Abstract
High temperature impairs grain filling by inhibiting the deposition of storage materials such as starch and protein. To comprehend its impact on grain filling metabolism in rice (Oryza sativa), levels of metabolites and transcripts related to central pathways of metabolism were simultaneously determined in developing caryopses exposed to high temperature (33°C/28°C) and a control temperature (25°C/20°C) during the milky stage. A capillary electrophoresis-based metabolomic analysis revealed that high temperature increased the accumulation of sucrose and pyruvate/ oxaloacetate-derived amino acids and decreased levels of sugar phosphates and organic acids involved in glycolysis/gluconeogenesis and the tricarboxylic acid (TCA) cycle, respectively. A transcriptomic analysis using a whole genome-covering microarray unraveled the possible metabolic steps causing the shortage of storage materials under the elevated temperature. Starch deposition might be impaired by down-regulation of sucrose import/degradation and starch biosynthesis, and/or up-regulation of starch degradation as well as inefficient ATP production by an inhibited cytochrome respiration chain, as indicated by the response of gene expression to high temperature. Amino acid accumulation might be attributed to the heat-stable import of amino acids into the caryopsis and/or repression of protein synthesis especially the tRNA charging step under high temperature. An atlas showing the effect of high temperature on levels of metabolites and gene expression in the central metabolic pathways is presented.
Keywords: Gene expression, Grain filling, High temperature, Metabolome, Rice, Starch
Introduction
High temperature impairs grain filling, leading to loss of yield for crops such as rice (Oryza sativa) and wheat (Triticum aestivum) through decreases in grain size and number (Wardlaw et al. 1989, Peng et al. 2004). Considering recent global warming, an understanding of the physiological processes underlying this decrease is of great importance. Rice of japonica cultivars shows a pronounced decline in grain size when exposed to temperatures >26°C during the first half of the grain filling process, the milky stage (Tashiro and Wardlaw 1991), and panicles are the most heat-sensitive parts (Sato and Inaba 1973, Morita et al. 2004). High temperature results in grains with a chalky endosperm as well as decreased weight. The chalky part contains immature starch granules as revealed by microscopic observation (Zakaria et al. 2002), suggesting the accumulation of starch to be impaired at high temperature. Since starch is a major component of grain, making up 60–70% of its weight, a shortage is considered a primary factor in the decrease of grain weight under high temperature.
A previous transcriptomic study showed repressed expression of genes for the biosynthesis of starch and induced expression of genes for starch-consuming enzymes at high temperature, confirming the accumulation of starch to be inhibited during ripening under high temperature (Yamakawa et al. 2007). However, a comprehensive knowledge of the metabolic status of sugars and sugar-related compounds, which are the precursors for the biosynthesis as well as products of the degradation of starch, is indispensable to understand the physiological basis of grain filling under high temperature.
For the simultaneous quantification of metabolites, a method of capillary electrophoresis–mass spectrometry (CE-MS) was developed by Soga et al. (2003), enabling the sensitive and reproducible analysis of a broad range of compounds: carbohydrates, organic acids, amino acids and nucleotides. By coupling this method with capillary electrophoresis–diode array detection (CE-DAD), a large number of primary metabolites including sugars, sugar phosphates, organic acids and amino acids were quantified in rice leaves and seeds (Sato et al. 2004, Takahashi et al. 2006). Thus, CE-MS is a powerful tool for metabolome analyses to determine the status of primary metabolism.
In the present study, we conducted a parallel analysis, the quantification of metabolites by CE-MS and determination of transcripts by a whole-genome microarray, to construct a metabolic atlas summarizing the impact of high temperature on central metabolic pathways in developing rice seeds, and revealed repression of starch deposition and induction of amino acid accumulation at elevated temperatures. Possible key metabolic steps relevant to starch shortage and the regulation of amino acid metabolism are discussed.
Results
The mature grains ripened under high temperature (33°C/28°C) had a decreased weight (20.60 ± 1.22 mg, mean ± SD of >50 grains) and severe chalky appearance, a hallmark of impaired starch granule development, compared with the grains ripened under the control temperature (25°C/20°C) (22.83 ± 0.89 mg), consistent with a previous study (Yamakawa et al. 2007).
To comprehend the impact of high temperatures on the central pathways of metabolism during grain filling, levels of metabolites and gene expression were determined for a set of rice plants ripened in a controlled environment using a grain filling system with incubators. Since a previous transcriptomic study using the system showed the caryopsis 10 days after flowering (DAF) under high temperature to be in almost the same developmental stage of grain filling, the milky stage, as that at 12 DAF under control conditions, with similar fresh weight and expression phase of genes for storage proteins such as glutelin, whose expression was preceded by the elevated temperature (Yamakawa et al. 2007), levels of metabolites and gene expression were compared at this time point. For mature kernels, brown grains harvested 40 DAF (high temperature) and 45 DAF (control) were compared in terms of metabolite levels.
According to public databases for metabolic pathways; KEGG, RiceCyc and RAP-DB, a metabolic map for the central metabolic pathways was depicted to show the metabolite levels determined by CE-MS and CE-DAD (Figs. 1–5 and Supplementary Fig. S1). For the genes encoding each enzyme, the extent of expression in the control plot and changes at high temperature are shown by the square size and color, respectively, of the heat map beside the reaction step. To quantify the impact of temperature at each step, the ratio of cumulative expression level (RCEL), which is the ratio of the sum of expression levels for all genes encoding a corresponding enzyme in the high temperature plot to that in the control plot, was defined as an index for the change in expression of all of the corresponding genes at high temperature, taking into account the difference in the extent of expression for each gene. Thus, major genes would make more of a contribution to the RCEL value than minor expressers among the isoforms in a multigene family.
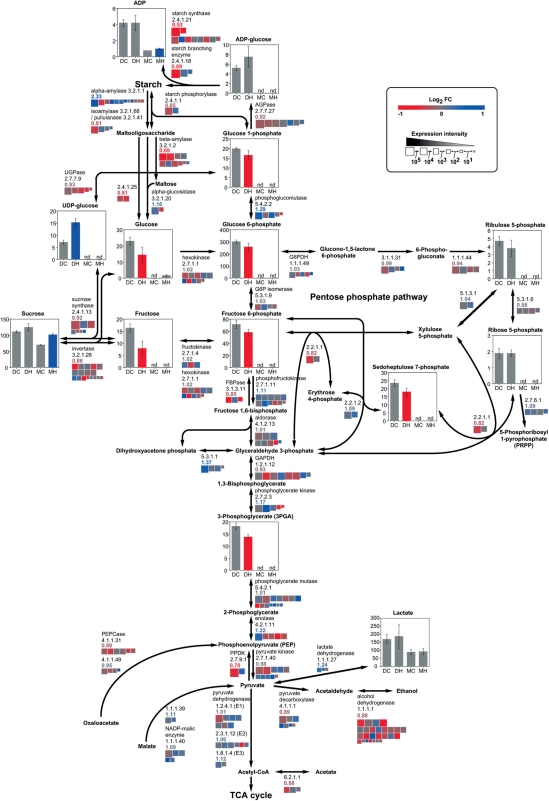
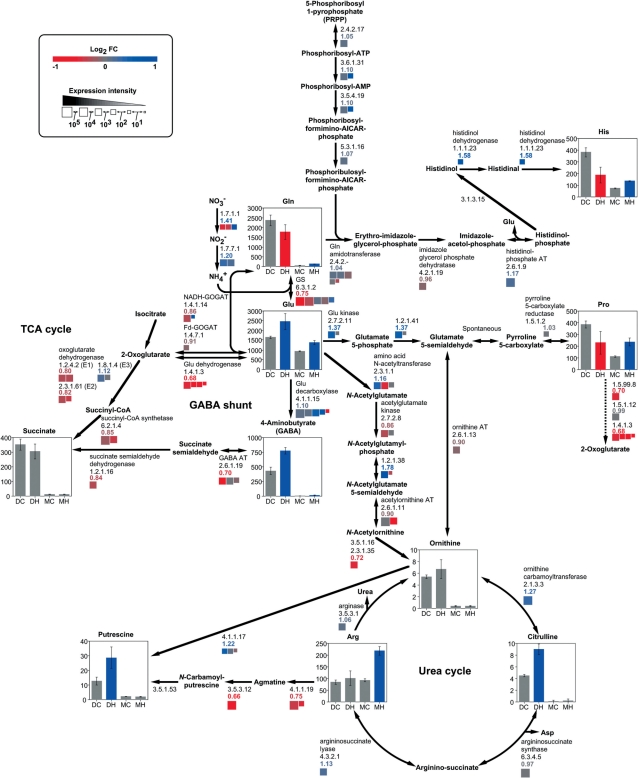
Fig. 1.
Levels of metabolites and expression of genes for starch/sugar metabolism in response to high temperature. Quantities (nmol g FW−1) of metabolites, whose values are the mean ± SD for four replicates, are shown for developing caryopses exposed to the control temperature, 25°C/20°C (DC), and to high temperature, 33°C/28°C (DH), and mature kernels ripened under the control temperature (MC) and high temperature (MH). Red and blue bars indicate a decrease and increase, respectively, significant at the 5% level as determined by the t test. The extent of expression of genes for the respective enzymes in the developing caryopsis and their response to high temperature are indicated for the average of three experiments by a heat map with the criterion shown. For each reaction step, the ratio of cumulative expression level (RCEL), which is the ratio of the sum of expression levels for all genes encoding a corresponding enzyme in the high temperature plot to that in the control plot, is indicated below the EC number as an index for the change in gene expression at high temperature.
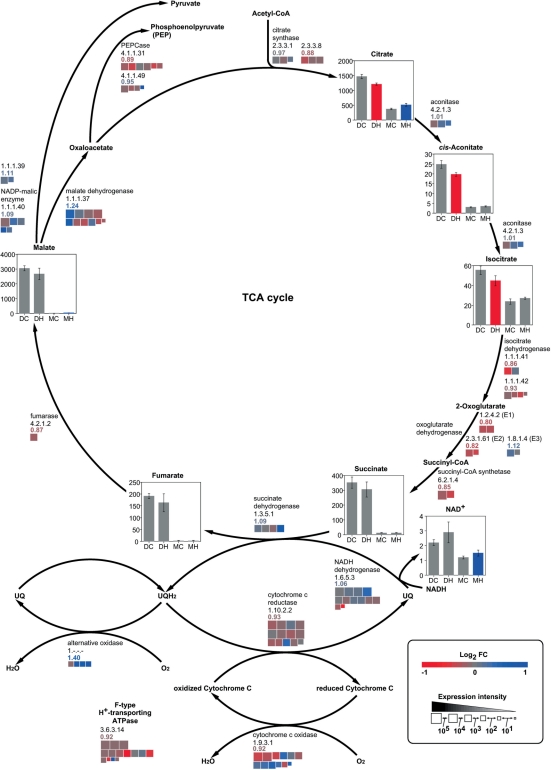
Fig. 2.
Levels of metabolites and expression of genes for the TCA cycle and respiration chain in response to high temperature. Quantities (nmol g FW−1) of metabolites and the extent and change in expression of genes are shown as described in the legend of Fig. 1.
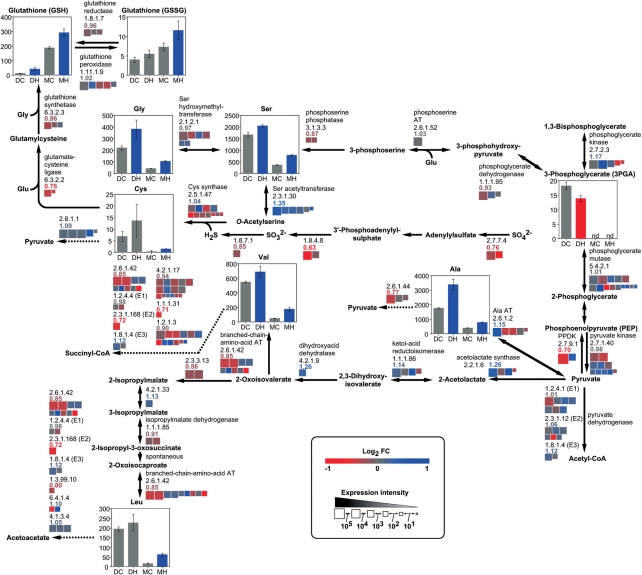
Fig. 3.
Levels of metabolites and expression of genes for metabolism of amino acids derived from 3-phosphoglycerate and pyruvate in response to high temperature. Quantities (nmol g FW−1) of metabolites and the extent and change in expression of genes are shown as described in the legend of Fig. 1.
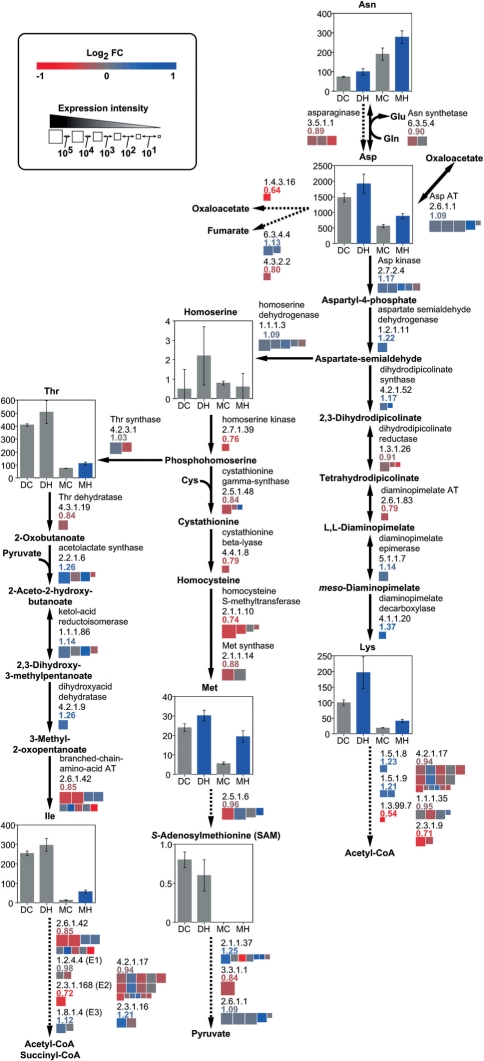
Fig. 4.
Levels of metabolites and expression of genes for metabolism of amino acids derived from oxaloacetate in response to high temperature. Quantities (nmol g FW−1) of metabolites and the extent and change in expression of genes are shown as described in the legend of Fig. 1.
Fig. 5.
Levels of metabolites and expression of genes for metabolism of amino acids derived from 2-oxoglutarate in response to high temperature. Quantities (nmol g FW−1) of metabolites and the extent and change in expression of genes are shown as described in the legend of Fig. 1.
Sucrose and amino acids accumulated and levels of sugar phosphates and organic acids decreased in high temperature-ripened caryopses
In the developing caryopsis, amounts of sucrose, UDP-glucose and ADP-glucose were increased, whereas those of glucose, fructose, sugar phosphates, except ribose 5-phosphate, and organic acids of the tricarboxlic acid (TCA) cycle were decreased at high temperature (Figs. 1, 2). Most amino acids including 4-aminobutyrate (GABA), a tripeptide, glutathione in both reduced (GSH) and oxidized (GSSG) forms, and amines such as putrescine increased in response to high temperature, with the exception of glutamine, proline, histidine and tyrosine, which showed a transient decrease in the developing phase (Figs. 3–5 and Supplementary Fig. S1). In mature kernels, sugar phosphates decreased below the level of detection in both temperature plots, while sucrose and some organic acids such as citrate, glutathione and amino acids remained at high levels in grains ripened under high temperature. Most nucleotides including ATP were not detected (Supplementary Table S1). The complete data set of metabolites quantified in the developing caryopsis and mature kernel is shown in Supplementary Table S1.
Expression of genes for the metabolism of carbohydrates and amino acids responded differently to high temperature
In the developing caryopsis, genes whose expression was up-regulated and down-regulated at high temperature co-existed for most of the reaction steps. However, those showing the highest levels of expression were down-regulated genes for two sucrose-utilizing enzymes, sucrose synthase and invertase, UDP-glucose pyrophosphorylase (UGPase), ADP-glucose pyrophosphorylase (AGPase), starch synthase and starch branching enzyme, with RCEL values of 0.92, 0.86, 0.93, 0.92, 0.53 and 0.69, respectively (Fig. 1). In contrast, the expression of major genes for starch-consuming α-amylase was induced, with an RCEL of 2.33, although that for β-amylase was repressed at high temperature. Two gluconeogenesis-related enzymes, fructose bisphosphatase (FBPase) and pyruvate orthophosphate dikinase (PPDK), were impaired by high temperature, with an RCEL of 0.85 and 0.70, respectively, while phosphofructokinase and pyruvate kinase catalyzing glycolysis were slightly induced or less affected (RCEL of 1.11 and 0.98, respectively). Most genes of the TCA cycle, cytochrome c respiratory chain and F-type H+-transporting ATPases were repressed to RCEL values of 0.80–0.93, whereas genes for alternative oxidases (AOXs) were mostly up-regulated to an RCEL of 1.40 in response to high temperature (Fig. 2). Similarly, genes in amino acid biosynthetic and metabolizing pathways were differentially regulated as the temperature rose (Figs. 3–5 and Supplementary Fig. S1). It was noteworthy that the expression of alanine aminotransferase (Ala AT), acetolactate synthase (ALS) and aspartate aminotransferase (Asp AT) at the branching point from glycolytic pathways was induced with an RCEL of 1.15, 1.26 and 1.09, respectively.
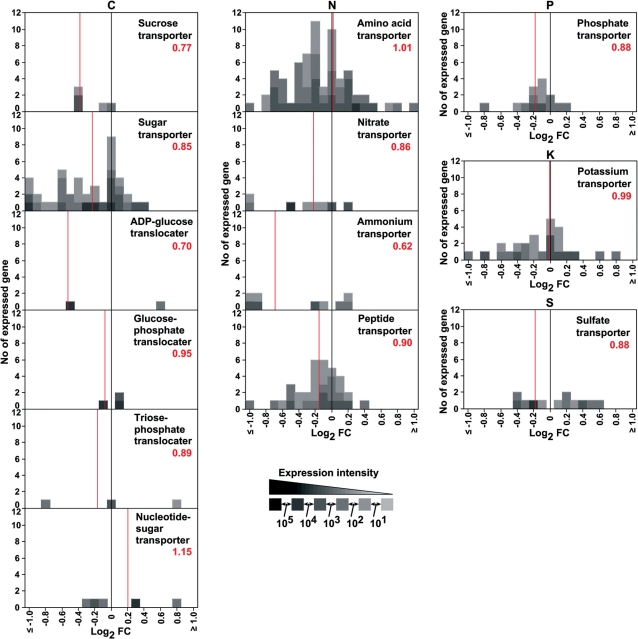
Expression of genes for sucrose transporter and ADP-glucose translocater, but not for amino acid transporter, was sensitive to high temperature
To obtain an insight into the impact of high temperature on the uptake of organic and inorganic compounds conveyed from source organs to the sink organ, the developing caryopsis, the expression profiles of genes for various transporters were summarized in histograms (Fig. 6). The expression of major genes (shown by dark gray boxes) for sucrose transporter, ADP- glucose translocater and ammonium transporter was impaired by high temperature, with a bulky RCEL of 0.77, 0.70 and 0.62, respectively, whereas that for amino acid transporter and potassium transporter was both up-regulated and down- regulated, keeping the RCEL value stable at around 1.00 over the temperature range examined. Phosphate and sulfate transporters showed a slightly repressed gene expression at high temperature.
Fig. 6.
Response of genes for various transporters to high temperature. A histogram for the ‘fold change (FC)’ value of all genes for each transporter is shown. The genes whose expression was induced or repressed >2-fold are included in the classes, ≤−1.0 and ≥1.0, respectively. The ratio of cumulative expression level (RCEL), which is the ratio of the sum of expression levels for all genes encoding a corresponding transporter in the high temperature plot to that in the control plot, is indicated by the red number and red line as an index for the impact on gene expression at high temperature. The extent of expression of each gene is indicated by the gray scale. Data are average values for three experiments.
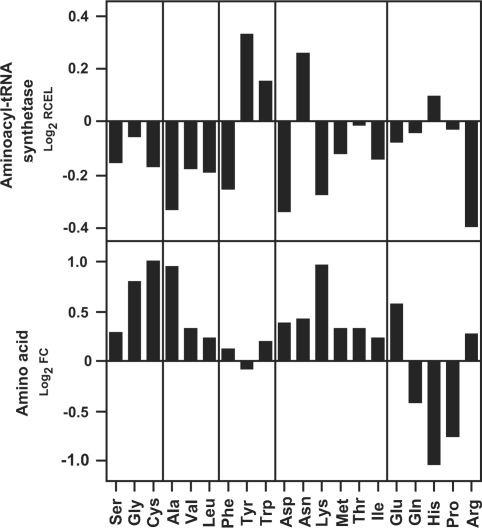
Expression of genes for aminoacyl-tRNA biosynthesis, transcription and translation was impaired by high temperature
Since the accumulation of amino acids in the high temperature-exposed caryopsis raised the possibility of inhibited protein synthesis, various steps of translation were evaluated for sensitivity to high temperature. The gene expression profiling revealed the charging of amino acids to tRNA to be impaired. As indicated by negative Log2 values of RCEL, the expression of aminoacyl-tRNA synthase genes for amino acids other than tyrosine, tryptophan, asparagine and histidine was repressed at high temperature (Fig. 7). Furthermore, a comparison of RCEL values in the histograms uncovered that the expression levels of genes for basal components of transcription (basal transcription factors and RNA polymerases) and translation (initiation factors, elongation factors, peptide chain release factors and ribosomal proteins) decreased slightly in response to high temperature (RCEL of 0.91–0.97), while those for proteolytic enzymes (proteases/peptidases) were less affected (RCEL of 1.00) (Supplementary Fig. S2).
Fig. 7.
Response of genes for aminoacyl-tRNA biosynthesis to high temperature. For aminoacyl-tRNA synthetase genes corresponding to each amino acid, the ratio of cumulative expression level (RCEL), which is the ratio of the sum of expression levels for all genes encoding aminoacyl-tRNA synthetase for a given amino acid in the high temperature plot to that in the control plot, is indicated in the upper panel as an index for the change in gene expression at high temperature. Alongside, the change ratio (FC, fold change) of free amino acid levels in the developing caryopsis is shown in the lower panel. Data are average values for three and four biologically independent experiments, respectively.
Genes for heat shock proteins were up-regulated while those for prolamin were down-regulated
For other responses, the expression of genes for groups of heat shock proteins (HSPs), especially many small HSPs (sHSPs) and HSP60s, was induced by high temperature (Supplementary Fig. S3). Among seed-specific storage proteins, the gene expression of prolamin, particularly 13 kD prolamin, was repressed under high temperature, while that for glutelin, the other type of storage protein, was less affected (Supplementary Fig. S4). These responses are consistent with previous observations (Yamakawa et al. 2007). For the enzymes responsible for redox modulation, genes for thioredoxin reductase were down-regulated to an RCEL of 0.87, whereas those for ascorbate peroxidase were up-regulated to an RCEL of 1.20 in response to the elevated temperature (Supplementary Fig. S5). The extracted data set of gene expression in the developing caryopsis is shown in Supplementary Table S2 and the complete set of microarray data was deposited to the GEO repository under accession number GSE20345.
Discussion
Accumulation of sucrose and amino acids—general response to high temperature?
In the present study, the accumulation of sucrose and amino acids other than glutamine, proline, histidine and tyrosine was induced, while levels of sugar phosphates and TCA cycle-related organic acids were decreased by high temperature in the developing caryopsis. Consist with this, Arabidopsis plants exposed to high temperature showed a rapid increase in levels of sucrose and amino acids derived from oxaloacetate and pyruvate (Kaplan et al. 2004), and Arabidopsis leaves subjected to a combination of drought and heat stress accumulated sucrose (Rizhsky et al. 2004). The accumulation of sucrose and amino acids might therefore be a general metabolic response to high temperature common to various organs in numerous plant species.
Possible key steps for inhibition of starch accumulation in the developing caryopsis exposed to high temperature
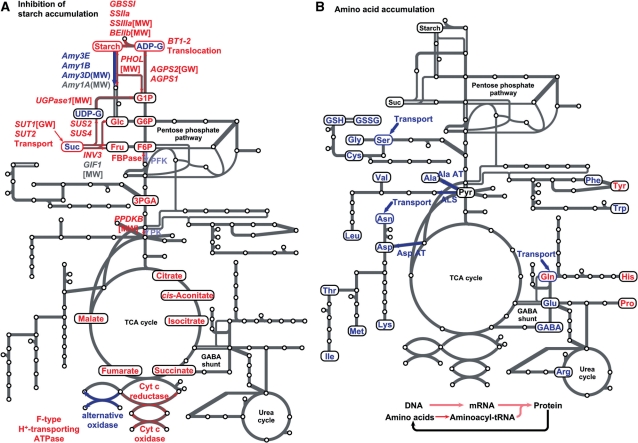
High temperature decreases the amount of starch deposited in a mature grain, since high temperature-ripened grain has a lower weight than control grain without a significant change in starch content, i.e. the ratio of starch weight to grain weight (Yamakawa et al. 2007). By comparing levels of metabolites and transcripts in developing caryopses exposed to high temperature and the control temperature, possible key steps related to the inhibition of starch accumulation were considered, as summarized in Fig. 8A.
Fig. 8.
Possible metabolic steps involved in (A) inhibition of starch accumulation and (B) induction of amino acid accumulation at high temperature. Up-regulated and down-regulated genes, metabolites and pathways in the developing caryopsis are indicated in blue and red, respectively. Grain phenotypes reported for the mutants and overexpressors of the genes are shown in brackets and parentheses, respectively. MW, milky white (chalky) kernel; GW, decreased grain weight.
The import of sucrose into endosperm cells might be impaired, because the expression of two major sucrose transporter genes, SUT1 and SUT2, was down-regulated 0.75- and 0.76-fold, respectively, under high temperature (Fig. 6; Supplementary Table S2). Since sucrose is an exclusive carbohydrate of phloem sap which is conveyed from source leaves to developing caryopses (Hayashi and Chino 1990), an increased level of sucrose in the high temperature plot (Fig. 1) raises the possibility that the uptake of sucrose is limited and excessive sucrose remains outside of endosperm cells in the high temperature-exposed caryopsis. Since the antisense suppression of SUT1 impaired grain filling with a decrease in seed weight (Scofield et al. 2002), the reduced expression of the sucrose transporter genes might be one of the factors restricting the deposition of starch in high temperature-ripened grain. A recent flux analysis by pulsed labeling with 13CO2 of rice plants after anthesis revealed impaired allocation of assimilated carbohydrates into the developing caryopsis and decreased synthesis of starch, accompanied by increased accumulation of sucrose under high temperature conditions (Ito et al. 2009).
Alternatively, the increase in sucrose content at high temperature might be attributable to a decrease in sucrolysis. The expression of many genes for two sucrolytic enzymes, sucrose synthase (especially SUS2 and SUS4) and invertase (especially INV3), was down-regulated by high temperature to an RCEL of 0.92 and 0.86, respectively (Fig. 1). Also, levels of monosaccharides produced by an invertase reaction, glucose and fructose, were decreased by high temperature. The mutation of a cell wall invertase gene, GIF1 (synonymous with CIN2), reportedly resulted in chalky and light grain accompanied by immature starch granules (Wang et al. 2008). However, down-regulation of the expression of invertases other than GIF1 might contribute to a shortage of starch, since GIF1 expression was not perturbed by high temperature (Supplementary Table S2).
The conversion of UDP-glucose to glucose 1-phosphate, the subsequent step in starch biosynthesis, was also impaired, as manifested by an increase in the former compound and decrease in the latter under high temperature (Fig. 1). Concomitantly, the expression of genes for the catalyzing enzyme UGPase was down-regulated to an RCEL of 0.93 as the temperature rose. Since a mutant deficient in an UGPase gene produced chalky grain (Woo et al. 2008), retardation of the UGPase reaction might hamper the accumulation of starch in the high temperature- exposed caryopsis, although the direction of the reaction in the developing endosperm remains to be confirmed.
The expression of major genes for AGPase, which produces ADP-glucose, the substrate for starch biosynthesis, was repressed in response to high temperature (Fig. 1). Since the mutation of one major gene, AGPS2, resulted in shrunken grain with severely inhibited starch synthesis (Kawagoe et al. 2005), the reduced expression of AGPase genes would restrain the deposition of starch. However, the increased levels of ADP-glucose in high temperature-exposed caryopses suggested other impaired steps later than the ADP-glucose-producing step.
One possible explanation for the accumulation of ADP- glucose is that the import of ADP-glucose into amyloplasts where starch is produced is repressed at high temperature. The expression of the gene for an ADP-glucose translocater, BT1-2, was repressed 0.69-fold by high temperature (Fig. 6).
Alternatively, starch synthesis was down-regulated, thus an excess of ADP-glucose remained. The expression levels of genes for starch synthases and branching enzymes, namely GBSSI, SSIIa, SSIIIa and BEIIb, decreased under high temperature (Fig. 1; Supplementary Table S2), although the japonica cultivar, Nipponbare, carries an inactive haplotype of SSIIa which is deficient in starch association (Umemoto et al. 2004). Repression of these genes directly impairs starch biosynthesis, as reported for mutants of SSIIIa and BEIIb, which produces chalky grain with malformed starch granules (Nishi et al. 2001, Fujita et al. 2007).
High temperature-ripened grains contained decreased levels of amylose and long chain-enriched amylopectin (Yamakawa et al. 2007). Since amylose is exclusively synthesized by GBSSI and the structure of amylopectin is governed by a balance of the branching activity of starch branching enzymes and the chain-elongating activity of soluble starch synthases, decreased gene expression (present data) and activity (Umemoto and Terashima 2002, Jiang et al. 2003) of GBSSI and BEIIb might contribute to the decrease in amylose content and chain elongation of amylopectin at high temperature, respectively. Mutations at both BEIIb and SSIIIa lead to alterations in the chain length distribution of amylopectin as well as chalky endosperm. Since BEIIb mutation resulted in long chain- enriched amylopectin (Nishi et al. 2001), down-regulation of expression of BEIIb appears to have more impact on the structure of amylopectin rather than that of SSIIIa whose mutation resulted in short chain-enriched amylopectin (Fujita et al. 2007). The chalky appearance of the BEIIb mutant, whose severity changed in a manner dependent on the mutant allele dosage and expression level, became prominent when the BEIIb protein amounts were lower than approximately half of the wild-type level (Nishi et al. 2001, Tanaka et al. 2004). In the present study, high temperature reduced expression of BEIIb to 61% of the control level (Supplementary Table S2). Thus, the decreased level of BEIIb expression might be one of the factors that potentially trigger production of chalky grains.
The expression of α-amylase genes might promote the degradation of deposited starch under high temperature. Amy1C, Amy3A, Amy3D and Amy3E were up-regulated at high temperature in the present study (Fig. 1; Supplementary Table S2), and Amy1A, Amy3D and Amy3E were induced in a previous study, in which the time course of their expression in the developing caryopsis was investigated (Yamakawa et al. 2007). Since the overexpression of Amy1A (synonymous with AmyI-1) and Amy3D (synonymous with AmyII-4) in transgenic rice resulted in chalky grain with decreased weight even when ripened under an ambient temperature (Asatsuma et al. 2006, Kitajima et al. 2009), these genes might regulate the accumulation of starch in developing seeds. However, the analysis using CE-DAD failed to detect maltooligosaccharide or maltose in extracts of high temperature-ripened caryopses and mature kernels. These oligosaccharides produced by the α-amylase reaction would be immediately metabolized to monosaccharides by enzymes such as the high temperature-induced α-glucosidase, although the expression of many β-amylase genes was repressed at the elevated temperature. Degradation of transitory starch in the leaves of Arabidopsis depends on starch phosphorylation (Smith et al. 2005). Expression of an α-glucan water dikinase (GWD) gene but not of a phosphoglucan water dikinase (PWD) gene was induced to a small extent by high temperature (RCEL of 1.11 and 0.95, respectively, Supplementary Table S2). Thus, GWD might phosphorylate the surface of starch granules, making them accessible for action of amylolytic enzymes, although the extent of phosphorylation of starch in the endosperm of developing cereals remains to be determined.
The decreased expression of the gene PHOL for starch phosphorylase (Fig. 1; Supplementary Table S2), which catalyzes a reversible reaction, i.e. the addition of a glucose unit of glucose 1-phosphate to the non-reducing end of an α-glucan and release of glucose 1-phosphate from the α-glucan, might be involved in the shortage of starch at high temperature, since a mutation in PHOL resulted in temperature-dependent changes in the appearance of grain and the abnormal accumulation of starch, producing chalky grain when ripening occurred at high temperature (30°C), and shrunken grain at 20°C (Satoh et al. 2008).
Gluconeogenesis might be inhibited by high temperature. For most reactions in the central metabolism of sugar phosphates, one enzyme catalyzes a reversible process in both directions, glycolysis and gluconeogenesis. However, the conversion of fructose 6-phosphate/fructose 1,6-bisphosphate as well as phosphoenolpyruvate/pyruvate is catalyzed by a pair of unidirectional enzymes working in the opposite direction, phosphofructokinase and FBPase, and pyruvate kinase and PPDK for glycolysis and gluconeogenesis, respectively. In these steps, the gene expression of gluconeogenesis-related FBPase and PPDK was impaired, with RCEL values of 0.85 and 0.70 under high temperature, respectively, while that of glycolysis-related phosphfructokinase and pyruvate kinase was slightly increased or less affected, with RCEL values of 1.11 and 0.98, respectively (Fig. 1), suggesting that glycolysis is superior to gluconeogenesis in preventing sugar phosphates from being supplied for starch biosynthesis, and rather they are consumed for the TCA cycle. The importance of gluconeogenesis in the accumulation of starch during grain filling was supported by the aberrant phenotype of the mutant for a PPDK gene, PPDKB, which produces chalky and light grains (Kang et al. 2005).
The respiration chain in mitochondria might be impaired at high temperature, since the expression of genes for cytochrome c oxidase, cytochrome c reductase and F-type H+-transporting ATPase was repressed to an RCEL of 0.92, 0.93 and 0.92, respectively (Fig. 2). High temperatures might slow down ATP production and ATP-requiring starch biosynthesis, although the amount of ATP in the developing caryopsis remains to be determined. The endogenous O2 concentration fell rapidly in developing sunflower seeds exposed to elevated temperatures (Rolletschek et al. 2007). Although the Km value of mitochondrial cytochrome c oxidase for O2 is as low as 0.08–0.16 μM (Hoshi et al. 1993, Millar et al. 1994), high temperature might cause the concentration of O2 in rice endosperm cells to decrease, diminishing ATP synthesis. Meanwhile, the expression of genes for AOX was induced to an RCEL of 1.40, which might be a response to prevent over-reduction of respiratory components that might otherwise result in the formation of harmful reactive oxygen species (ROS) (Purvis and Shewfelt 1993, Wagner and Krab 1995). The dominance of non-protonmotive AOX against cytochrome c oxidase lowers the efficiency of ATP production, and might further impair starch biosynthesis.
Possible key steps in the accumulation of amino acids in developing caryopses exposed to high temperature
The accumulation of amino acids, especially those derived from pyruvate and oxaloacetate, in the high temperature-exposed caryopsis might be caused by import exceeding usage, as summarized in Fig. 8B. A large proportion of assimilated nitrogen was transported from source organs to the developing caryopsis as amino acids such as glutamine, asparagine and serine (Hayashi and Chino 1990). The relatively stable expression of genes for amino acid transporters under high temperature, which kept the RCEL value at 1.01 (Fig. 6), might enable a constant supply of glutamine, asparagine and serine, and the accumulation of other amino acids synthesized from these, such as glutamate, arginine, aspartate, threonine, isoleucine, methionine, lysine, glycine and cysteine in the developing caryopsis.
The abundance of alanine, valine and leucine, which are derived from pyruvate, might be attributed to the increased expression of genes for Ala AT and ALS under high temperature. The RCEL values for Ala AT and ALS, which produce alanine and 2-acetolactate, a precursor for the biosynthesis of valine and leucine, were 1.15 and 1.26, respectively, while those for other pyruvate-metabolizing enzymes, PPDK and pyruvate dehydrogenase, were 0.70–1.12 (Fig. 3). Similarly, the RCEL value for Asp AT, which produces aspartate from oxaloacetate, was 1.09 (Fig. 4), while those for other oxaloacetate-metabolizing enzymes such as citrate synthase and phosphoenolpyruvate carboxylase were 0.88–0.97 (Fig. 2). The enhanced expression of these enzymes at the point branching from the central pathway of glycolysis/gluconeogenesis or the TCA cycle suggested increased synthesis of these amino acids from carbohydrates. Also, levels of sugar phosphates and organic acids involved in glycolysis and the TCA cycle were decreased whereas levels of amino acids derived from pyruvate and oxaloacetate were increased by high temperature.
Reduced synthesis of aminoacyl-tRNA might cause the accumulation of free amino acids not used for protein synthesis, since the gene expression of aminoacyl-tRNA synthetases for most amino acids was impaired at high temperature (Fig. 7). Although the gene expression of aminoacyl-tRNA synthetases corresponding to tyrosine, tryptophan, asparagine and histidine was up-regulated, the levels of these amino acids showed a decrease or slight increase in the developing caryopsis exposed to high temperature.
Reduced transcription and translation might slow down the incorporation of free amino acids into proteins. The expression of genes for basal factors for transcription (transcription factor and RNA polymerase) and translation (translation factors and ribosomal protein) was slightly repressed by high temperature, with RCEL values of 0.91–0.97, whereas that of genes for proteolytic enzymes (protease/peptidase) was not repressed, with an RCEL of 1.00 (see histograms in Supplementary Fig. S2). Thus, the amounts of free amino acids supplied by protein degradation might be too great for incorporation into proteins, leading to accumulation in the high temperature- exposed caryopsis.
Our previous determination of storage proteins by SDS–PAGE analysis revealed a specific decrease of 13 kDa prolamin in high temperature-ripened grains (Yamakawa et al. 2007), which was confirmed by the decreased expression of genes encoding it in the present transcriptomic study (Supplementary Fig. S4). Most of 13 kDa prolamin peptides are rich in glutamine (making up 16.2% of their constituents on average, ranging from 3.4 to 19.2%), leucine (12.4% on average, ranging from 7.7 to 15.7%) and alanine (11.6% on average, ranging from 9.0 to 12.7%). However, these amino acids that were not incorporated into the storage protein did not agree with those abundant in high temperature-ripened grains. The reason for this discrepancy remains to be elucidated. Glutamine not used for prolamin synthesis might be converted into other metabolites such as glutamate, although expression of many genes for converting enzymes, GS and GOGAT, were impaired at high temperature.
Accumulation of GABA and glutathione in developing caryopsis exposed to high temperature—response to regulate redox status
The accumulation of GABA in response to high temperature (Fig. 5) might be a response to stress. The gene expression of the GABA-producing enzyme glutamate decarboxylase was slightly increased to an RCEL of 1.10 at high temperature, while that for GABA-metabolizing enzymes, GABA aminotransferase and succinate semialdehyde dehydrogenase, was impaired to an RCEL of 0.70 and 0.84, respectively (Fig. 5). Since high temperature decreased the endogenous O2 concentration in developing sunflower seeds (Rolletschek et al. 2007) and low levels of O2 induced GABA production (Reggiani et al. 1988) by activating glutamate decarboxylase (Aurisano et al. 1995), glutamate decarboxylase might be activated in the caryopsis at high temperature. In Arabidopsis, mutants with a disrupted succinate semialdehyde dehydrogenase were sensitive to heat stress, because they were unable to scavenge ROS (Bouché et al. 2003). Glutamate decarboxylase is indispensable to cope with oxidative stress in yeast (Coleman et al. 2001). Furthermore, GABA itself showed hydroxyl radical-scavenging activity in vitro (Smirnoff and Cumbes 1989). Taken together, these studies suggest the possible involvement of the GABA shunt in tolerance to oxidative stress.
Another redox-related compound, glutathione, accumulated in response to high temperature, although the expression of genes for the enzymes that produce it, glutamate-cysteine ligase and glutathione synthetase, was repressed (Fig. 3). Glutathione is a major redox buffer in the cell and might have roles in the detoxification of peroxides and scavenging of ROS. The reduced form of glutathione (GSH) serves as an electron donor to dehydroascorbate reductase for the maintenance of a reduced pool of ascorbate, which is used by ascorbate peroxidase for the removal of H2O2. The ratio of GSH to oxidized glutathione (GSSG) increased and the gene expression of ascorbate peroxidase was up-regulated, with an RCEL value of 1.20 in the caryopsis at high temperature (Supplementary Fig. S5).
Based on parallel analyses of the transcriptome and metabolome, an atlas showing changes in metabolic status in developing rice seeds in response to high temperature was proposed, which might be useful for comprehending the response in other crops such as wheat, whose yield and quality are impaired by high temperature during grain filling.
The shortage of starch in the high temperature-exposed caryopsis is presumably caused by a combination of (i) impaired import and/or degradation of sucrose; (ii) decreased synthesis of starch; (iii) increased degradation of starch; (iv) glycolysis exceeding gluconeogenesis; and (v) decreased respiration efficiency. Genetic approaches to enhancing steps in the biosynthetic process that were repressed at high temperature and to suppressing starch-consuming enzymes might ameliorate the accumulation of starch under high temperatures.
The accumulation of amino acids at elevated temperatures is thought to be attributable to (i) heat-stable uptake of amino acids; (ii) increased amino acid biosynthesis from carbohydrates; (iii) decreased charging of free amino acids to tRNA; and/or (iv) decreased protein synthesis inferior to proteolysis. The amount of amino acids has a relatively small impact on yield but greatly affects grain quality, especially palatability and nutritional value. Further analyses of the physicochemical properties of high temperature-ripened grains are required.
In the present study, whole caryopses or grains were used to examine metabolites and transcripts. Some part of the metabolites and gene expression might be attributed to the bran layer and embryo, tissues other than starchy endosperm. It should be noted that further metabolite profiling that resolves organelle-specific aspects as well as organ-specific transcript profiling is necessary to obtain more insight into the localization of the respective metabolic reactions. Determination of protein levels and activities of selected enzymes is also required for further understanding of the dynamic metabolism. Furthermore, the present study was conducted with the samples harvested during the daytime. However, high temperature during the night has more impact on the decrease of grain weight (Morita et al. 2004). Although the expression levels of several starch/storage protein biosynthetic genes and sugar levels showed diurnal changes in the endosperm of cereal developing caryopsis (Ciceri et al. 1999, Carman and Bishop 2004, Mutisya et al. 2009), the time of the day when starch/protein synthesis and sucrose/amino acid transport are active is not known. Determination of metabolites and gene expression during the night would shed more light on the mechanism underlying the decrease of grain weight.
The expression of HSP and thioredoxin reportedly conferred tolerance to heat stress (Katiyar-Agarwal et al. 2003, Lee et al. 2009, Park et al. 2009). In the developing caryopsis, several HSPs and redox-related molecules were expressed at high temperature. The effect of the expression of these genes on grain filling impaired under high temperatures remains to be elucidated. To develop crops whose yield and quality are not impaired by high temperatures, further characterization of the candidate genes proposed in the present study using corresponding mutants and transgenic plants is required.
Materials and Methods
Plant materials
Rice (O. sativa ssp. japonica) ‘Nipponbare’ was grown at 27°C/22°C (12 h day/12 h night photoperiod) until flowering in a plant incubator (model FLI-301NH; Eyela, Tokyo, Japan) equipped with a sodium lamp, which allows illumination at an intensity of 560 μmol photons m−2 s−1. Six plants were grown in a plastic container (15 × 10 × 6 cm) filled with 600 ml of rice nursery culture soil (containing 0.15 g each of nitrogen, phosphate and potassium), and each plant was restricted to the main culm by the removal of tillers. Approximately 15–20 d before heading, 3 g of a fertilizer (containing 0.18 g of nitrogen, 0.24 g of phosphate, 0.18 g of potassium and 0.06 g of magnesium) was supplied per container. At 5 DAF, the plants were transferred to either a 33°C/28°C or 25°C/20°C chamber for high temperature or control treatment, respectively, and then the temperature was maintained at 25°C/20°C from 20 DAF to maturity. At 10 and 12 DAF for high temperature and control plots, respectively, developing caryopses were detached from the ear at midday (6 h after illumination was started), immediately frozen in liquid nitrogen and stored at −80°C. At 40 and 45 DAF, caryopses were dehulled, frozen and stored at −80°C as mature kernels.
For each replicate of all metabolomic and transcriptomic analyses, the well-developed caryopses located on the primary rachis branches at the upper half of two panicles excised from the independently grown plants, that are sufficient amounts for one extraction of RNA or metabolites, were pooled and dealt with as one biological replicate.
Gene expression profiling by DNA microarray analysis
Total RNA was extracted from 12 developing caryopses harvested from 25°C/20°C- and 33°C/28°C-treated plants using the method of Chang et al. (1993) (three biologically independent replicates, each). The yield and RNA purity were determined spectrophotometrically. Integrity was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Total RNA (400 ng) was labeled with Cy3 or Cy5 using an Agilent Quick Amp Labeling kit (Agilent Technologies). Fluorescently labeled targets were hybridized to Agilent rice 44-K oligo DNA microarrays (Agilent Technologies). The hybridization and washing were performed according to the manufacturer’s instructions, and hybridized microarrays were scanned using an Agilent Microarray Scanner (Agilent Technologies). Feature Extraction software (version 9.1; Agilent Technologies) was employed for the data extraction processes.
Metabolite quantification by CE-MS
Seven developing caryopses or 10 mature kernels (frozen) (four biological replicates, each) were weighed and macerated in a Multi-beads Shocker (Yasuikikai, Osaka, Japan), and ice-cooled methanol (20 and 40 μl mg−1 of developing caryopsis and mature kernel, respectively), containing 10 μM Internal Standard Solution 1 (Human Metabolome Technologies, Tsuruoka, Japan) and 500 μM galactose as an internal standard, was immediately added to inactivate enzymes. A 1 ml aliquot of the homogenate was mixed with 1 ml of chloroform and 400 μl of ice-cold Milli-Q water. After centrifugation, the separated methanol–water layer (750 μl) was ultrafiltrated through a Millipore 5 kDa cut-off filter to remove proteins. The filtrate was lyophilized, dissolved in 50 μl of Milli-Q water and analyzed by CE-MS and CE-DAD.
CE-MS and CE-DAD experiments were performed using an Agilent CE system equipped with a time-of-flight mass spectrometer (TOF-MS) (Agilent Technologies) and an Agilent CE system with a built-in diode array detector (Agilent Technologies), respectively.
Cationic metabolites were analyzed with a fused silica capillary (50 μm i.d. × 80 cm total length), with Cation Buffer Solution (Human Metabolome Technologies) as the electrolyte. The sample was injected at a pressure of 5.0 kPa for 10 s (approximately 10 nl). The applied voltage was set at 30 kV. Electrospray ionization-mass spectrometry (ESI-MS) was conducted in the positive ion mode, and the capillary voltage was set at 4,000 V. The spectrometer was scanned from m/z 50 to 1,000. Other conditions were as in the cation analysis (Soga and Heiger 2000).
Anionic metabolites were analyzed with a fused silica capillary (50 μm i.d. × 80 cm total length), with Anion Buffer Solution (Human Metabolome Technologies) as the electrolyte. The sample was injected at a pressure of 5.0 kPa for 25 s (approximately 25 nl). The applied voltage was set at 30 kV. ESI-MS was conducted in the negative ion mode, and the capillary voltage was set at 3,500 V. The spectrometer was scanned from m/z 50 to 1,000. Other conditions were as in the anion analysis (Soga et al. 2007).
Sugars were analyzed with a fused silica capillary (50 μm i.d. × 112.5 cm total length, 104 cm effective length), with Basic Anion Buffer for HPCE (Agilent Technologies) as the electrolyte. The sample was injected at a pressure of 5.0 kPa for 6 s (approximately 6 nl). The applied voltage was set at −30 kV. Sugars were detected by indirect UV detection using a diode array detector. The signal wavelength was set at 390 nm with a reference at 210 m. Other conditions were as in the sugar analysis (Soga and Ross 1999).
Metabolites in the samples were identified by comparison of the migration time and m/z ratio with those of authentic standards, in which the difference of ±0.5 min and ±10 p.p.m. was permitted, respectively, and quantified by comparing their peak areas with those of the authentic standards using ChemStation software (Agilent Technologies).
Data analysis and construction of the metabolic atlas
Functional annotations of gene sets representing various metabolic pathways were determined by reference to databases such as the KEGG pathway (http://www.genome.jp/kegg/ pathway.html), RiceCyc (http://www.gramene.org/pathway/ricecyc.html) and RAP-DB (http://rapdb.dna.affrc.go.jp/) to construct a rice central metabolic map. A full list of gene sets with corresponding function, IRGSP accessions and expression data is available as Supplementary data at PCP online (Supplementary Table S2). Since the rice 44-K oligo DNA microarray often contains several features for one given gene, the geometric mean of the expression intensity data from all the corresponding features corresponding to the same gene was taken in such cases.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan [Genomics for Agricultural Innovation, Molecular cloning and characterization of agronomically important genes of rice, IPG-0020]; National Agriculture and Food Research Organization [Development of innovative crops through the molecular analysis of useful genes, No. 1211].
Supplementary Material
Acknowledgments
The authors thank Y. Nagamura and R. Motoyama (National Institute of Agrobiological Sciences) for technical help on microarray analysis, and T. Umemoto (National Institute of Crop Science) for reading the manuscript.
Glossary
Abbreviations
- AGPase
ADP-glucose pyrophosphorylase
- Ala AT
alanine aminotransferase
- ALS
acetolactate synthase
- AOX
alternative oxidase
- APX
ascorbate peroxidase
- Asp AT
aspartate aminotransferase
- CE-DAD
capillary electrophoresis–diode array detection
- CE-MS
capillary electrophoresis–mass spectrometry
- DAF
days after flowering
- ESI-MS
electrospray ionization-mass spectrometry
- FBPase
fructose bisphosphatase
- GABA
4-aminobutyrate
- HSP
heat shock protein
- PPDK
pyruvate orthophosphate dikinase
- RCEL
ratio of cumulative expression level
- ROS
reactive oxygen species
- UGPase
UDP-glucose pyrophosphorylase.
Footnotes
The complete set of microarray data reported in this paper has been deposited in the Gene Expression Omnibus (GEO) repository under accession number GSE20345.
References
- Asatsuma S, Sawada C, Kitajima A, Asakura T, Mitsui T. α-Amylase affects starch accumulation in rice grains. J. Appl. Glycosci. 2006;53:187–192. [Google Scholar]
- Aurisano N, Bertani A, Reggiani R. Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol. 1995;36:1525–1529. [Google Scholar]
- Bouché N, Fait A, Bouchez D, Møller SG, Fromm H. Mitchondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl Acad. Sci. USA. 2003;100:6843–6848. doi: 10.1073/pnas.1037532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman JG, Bishop DL. Diurnal O2 and carbohydrate levels in wheat kernels during embryony. J. Plant Physiol. 2004;161:1003–1010. doi: 10.1016/j.jplph.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993;11:113–116. [Google Scholar]
- Ciceri P, Locatelli F, Genga A, Viotti A, Schmidt RJ. The activity of the maize Opaque2 transcriptional activator is regulated diurnally. Plant Physiol. 1999;121:1321–1327. doi: 10.1104/pp.121.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ST, Fang TK, Rovinsky SA, Turano FJ, Moye-Rowley WS. Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 2001;276:244–250. doi: 10.1074/jbc.M007103200. [DOI] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Kondo T, Saito K, Utsumi Y, Tokunaga T, et al. Characterization of SSIIIa-deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007;144:2009–2023. doi: 10.1104/pp.107.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Chino M. Chemical composition of phloem sap from the uppermost internode of the rice plant. Plant Cell Physiol. 1990;31:247–251. [Google Scholar]
- Hoshi Y, Hazeki O, Tamura M. Oxygen dependence of redox state of copper in cytochrome oxidase in vitro. J. Appl. Physiol. 1993;74:1622–1627. doi: 10.1152/jappl.1993.74.4.1622. [DOI] [PubMed] [Google Scholar]
- Ito S, Hara T, Kawanami Y, Watanabe T, Thiraporn K, Ohtake N, et al. Carbon and nitrogen transport during grain filling in rice under high-temperature conditions. J. Agron. Crop Sci. 2009;195:368–376. [Google Scholar]
- Jiang H, Dian W, Wu P. Effect of high temperature on fine structure of amylopectin in rice endosperm by reducing the activity of the starch branching enzyme. Phytochemistry. 2003;63:53–59. doi: 10.1016/s0031-9422(03)00005-0. [DOI] [PubMed] [Google Scholar]
- Kang H-G, Park S, Matsuoka M, An G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB) Plant J. 2005;42:901–911. doi: 10.1111/j.1365-313X.2005.02423.x. [DOI] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, et al. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004;136:4159–4168. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Agarwal M, Grover A. Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol. Biol. 2003;51:677–686. doi: 10.1023/a:1022561926676. [DOI] [PubMed] [Google Scholar]
- Kawagoe Y, Kubo A, Satoh H, Takaiwa F, Nakamura Y. Roles of isoamylase and ADP-glucose pyrophosphorylase in starch granule synthesis in rice endosperm. Plant J. 2005;42:164–174. doi: 10.1111/j.1365-313X.2005.02367.x. [DOI] [PubMed] [Google Scholar]
- Kitajima A, Asatsuma S, Okada H, Hamada Y, Kaneko K, Nanjo Y, et al. The rice α-amylase glycoprotein is targeted from the golgi apparatus through the secretory pathway to the plastids. Plant Cell. 2009;21:2844–2858. doi: 10.1105/tpc.109.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JR, Lee SS, Jang HH, Lee YM, Park JH, Park S-C, et al. Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein, AtTDX. Proc. Natl Acad. Sci. USA. 2009;106:5978–5983. doi: 10.1073/pnas.0811231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Bergersen FJ, Day DA. Oxygen affinity of terminal oxidases in soybean mitochondria. Plant Physiol. Biochem. 1994;32:847–852. [Google Scholar]
- Morita S, Shiratsuchi H, Takahashi J, Fujita K. Effect of high temperature on grain ripening in rice plants. Analysis of the effects of high night and high day temperatures applied to the panicle and other parts of the plant. Jpn J. Crop Sci. 2004;73:77–83. [Google Scholar]
- Mutisya J, Sun C, Rosenquist S, Baguma Y, Jansson C. Diurnal oscillation of SBE expression in sorghum endosperm. J. Plant Physiol. 2009;166:428–434. doi: 10.1016/j.jplph.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Nishi A, Nakamura Y, Tanaka N, Satoh H. Biochemical and genetic analysis of the effects of amylase-extender mutation in rice endosperm. Plant Physiol. 2001;127:459–472. [PMC free article] [PubMed] [Google Scholar]
- Park SK, Jung YJ, Lee JR, Lee YM, Jang HH, Lee SS, et al. Heat-shock and redox-dependent functional switching of an h-type Arabidopsis thioredoxin from a disulfide reductase to a molecular chaperone. Plant Physiol. 2009;150:552–561. doi: 10.1104/pp.109.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, et al. Rice yields decline with higher night temperature from global warming. Proc. Natl Acad. Sci. USA. 2004;101:9971–9975. doi: 10.1073/pnas.0403720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol. Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683–1696. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiani R, Cantu’ CA, Brambilla I, Bertani A. Accumulation and interconversion of amino acids in rice roots under anoxia. Plant Cell Physiol. 1988;29:981–987. [Google Scholar]
- Rolletschek H, Borisjuk L, Sánchez-García A, Gotor C, Romero LC, Martínez-Rivas JM, et al. Temperature-dependent endogenous oxygen concentration regulates microsomal oleate desaturase in developing sunflower seeds. J. Exp. Bot. 2007;58:3171–3181. doi: 10.1093/jxb/erm154. [DOI] [PubMed] [Google Scholar]
- Sato K, Inaba K. High temperature injury of ripening in rice plant. II. Ripening of rice grains when the panicle and straw were separately treated under different temperature. Proc. Crop Sci. Soc. Jpn. 1973;42:214–219. [Google Scholar]
- Sato S, Soga T, Nishioka T, Tomita M. Simultaneous determination of the main metabolites in rice leaves using capillary electrophoresis mass spectrometry and capillary electrophoresis diode array detection. Plant J. 2004;40:151–163. doi: 10.1111/j.1365-313X.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- Satoh H, Shibahara K, Tokunaga T, Nishi A, Tasaki M, Hwang S-K, et al. Mutation of the plastidial α-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell. 2008;20:1833–1849. doi: 10.1105/tpc.107.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield GN, Hirose T, Gaudron JA, Upadhyaya NM, Ohsugi R, Furbank RT. Antisense suppression of the rice sucrose transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct. Plant Biol. 2002;29:815–826. doi: 10.1071/PP01204. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Smith AM, Zeeman SC, Smith SM. Starch degradation. Annu. Rev. Plant Biol. 2005;56:73–98. doi: 10.1146/annurev.arplant.56.032604.144257. [DOI] [PubMed] [Google Scholar]
- Soga T, Heiger DN. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2000;72:1236–1241. doi: 10.1021/ac990976y. [DOI] [PubMed] [Google Scholar]
- Soga T, Ishikawa T, Igarashi S, Sugawara K, Kakazu Y, Tomita M. Analysis of nucleotides by pressure-assisted capillary electrophoresis-mass spectrometry using silanol mask technique. J. Chromatogr. A. 2007;1159:125–133. doi: 10.1016/j.chroma.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003;2:488–494. doi: 10.1021/pr034020m. [DOI] [PubMed] [Google Scholar]
- Soga T, Ross GA. Simultaneous determination of inorganic anions, organic acids, amino acids and carbohydrates by capillary electrophoresis. J. Chromatogr. A. 1999;837:231–239. [Google Scholar]
- Takahashi H, Hayashi M, Goto F, Sato S, Soga T, Nishioka T, et al. Evaluation of metabolic alteration in transgenic rice overexpressing dihydroflavonol-4-reductase. Ann. Bot. 2006;98:819–825. doi: 10.1093/aob/mcl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M, et al. The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotechnol. J. 2004;2:507–516. doi: 10.1111/j.1467-7652.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Wardlaw IF. The effect of high temperature on the accumulation of dry matter, carbon and nitrogen in the kernel of rice. Aust. J. Plant Physiol. 1991;18:259–265. [Google Scholar]
- Umemoto T, Aoki N, Lin H, Nakamura Y, Inouchi N, Sato Y, et al. Natural variation in rice starch synthase IIa affects enzyme and starch properties. Funct. Plant Biol. 2004;31:671–684. doi: 10.1071/FP04009. [DOI] [PubMed] [Google Scholar]
- Umemoto T, Terashima K. Activity of granule-bound starch synthase is an important determinant of amylose content in rice endosperm. Funct. Plant Biol. 2002;29:1121–1124. doi: 10.1071/PP01145. [DOI] [PubMed] [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants: role and regulation. Physiol. Plant. 1995;95:318–325. [Google Scholar]
- Wang E, Wang J, Zhu X, Hao W, Wang L, Li Q, et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008;40:1370–1374. doi: 10.1038/ng.220. [DOI] [PubMed] [Google Scholar]
- Wardlaw IF, Dawson IA, Munibi P, Fewster R. The tolerance of wheat to high temperatures during reproductive growth. I. Survey procedures and general response patterns. Aust. J. Agric. Res. 1989;40:1–13. [Google Scholar]
- Woo M-O, Ham T-H, Ji H-S, Choi M-S, Jiang W, Chu S-H, et al. Inactivation of the UGPase1 gene causes genic male sterility and endosperm chalkiness in rice (Oryza sativa L.) Plant J. 2008;54:190–204. doi: 10.1111/j.1365-313X.2008.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Hirose T, Kuroda M, Yamaguchi T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol. 2007;144:258–277. doi: 10.1104/pp.107.098665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria S, Matsuda T, Tajima S, Nitta Y. Effect of high temperature at ripening stage on the reserve accumulation in seed in some rice cultivars. Plant Prod. Sci. 2002;5:160–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.