, Sanes JR. Birthdays of retinal amacrine cell subtypes are systematically related to their molecular identity and soma position. J Comp Neurol. 2009;517:737–750. doi: 10.1002/cne.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin KJ, Moreira EF, Huang H, Yu N, Adler R. Molecular dynamics of photoreceptor synapse formation in the developing chick retina. J Comp Neurol. 2008;506:822–837. doi: 10.1002/cne.21582. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–40. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wassle H. Decomposing a cone’s output (Parallel processing) In: Albright R.H.M.a.T.D., editor. The Senses: A Comprehensive Reference. Elsevier; Amsterdam: 2008. pp. 313–339. [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proc R Soc Lond B Biol Sci. 1978;200:441–461. doi: 10.1098/rspb.1978.0026. [DOI] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–80. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederman SD, Shoemaker PA, O’Carroll DC. A model for the detection of moving targets in visual clutter inspired by insect physiology. PLoS ONE. 2008;3:e2784. doi: 10.1371/journal.pone.0002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhrn JC, Puelles L, Nakagawa S, Takeichi M, Redies C. Cadherin expression in the retina and retinofugal pathways of the chicken embryo. J Comp Neurol. 1998;396:20–38. [PubMed] [Google Scholar]
- Xiao T, Baier H. Lamina-specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet. Nat Neurosci. 2007;10:1529–1537. doi: 10.1038/nn2002. [DOI] [PubMed] [Google Scholar]
- Xie B, Charlton-Perkins M, McDonald E, Gebelein B, Cook T. Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development. 2007;134:4243–4253. doi: 10.1242/dev.012781. [DOI] [PubMed] [Google Scholar]
- Xu HP, Tian N. Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye opening. J Comp Neurol. 2007;503:244–259. doi: 10.1002/cne.21379. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Target-independent diversification and target-specific projection of chemically defined retinal ganglion cell subsets. Development. 1995;121:3763–3776. doi: 10.1242/dev.121.11.3763. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Versican in the developing brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J Neurosci. 2005;25:8457–8467. doi: 10.1523/JNEUROSCI.1976-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Synaptic Localization And Function Of Sidekick Recognition Molecules Require Magi Scaffolding Proteins. J. Neuroscience. 2010 doi: 10.1523/JNEUROSCI.6319-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Dulac C, Roth KA, Sanes JR. Labeled lines in the retinotectal system: markers for retinorecipient sublaminae and the retinal ganglion cell subsets that innervate them. Mol Cell Neurosci. 2006;33:296–310. doi: 10.1016/j.mcn.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Holub AD, McLaughlin T, Sejnowski TJ, O’Leary DD. Computational modeling of retinotopic map development to define contributions of EphA-ephrinA gradients, axon-axon interactions, and patterned activity. J Neurobiol. 2004;59:95–113. doi: 10.1002/neu.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S, Noda M. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS One. 2009;4:e4320. doi: 10.1371/journal.pone.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nikolaev A, Wardill TJ, O’Kane CJ, de Polavieja GG, Juusola M. Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: I dynamics. PLoS One. 2009;4:e4307. doi: 10.1371/journal.pone.0004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. J Physiol. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nern A, Zipursky SL, Frye MA. Peripheral visual circuits functionally segregate motion and phototaxis behaviors in the fly. Curr Biol. 2009;19:613–619. doi: 10.1016/j.cub.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu Rev Neurosci. 1994;17:373–397. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]
- Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Zuker CS, Montell C, Jones K, Laverty T, Rubin GM. A rhodopsin gene expressed in photoreceptor cell R7 of the Drosophila eye: homologies with other signal-transducing molecules. J Neurosci. 1987;7:1550–1557. doi: 10.1523/JNEUROSCI.07-05-01550.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]