Abstract
Objectives
The purpose of this study was to examine the association of progressive versus stable peripheral arterial disease (PAD) with the risk of future cardiovascular disease (CVD) events.
Background
An independent association between PAD, defined by low values of the ankle-brachial index (ABI), and future CVD risk has been demonstrated. However, the prognostic significance of declining versus stable ABI has not been studied.
Methods
We recruited 508 subjects (59 women, 449 men) from 2 hospital vascular laboratories in San Diego, California. ABI and CVD risk factors were measured at Visit 2 (1990 to 1994). ABI values from each subject’s earliest vascular laboratory examination (Visit 1) were abstracted from medical records. Mortality and morbidity were tracked for 6 years after Visit 2 using vital statistics and hospitalization data.
Results
In multivariate models adjusted for CVD risk factors, very low (<0.70) and, in some cases, low (0.70 ≤ ABI <0.90) Visit 2 ABIs were associated with significantly elevated all-cause mortality, CVD mortality, and combined CVD morbidity/mortality at 3 and 6 years. Decreases in ABI of more than 0.15 between Visit 1 and Visit 2 were significantly associated with an increased risk of all-cause mortality (risk ratio [RR]: 2.4) and CVD mortality (RR: 2.8) at 3 years, and CVD morbidity/mortality (RR: 1.9) at 6 years, independent of Visit 2 ABI and other risk factors.
Conclusions
Progressive PAD (ABI decline >0.15) was significantly and independently associated with increased CVD risk. Patients with decreasing ABI may be candidates for more intensive cardiovascular risk factor management.
Keywords: peripheral vascular disease, cardiovascular diseases, risk factors, morbidity, mortality
The association of peripheral arterial disease (PAD) with future cardiovascular disease (CVD) events and CVD and total mortality has been demonstrated in multiple studies. More recent studies using the ankle-brachial index (ABI), the ratio of the ankle to the arm blood pressure, and other noninvasive tests have shown the mortality association to be based largely on increased CVD, and independent of traditional CVD risk factors (1–15).
Most studies have used a dichotomous definition of PAD based on an ABI cut point of 0.90. Although some studies stratified ABI into additional categories for survival curve analysis, only a few have used additional ABI categories in full multivariate analysis. Many studies excluded patients with unusually high ABIs (e.g., ≥1.40), because such ABIs may reflect medial arterial calcification (MAC), which precludes accurate ABI assessment.
Previous studies of the association of PAD with other CVD outcomes used ABI at baseline, and did not address the potential additional significance of changes in ABI over time. The progression of PAD itself has received relatively little attention (16–19). The prognostic significance of PAD progression for incident CVD events is unknown.
In the present study, the association between PAD, as measured by the ABI, with CVD morbidity and mortality was assessed in a group of vascular laboratory patients. The association of Visit 2 ABI with incident CVD morbidity and mortality was first examined, and the additional prognostic significance of changes in ABI between Visits 1 and 2 was then explored.
Methods
Subjects were recruited for Visit 2 in 1990 to 1994 from patients who had been seen in the previous 10 years for noninvasive lower extremity arterial testing at the vascular laboratories of the San Diego Veterans Administration Medical Center (SDVAMC) or the University of California San Diego Medical Center (UCSDMC). Each patient’s first vascular laboratory examination constituted Visit 1 for that patient. Of 2,265 patients having such visits, 481 were deceased and another 1,272 could not be located or declined to participate. Informed consent was obtained from the remaining 512 patients, who were examined for this study (Visit 2). A previous analysis of this cohort found that participants had slightly less advanced PAD than did surviving nonparticipants and included a higher percentage of women (13% vs. 8%), but were similar with respect to age (17).
At Visits 1 and 2, systolic brachial pressure was measured in both arms sphygmomanometrically with detection at the third finger by photoplethysmography, and ankle systolic blood pressure was similarly measured with detection at the toe (20,21). At Visit 2, subjects completed a health history questionnaire and the San Diego Claudication Questionnaire (SDCQ) (22). Basic laboratory, anthropometric, and physiologic measurements were obtained. Information from the earliest prior vascular laboratory visit (Visit 1) was abstracted for all subjects. The ABI at Visit 1 was used to calculate the change in ABI in the period before Visit 2 (mean ± SD: 5.0 ± 2.4 years). Four subjects failed to provide a Social Security number, the primary identifier used for morbidity and mortality follow-up (see the following text); these subjects were excluded from subsequent analysis. This resulted in a final group of 508 subjects.
Mortality in the study cohort after Visit 2 was identified using Social Security Administration data available through the end of 2002. A certified nosologist coded the causes of death based on death certificates. CVD morbidity, defined as inpatient hospitalization, was identified from 2 sources. Electronic hospital admission records for study participants were obtained from the SDVAMC. In addition, hospitalizations were identified from data collected by the California Office of Statewide Health Planning and Development, which include all admissions to all nonfederal hospitals in the state. Hospital discharge codes have been shown to agree well with independent assessment for cardiovascular diagnoses in Medicare patients (23). To protect patient privacy, data processing that involved the Office of Statewide Health Planning and Development data was carried out on remote secure servers by Health Information Solutions of Rocklin, California under contract with the Office of Statewide Health Planning and Development.
Three end points were analyzed: all cause mortality, CVD mortality, and combined CVD morbidity and mortality. Mortality was classified based on listed causes of death on the death certificate. Morbidity was classified based on the principal hospitalization diagnosis. A morbidity/mortality end point was identified as the earliest CVD morbidity or mortality event for a subject. CVD mortality and morbidity were identified by International Classification of Disease-9 (mortality) or International Classification of Disease-9-Clinical Modification (morbidity) codes in the range 401 to 437.9, excluding 412.
The ABI for each leg was computed as the ratio of the ankle pressure for that leg to the higher of the left and right brachial pressures. The higher brachial pressure was used because of the strong correlation between PAD and subclavian stenosis (24). The lower ABI value of the 2 legs was used. The ABI was categorized into five ranges: <0.70, 0.70 ≤ ABI <0.90, 0.90 ≤ ABI <1.00, 1.00 ≤ ABI <1.40, and ≥1.40. In addition, the set of models evaluating PAD progression and outcome had the change in ABI from Visit 1, categorized into 3 ranges: <−0.15, −0.15 to +0.15, and >+0.15. This 0.15 cut point has been used to represent clinically significant ABI change in several studies (16–18). In these latter models, subjects with Visit 1 or 2 ABI values ≥1.4 were excluded, since ABI change could be biased by MAC. The Visit 1 ABI value was also the lower of the 2 legs.
The following baseline cardiovascular risk factors were included in the analysis: age, gender, race (non-Hispanic white vs. other), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, (log) triglycerides, self-reported history of coronary heart disease (myocardial infarction, coronary artery bypass graft, or percutaneous transluminal coronary angioplasty), and self-reported history of stroke. Diabetes was included as a dichotomous variable based on plasma glucose ≥126 mg/dl among subjects reporting having fasted for 8 or more hours before their examination, or self-reported use of insulin or oral hypoglycemics (25). Hypertension was included as a dichotomous variable based on systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-reported use of hypertension medication (26). Smoking was included in models as 2 separate variables: pack-years and smoking status (current/former/never). The SDCQ was included as a 5-level categorical variable, based on the most symptomatic leg (22). Models involving the change in ABI were also adjusted for the time elapsed between ABI measurements. At Visit 2, 32.9% of subjects reported earlier revascularization for PAD, which was coded as a dichotomous variable.
Subjects were tabulated by morbidity and mortality status, and age-adjusted mean values of risk factors were calculated within these groups. To test for proportional hazards over follow-up time, initially Cox models for the 3 end points (all-cause mortality, CVD mortality, and combined morbidity and mortality from CVD) were fit for 3- and 6-year durations from the time of Visit 2. The relative hazard for all-cause and CVD mortality decreased over time, so separate logistic models at 3 and 6 years of follow-up were used for mortality analyses. For combined CVD morbidity and mortality, hazards remained proportional during follow-up so Cox models were fit for full (6-year) follow-up only. The odds ratios from the logistic models and hazard ratios from the Cox models are both subsequently referred to as “risk” ratios. Visit 2 ABI was considered in a first set of models; Visit 2 ABI and change in ABI from Visit 1 were both included in a second set of models. Simultaneous inclusion in models of the Visit 2 ABI category and the change category controls for regression to the mean and provides an estimate of the risk associated with ABI change independent of the Visit 2 ABI. All models were adjusted for the CVD risk factors previously described above. Separate gender-specific models were run for men only (numbers were too small for separate models for women). Models excluding subjects with previous revascularization for PAD were also fit and were compared with the models using statistical adjustment for revascularization for PAD. Separate models were also run for subjects with or without a history of coronary heart disease or stroke.
Smaller numbers necessitated simplified variables in these stratified models: the SDCQ was reduced to a single dichotomous variable (exertional leg pain, yes/no), and ABI was reduced to a 3-level variable (<0.70, 0.70 ≤ ABI <0.90, and 0.90 ≤ ABI <1.40) with values ≥1.40 excluded. These simplified models were also used to test for statistical interaction of history of coronary heart disease and stroke with ABI and ABI change in the whole cohort. Interactions of ABI and ABI change with history of revascularization for PAD were also modeled. All analyses were performed using SAS software (SAS Institute, Inc., Cary, North Carolina).
The study protocol was approved by the institutional review boards of SDVAMC and the University of California San Diego, and the California State Committee for the Protection of Human Subjects.
Results
Three years after Visit 2, 302 of 508 subjects (59.4%) were alive and had not been hospitalized for CVD (reference group). There were 121 (23.8%) who had been hospitalized for a principal diagnosis of CVD but had not died of CVD. Sixty-five (12.8%) were dead due to CVD, and the remaining 20 (3.9%) were dead from another cause without previous CVD hospitalization.
Table 1 shows that subjects with CVD hospitalization and deceased subjects were somewhat older at baseline than subjects in the reference group. The groups did not differ significantly with respect to claudication symptoms. At baseline, the age-, gender- and ethnic-adjusted percentage of reference group subjects who had very low ABI (<0.70) was 32.2%; the corresponding proportion was significantly higher among subjects hospitalized for nonfatal CVD (44.3%; p < 0.05) as well as among subjects who died of CVD (63.2%; p < 0.0001). The adjusted proportion of patients with “normal” ABI (1.00 ≤ ABI <1.40) was higher in the reference group (39.4%) than it was among subjects hospitalized for nonfatal CVD (29.2%, p = 0.05) and among subjects dying of CVD (10.7%, p < 0.0001). The proportions of subjects in the other Visit 2 ABI categories did not differ significantly across groups.
Table 1.
Demographic and Peripheral Arterial Disease Characteristics by 3-Year Morbidity/Mortality Status, 508 Vascular Laboratory Patients, San Diego, California, Recruited 1990 to 1994
| Variable* | Alive, No CVD Hospitalization |
CVD Hospitalization But No CVD Death |
Dead From CVD | No CVD Hospitalization, Dead From Non-CVD Cause |
|---|---|---|---|---|
| n | 302 | 121 | 65 | 20 |
| Age (yrs) | 67.2 | 69.1§ | 70.2§ | 69.1 |
| Gender (% female) | 14.6 | 7.4§ | 6.2 | 10.0 |
| Race (% other than non-Hispanic white) | 13.2 | 10.7 | 10.8 | 15.0 |
| San Diego Claudication Questionnaire (% of subjects)† |
||||
| No pain | 27.9 | 20.7 | 21.2 | 20.2 |
| Pain at rest | 24.4 | 29.6 | 34.8 | 30.1 |
| Non-calf pain | 2.7 | 2.2 | 6.4 | 4.0 |
| Non-rose calf pain | 13.8 | 17.8 | 17.3 | 14.7 |
| Rose | 29.6 | 28.4 | 18.6 | 29.2 |
| Visit 2 ABI (%) | ||||
| ≥1.40‡ | 1.3 | 1.7 | 3.1 | 0.0 |
| 1.00 ≤ ABI <1.40 | 39.4 | 29.2 | 10.7¶ | 26.6 |
| 0.90 ≤ ABI <1.00 | 9.3 | 8.4 | 6.4 | 14.9 |
| 0.70 ≤ ABI <0.90 | 17.7 | 16.4 | 16.6 | 24.7 |
| <0.70 | 32.2 | 44.3§ | 63.2¶ | 33.8 |
| ABI change, Visit 1 to Visit 2 (%) | ||||
| >+0.15 | 25.9 | 21.9 | 15.1 | 19.9 |
| Between −0.15 and +0.15 | 63.1 | 58.0 | 55.0 | 61.1 |
| <−0.15 | 11.0 | 20.2§ | 29.9∥ | 19.0 |
Variables other than age, gender, and race are adjusted for age, gender, and race, except as noted.
SDCQ categories are listed from best to worst, as used to determine worst leg.
Data too sparse for age/gender/race adjustment by logistic regression; percentages are raw, not significantly different per Fisher’s exact test. Significantly different from “Alive, no CVD hospitalization:”
p < 0.05;
p < 0.001;
p < 0.0001.
ABI = ankle-brachial index; CVD = cardiovascular disease; SDCQ = San Diego Claudication Questionnaire.
Compared with the reference group, a significantly higher adjusted proportion of subjects hospitalized with nonfatal CVD had decreases in ABI of more than 0.15 between Visits 1 and 2 (20.2% vs. 11.0%, p < 0.05). This was even more pronounced for subjects who died of CVD (29.9%, p < 0.001).
Table 2 compares the 4 morbidity/mortality groups on other risk variables. Compared with the reference group, subjects with CVD hospitalization and CVD decedents had significantly more hypertension and diabetes, as well as Visit 2 coronary heart disease. CVD decedents also had a greater stroke prevalence at Visit 2 (27.4% vs. 14.2%, p < 0.05).
Table 2.
CVD Risk Factors by 3-Year Morbidity/Mortality Status, 508 Vascular Laboratory Patients, San Diego, California, Recruited 1990 to 1994
| Variable at Visit 2* | Alive, No CVD Hospitalization |
CVD Hospitalization But No CVD Death |
Dead From CVD | No CVD Hospitalization, Dead From Non-CVD Cause |
|---|---|---|---|---|
| n | 302 | 121 | 65 | 20 |
| BMI (kg/m2) | 26.9 | 27.8 | 26.3 | 26.7 |
| HDL cholesterol (mg/dl) | 46.9 | 45.0 | 43.9 | 45.7 |
| LDL cholesterol (mg/dl) | 131.5 | 131.5 | 133.3 | 112.1† |
| Log triglycerides (mg/dl) | 4.9 | 5.0 | 4.9 | 4.8 |
| Diabetes (%) | 26.5 | 45.1‡ | 50.4‡ | 40.2 |
| Hypertension (%) | 74.5 | 90.8‡ | 88.8† | 74.5 |
| Pack-years, n | 48.1 | 47.5 | 52.3 | 54.2 |
| Current smokers (%) | 28.6 | 29.0 | 30.2 | 30.2 |
| Past smokers (%) | 57.0 | 55.1 | 47.1 | 65.0 |
| Coronary heart disease history (%) | 31.8 | 52.4‡ | 48.8† | 9.5† |
| Stroke history (%) | 14.2 | 20.5 | 27.4† | 29.5 |
Variables are adjusted for age, gender, and race. Significantly different from “Alive, no CVD hospitalization:”
p < 0.05;
p < 0.001.
BMI = body mass index; CVD = cardiovascular disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Of 508 subjects, 482 (94.9%) had complete information on all adjustment variables and Visit 2 ABI and were included in the multivariate models presented in Figure 1. The 26 subjects with missing data did not differ significantly from the other subjects with respect to age, gender, race, or Visit 2 ABI.
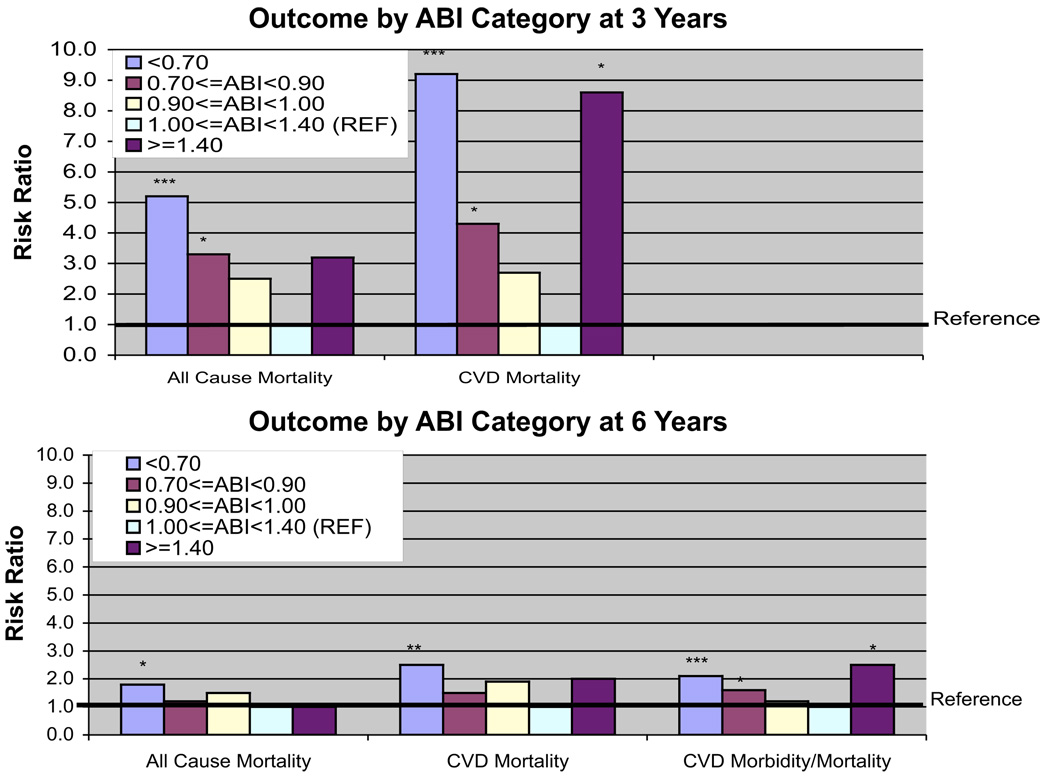
Figure 1. 3- and 6-Year All-Cause Mortality and CVD Events by ABI Category, Vascular Laboratory Patients, San Diego, California, Recruited 1990 to 1994.
Mortality model are logistic, morbidity/mortality model is Cox. Models adjusted for age, gender, race, body mass index, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, log triglycerides, diabetes, hypertension, pack-years smoking, smoking status (current/past/never), history of coronary heart disease, history of stroke, claudication status and history of surgery for peripheral artery disease. *p < 0.05; **p < 0.01; ***p < 0.001. ABI = ankle-brachial index; CVD = cardiovascular disease.
Figure 1 shows risk ratios (RRs) for Visit 2 ABI as a 5-level categorical variable, for 3 end points: all-cause mortality, CVD mortality, and CVD morbidity/mortality. All models were fully adjusted for CVD risk factors as well as claudication and peripheral revascularization history. Results for 3- and 6-year follow-ups are shown for mortality. Compared with subjects in the reference category (1.00 ≤ ABI <1.40), subjects with very low ABI (<0.70) had a significantly elevated risk of events for all end points at both 3- and 6-year follow-ups. Risk ratios ranged from 1.8 (p = 0.03) for 6-year all-cause mortality to 9.2 (p < 0.0001) for 3-year CVD mortality.
Subjects with low ABI (0.70 ≤ ABI < 0.90) had significantly elevated risks of 3-year all-cause mortality (RR: 3.3, p < 0.01), 3-year CVD mortality (RR: 4.3, p = 0.01), and 6-year CVD morbidity/mortality (RR: 1.6, p < 0.05). Subjects with ABI in the 0.90 ≤ ABI <1.00 range had risk ratio estimates >1 for all end points and durations, but these results did not achieve statistical significance.
There were only 8 subjects in the high ABI category (≥1.40). Nonetheless, for 3-year CVD mortality, a statistically significant risk ratio of 8.6 (p = 0.03) was observed. Significantly elevated risk in the high ABI category was also observed for 6-year CVD morbidity/mortality (RR: 2.5, p < 0.05). Risk ratio point estimates for all other end points and durations were >1, but did not achieve statistical significance.
Table 3 shows risk ratios and p values for the change in ABI between Visits 1 and 2. Adjusted for Visit 2 ABI and other potential confounders, compared with an ABI change of <0.15, a decrease in ABI of >0.15 was significantly associated with 3-year all-cause mortality (RR: 2.4, p = 0.01) and 3-year CVD mortality (RR: 2.8, p < 0.01). Estimated risk ratios at 6 years were >1, but did not achieve statistical significance for any of the outcomes.
Table 3.
Association of 3- and 6-Year CVD Outcomes With Visit 2 ABI and Change From Visit 1 in ABI, Vascular Laboratory Patients, San Diego, California, Recruited 1990 to 1994*
| All-Cause Mortality |
CVD Mortality |
CVD Morbidity/Mortality |
||||
|---|---|---|---|---|---|---|
| ABI | RR (95% CI) | p Value | RR (95% CI) | p Value | RR (95% CI) | p Value |
| 3 yrs | ||||||
| Visit 2 ABI | ||||||
| 1.00 ≤ ABI <1.40 | 1.0 | 1.0 | ||||
| 0.90 ≤ ABI <1.00 | 2.2 (0.7–6.7) | 0.17 | 2.2 (0.5–10.0) | 0.30 | ||
| 0.70 ≤ ABI <0.90 | 2.5 (1.0–6.3) | 0.05 | 2.9 (0.9–9.4) | 0.08 | ||
| <0.70 | 3.9 (1.7–8.9) | 0.001 | 6.6 (2.4–18.6) | <0.001 | ||
| ABI change | ||||||
| Increase ≥0.15 | 0.8 (0.3–1.7) | 0.53 | 0.8 (0.3–2.1) | 0.65 | ||
| Change <0.15 | 1.0 | 1.0 | ||||
| Decrease ≥0.15 | 2.4 (1.2–4.8) | 0.01 | 2.8 (1.3–6.0) | 0.01 | ||
| 6 yrs | ||||||
| Visit 2 ABI | ||||||
| 1.00 ≤ ABI <1.40 | 1.0 | 1.0 | 1.0 | |||
| 90 ≤ ABI <1.00 | 1.5 (0.7–3.5) | 0.31 | 1.8 (0.7–4.7) | 0.24 | 1.1 (0.6–1.9) | 0.70 |
| 0.70 ≤ ABI <0.90 | 1.1 (0.5–2.2) | 0.81 | 1.4 (0.6–3.0) | 0.40 | 1.5 (1.0–2.3) | 0.04 |
| <0.70 | 1.6 (0.9–3.0) | 0.12 | 2.4 (1.2–4.7) | 0.02 | 1.9 (1.3–2.7) | 0.001 |
| ABI change | ||||||
| Increase ≥0.15 | 0.8 (0.5–1.5) | 0.53 | 1.2 (0.6–2.2) | 0.66 | 1.0 (0.7–1.4) | 0.99 |
| Change <0.15 | 1.0 | 1.0 | 1.0 | |||
| Decrease ≥0.15 | 1.2 (0.6–2.2) | 0.61 | 1.2 (0.6–2.4) | 0.56 | 1.3 (0.9–1.8) | 0.21 |
Mortality models are logistic; morbidity/mortality models are Cox. Models include Visit 2 ABI and ABI change and are adjusted for age, gender, race, body mass index, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, log triglycerides, diabetes, hypertension, pack-years smoking, smoking status (current/past/never), history of coronary heart disease, history of stroke, claudication status and peripheral revascularization.
CI = confidence interval; RR = risk ratio; other abbreviations as in Table 1.
The ABI change models in Table 3 were adjusted for Visit 2 ABI using the categorical ABI variable. In the ABI change models, very low Visit 2 ABI (<0.70) was significantly associated with higher risk of all 3 outcomes at all durations except all-cause mortality at 6 years. Compared with the models in Figure 1, the RR estimates for low and very low Visit 2 ABI were generally lower in Table 3, suggesting that associations for Visit 2 ABI in Figure 1 were partially due to an ABI decline from Visit 1.
In multivariate models that excluded subjects who had undergone procedures for peripheral arterial disease before Visit 2 (not shown), the observed associations were similar in direction and magnitude to the models presented previously. This was also true of models in men only (not shown).
Separate models were also run based on history of coronary heart disease (CHD) or stroke. These stratified models also showed independent and significant contributions of Visit 2 ABI and change in ABI to CVD morbidity and mortality.
In models including statistical interaction between selected variables, ABI and ABI change demonstrated no significant interactions with history of CHD and stroke, corrected for the large number of potential interactions tested. The same was true of interactions of ABI with ABI change, and of ABI and ABI change with history of revascularization for PAD.
Discussion
The observed association of low ABI values with future risk of cardiovascular disease morbidity and mortality in the present study is generally consistent with that reported in other studies (1−15), as is the predictive value of a high (≥1.40) ABI (14,15,27).
In the present study, without including ABI change variables, RR point estimates increased monotonically with lower ABI, with the <0.70 category RR reaching statistical significance in all but 1 model. A number of studies have presented evidence for a dose-response relationship between lower ABI and future CVD events. In some cases this evidence was limited to Kaplan-Meier curves without full multivariate adjustment (6,8,9). Using a Cox model with multivariate adjustment, Tsai et al. (12) reported a borderline significant trend for ABI categories for the end point of ischemic stroke. Other studies using Cox models with multivariate adjustment have found such a trend toward higher CVD risk in successively lower ranges of ABI, but did not find all the successive categories to be significantly different or, in some cases, even monotonically increasing (13–15).
Decreases in ABI of more than 0.15 were associated with a significantly higher risk of events, independent of both Visit 2 ABI and time elapsed between Visits 1 and 2. In the present study, 16.7% of subjects had such decreases, suggesting that this is reasonably common in a vascular laboratory population. Since only two-thirds of subjects met a standard PAD definition at Visit 2, these results refer to overall progression of peripheral atherosclerosis in a vascular laboratory cohort, rather than progression of clinical PAD per se. It has been previously observed that progression of coronary atherosclerosis is predictive of subsequent coronary events (28,29). To our knowledge, the present study is the first to demonstrate the independent significance of PAD progression for incident CVD events.
Cronenwett et al. (16) compared patients whose PAD either remained stable or progressed, based on reduced walking tolerance. They found that the change in mean ABI over the observation period was significantly different in the 2 groups, with a larger decrease among the patients whose symptoms progressed, but that the mean ABI at the start of the period did not differ between the 2 groups. The same was true comparing patients undergoing surgery for PAD with those not having operations. Cronenwett concluded that initial ABI is “an unsuitable criterion to accurately predict outcome,” but that “attention to a deteriorating ABI” is necessary to plan timely operative intervention. This parallels the association of change in ABI with CVD risk demonstrated in the present study, although in this study low ABI remained predictive in many models.
The ability of ABI to predict CVD outcomes even in a high-morbidity, high-mortality vascular laboratory population is itself a new finding; previously, this has been demonstrated in such a population only with respect to all-cause mortality (30). This is of practical clinical interest, since at present most ABI measurements are taken in such settings.
In separate models, subjects with and without history of CHD or stroke showed associations similar to the whole group. Newman et al. (11) also reported associations between low ABI and CVD in separate analyses of subjects with and without a baseline history of disease. Although many earlier studies excluded subjects with baseline CVD, an association between PAD and adverse outcomes has been demonstrated in several cohorts consisting exclusively of subjects with CVD, such as myocardial infarction (31,32) and angina patients (33).
In most previous studies, high ABI values (e.g., ≥1.40) were excluded from analysis, because they were thought to be due to MAC, which makes arteries difficult to compress and yields erroneously high ankle blood pressures. MAC is most common in patients with diabetes and renal disease, and has been shown to be independently associated with cardiovascular disease morbidity and mortality in these groups (34,35). These findings are consistent with our results, and with results from population studies (14,15,27). Thus, a high ABI predicts high CVD risk, perhaps because a majority of such patients have underlying PAD (36,37).
The present study has a number of potential limitations. Factors that predispose patients to participate in a clinical study may skew recruitment in unknown ways, although the available information on nonparticipants did not suggest strong selection effects (17). Morbidity follow-up did not include hospitalization outside California or at Veterans Administration facilities other than SDVAMC, although these numbers are likely to be small. The time elapsed between the Visit 1 and 2 ABI measurements should ideally have been uniform. Since this was not feasible, models included adjustment for differences in the time elapsed between ABI measurements.
Conclusions
These data provide the first evaluation of the relation of PAD progression to subsequent CVD morbidity and mortality, and show a consistent and significant association, independent of the severity of PAD and traditional CVD risk factors. The present study also confirms the independent association of very low and low ABI (<0.70 and 0.70 ≤ ABI <0.90) with an increased risk of all-cause mortality, CVD mortality, and combined CVD morbidity/mortality, with a greater increase in risk in the lowest ABI group, as well as the association of high ABI values (≥1.40) with an increased CVD event risk. In addition to the known prognostic significance of a low ABI, and more recently, a high ABI, the evidence here shows a deteriorating ABI also independently carries a poor prognosis.
Acknowledgment
The authors are indebted to Ms. Julie Denenberg for assistance with statistical analysis.
This research was supported by National Institutes of Health Grant HL42973, NIH-NCRR General Clinical Research Center Program Grant M01 RR00827, and American Heart Association Grant-in-Aid No. 0050002N.
Abbreviations and Acronyms
- ABI
ankle-brachial index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- MAC
medial arterial calcification
- PAD
peripheral arterial disease
- RR
risk ratio
- SDCQ
San Diego Claudication Questionnaire
Footnotes
William Hiatt, MD, served as Guest Editor for this article.
REFERENCES
- 1.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation. 1985;72:768–773. doi: 10.1161/01.cir.72.4.768. [DOI] [PubMed] [Google Scholar]
- 2.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, Feigelson HS. Coronary disease and stroke in patients with large-vessel peripheral arterial disease. Drugs. 1991;42 Suppl 5:16–21. doi: 10.2165/00003495-199100425-00005. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 6.Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–469. [PubMed] [Google Scholar]
- 7.Kornitzer M, Dramaix M, Sobolski J, Degre S, De Backer G. Ankle/arm pressure index in asymptomatic middle-aged males: an independent predictor of ten-year coronary heart disease mortality. Angiology. 1995;46:211–219. doi: 10.1177/000331979504600304. [DOI] [PubMed] [Google Scholar]
- 8.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–1444. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AB, Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc. 1997;45:1472–1478. doi: 10.1111/j.1532-5415.1997.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 10.Jager A, Kostense PJ, Ruhe HG, et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:617–624. doi: 10.1161/01.atv.19.3.617. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 12.Tsai AW, Folsom AR, Rosamond WD, Jones DW. Ankle-brachial index and 7-year ischemic stroke incidence: the ARIC study. Stroke. 2001;32:1721–1724. doi: 10.1161/01.str.32.8.1721. [DOI] [PubMed] [Google Scholar]
- 13.Hooi JD, Stoffers HE, Kester AD, Van RJ, Knottnerus JA. Peripheral arterial occlusive disease: prognostic value of signs, symptoms, and the ankle-brachial pressure index. Med Decis Making. 2002;22:99–107. doi: 10.1177/0272989X0202200208. [DOI] [PubMed] [Google Scholar]
- 14.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 15.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 16.Cronenwett JL, Warner KG, Zelenock GB, et al. Intermittent claudication. Current results of nonoperative management. Arch Surg. 1984;119:430–436. doi: 10.1001/archsurg.1984.01390160060012. [DOI] [PubMed] [Google Scholar]
- 17.Bird CE, Criqui MH, Fronek A, Denenberg JO, Klauber MR, Langer RD. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc Med. 1999;4:15–21. doi: 10.1177/1358836X9900400103. [DOI] [PubMed] [Google Scholar]
- 18.Nicoloff AD, Taylor LM, Jr, Sexton GJ, et al. Relationship between site of initial symptoms and subsequent progression of disease in a prospective study of atherosclerosis progression in patients receiving long-term treatment for symptomatic peripheral arterial disease. J Vasc Surg. 2002;35:38–46. discussion 46-37. [PubMed] [Google Scholar]
- 19.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–2629. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 20.Fronek A, Johansen KH, Dilley RB, Bernstein EF. Noninvasive physiologic tests in the diagnosis and characterization of peripheral arterial occlusive disease. Am J Surg. 1973;126:205–214. doi: 10.1016/s0002-9610(73)80154-0. [DOI] [PubMed] [Google Scholar]
- 21.Thulesius O. Principles of pressure measurement. In: Bernstein EF, editor. Non-invasive Diagnostic Techniques in Vascular Disease. 3rd edition. St Louis, MO: CV Mosby; 1985. pp. 77–82. [Google Scholar]
- 22.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 27.Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mack WJ, Hodis HN. Efficacy of interventions designed to inhibit the progression of coronary atherosclerosis. Diabetes Res Clin Pract. 1996;30 Suppl:37–53. doi: 10.1016/s0168-8227(96)80037-4. [DOI] [PubMed] [Google Scholar]
- 29.Azen SP, Mack WJ, Cashin-Hemphill L, et al. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation. 1996;93:34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 30.McDermott MM, Feinglass J, Slavensky R, Pearce WH. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med. 1994;9:445–449. doi: 10.1007/BF02599061. [DOI] [PubMed] [Google Scholar]
- 31.Pardaens J, Lesaffre E, Willems JL, De Geest H. Multivariate survival analysis for the assessment of prognostic factors and risk categories after recovery from acute myocardial infarction: the Belgian situation. Am J Epidemiol. 1985;122:805–819. doi: 10.1093/oxfordjournals.aje.a114164. [DOI] [PubMed] [Google Scholar]
- 32.Behar S, Zion M, Reicher-Reiss H, Kaplinsky E, Goldbourt U. Short-and long-term prognosis of patients with a first acute myocardial infarction with concomitant peripheral vascular disease. SPRINT Study Group. Am J Med. 1994;96:15–19. doi: 10.1016/0002-9343(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 33.Eagle KA, Rihal CS, Foster ED, Mickel MC, Gersh BJ. Long-term survival in patients with coronary artery disease: importance of periph-eral vascular disease. The Coronary Artery Surgery Study (CASS) Investigators. J Am Coll Cardiol. 1994;23:1091–1095. doi: 10.1016/0735-1097(94)90596-7. [DOI] [PubMed] [Google Scholar]
- 34.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 35.Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predictscardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 36.Suominen V, Rantanen T, Venermo M, Saarinen J, Salenius J. Prevalence and risk factors of PAD among patients with elevated ABI. Eur J Vasc Endovasc Surg. 2008;35:709–714. doi: 10.1016/j.ejvs.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Aboyans A, Ho E, Denenberg JO, Ho LA, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and non-diabetic subjects. J Vasc Surg. 2008 Aug 8; doi: 10.1016/j.jvs.2008.06.005. [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]