Abstract
Background
Pandemic influenza A (H1N1) virus emerged during 2009. To help clinicians triage adults with acute respiratory illness, a scoring system for influenza-like illness (ILI) was implemented at Hospital Civil de Guadalajara, Mexico.
Methods
A medical history, laboratory and radiology results were collected on emergency room (ER) patients with acute respiratory illness to calculate an ILI-score. Patients were evaluated for admission by their ILI-score and clinicians' assessment of risk for developing complications. Nasal and throat swabs were collected from intermediate and high-risk patients for influenza testing by RT-PCR. The disposition and ILI-score of those oseltamivir-treated versus untreated, clinical characteristics of 2009 pandemic influenza A (H1N1) patients versus test-negative patients were compared by Pearson's Χ2, Fisher's Exact, and Wilcoxon rank-sum tests.
Results
Of 1840 ER patients, 230 were initially hospitalized (mean ILI-score = 15), and the rest were discharged, including 286 ambulatory patients given oseltamivir (median ILI-score = 11), and 1324 untreated (median ILI-score = 5). Fourteen (1%) untreated patients returned, and 3 were hospitalized on oseltamivir (median ILI-score = 19). Of 371 patients tested by RT-PCR, 104 (28%) had pandemic influenza and 42 (11%) had seasonal influenza A detected. Twenty (91%) of 22 imaged hospitalized pandemic influenza patients had bilateral infiltrates compared to 23 (38%) of 61 imaged hospital test-negative patients (p<0.001). One patient with confirmed pandemic influenza presented 6 days after symptom onset, required mechanical ventilation, and died.
Conclusions
The triaging system that used an ILI-score complimented clinicians' judgment of who needed oseltamivir and inpatient care and helped hospital staff manage a surge in demand for services.
Introduction
The severity of seasonal influenza epidemics is unpredictable and influenced by the predominant circulating virus strains and level of immunity in the population [1]. During peak community influenza activity, hospitals and emergency rooms may be overwhelmed by patients presenting with influenza-like illness (ILI) and more severe disease [2], [3]. Illness attack rates may be higher among most age groups during pandemics than observed for seasonal influenza due to limited immunity among exposed populations [4]. The re-emergence of highly pathogenic avian influenza A (H5N1) virus among poultry with sporadic transmission to exposed persons and the resulting high mortality has stimulated global influenza pandemic preparedness [5].
Key features of pandemic influenza planning are developing strategies to meet expected increased demand for patient care, and how to allocate limited resources, including ventilators and critical care [6]–[9]. Guidance has been developed for clinical triage of patients with ILI, including special populations (e.g. children, pregnant women), during a pandemic [10]–[12]. A key clinical decision is determining which ill persons can be managed as outpatients and which require hospitalization. Scoring systems, with varying predictive power, have been developed to determine who will require hospitalization, need ICU care, require a ventilator, or is at high risk of death (e.g. CURB-65)[13]–[15].
The emergence of 2009 pandemic influenza A (H1N1) virus has presented a great challenge for clinicians throughout the world [16]. Overwhelming demand for medical care by patients with ILI and limited availability of oseltamivir necessitated that clinicians rapidly triage patients for outpatient care or hospital admission. These challenges are compounded by the need for early oseltamivir treatment of influenza patients for optimal efficacy [17]. At the Hospital Civil de Guadalajara, Fray Antonio Alcalde (HCGFAA), Mexico, clinicians from the Adult Infectious Diseases Unit used a modified ILI scoring system to systematically triage adult patients with respiratory complaints and determine who would be prioritized for hospitalization and antivirals. We describe this triaging system during the peak 2009 pandemic in Guadalajara (April–August, 2009).
Methods
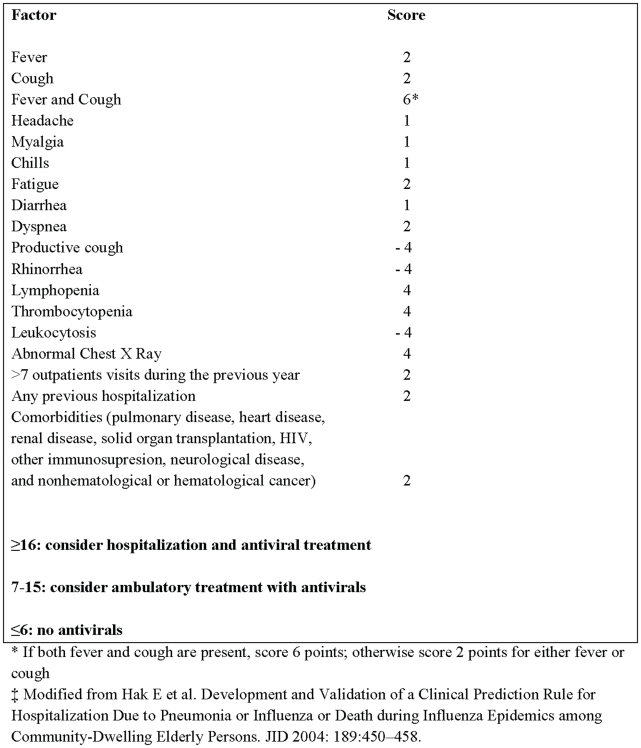
HCGFAA is a 1000-bed tertiary care facility with a 30-bed infectious diseases unit. In response to high demand for emergency medical services among adult patients with acute respiratory complaints, infectious disease specialists implemented an ILI scoring system on April 25, 2009. This scoring system was adapted from a system developed by Hak et al in the United States for hospitalization decision-making among elderly patients with pneumonia or influenza during influenza epidemics [18]. In the emergency room (ER), a questionnaire was used to record patients' demographics, signs and symptoms, history of health care utilization, chronic medical conditions, laboratory, and radiology findings to calculate patients' ILI-scores (Figure 1). Clinicians used an ILI-score ≥16 (high-risk), their judgment of patients' severity of illness and proximity to the hospital to decided whether to admit the patient and treat them with oseltamivir. Patients with intermediate ILI-scores (7–15) were discharged from the ER, treated with oseltamivir and followed daily by phone for 10 days. Those with low ILI-scores (≤6) were discharged without antiviral treatment, and instructed to return if their symptoms worsened.
Figure 1. Influenza Scoring System at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico‡.
Nasal and throat swab specimens were collected from all high-risk and intermediate-risk patients. Swabs were combined in phosphate-buffered saline viral transport media and split into aliquots for influenza testing. One aliquot was tested by rapid diagnostic test (QuickVue Influenza Test, Quidel, San Diego, CA) and immunofluorescence at the hospital. A second aliquot was sent frozen at −70°C to the National Public Health (InDRE) laboratory in Mexico City. InDRE tested the samples with real-time RT-PCR (rRT-PCR) using a multiplex assay and 4 sets of primers (i.e. influenza A, universal swine, 2009 pandemic influenza A (H1N1), and a control for human genetic material) [19]. Each hospitalized patient had a chest x-ray and a chest CT scan performed at admission.
Clinicians prescribed standard doses of oseltamivir 75 mg BID for five days [17]. Hospitalized patients assessed to have severe illness received 150 mg of oseltamivir PO BID ×5 days, amantadine 300 mg PO BID ×10 days, broad spectrum antibiotics (e.g. linezolid), and paracetamol. Patients were discharged when afebrile and without dyspnea.
Patients' demographics, clinical presentation, treatments, and outcome data were entered into an SPSS database. The ILI-score, treatment, disposition, and virology results of triaged patients were compared by Pearson's Χ2, Fisher's Exact, Student t-tests, and Wilcoxon rank-sum tests.
The study was approved by the research ethics committee of the Hospital Civil de Guadalajara, Fray Antonio Alcalde and the final draft for publication was also approved by the research ethics committee of the Hospital Civil de Guadalajara, Fray Antonio Alcalde. Investigators kept the datasets in password protected systems and presented data without identifiers to protect the anonymity of case-patients.
Results
Disposition of Triaged Patients
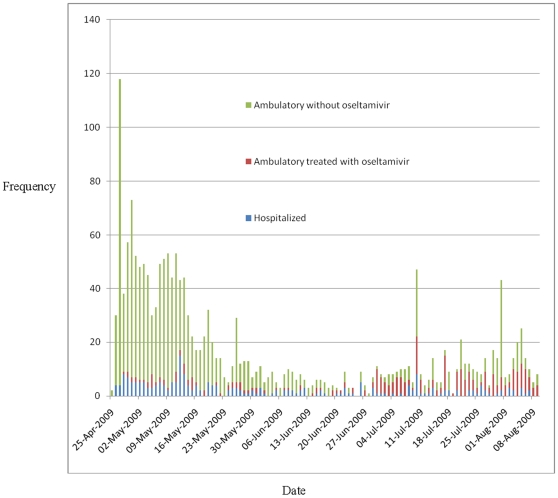
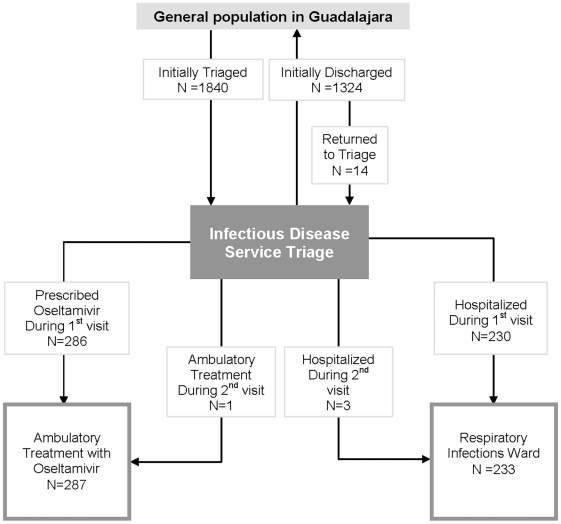
During April 25–August 9, hospital staff triaged 1840 persons with acute respiratory infections (Figure 2). Patients' median age was 29 years [IQR 22–41 years] and 55% were female. Of the 1840 ER patients, 167 (9.1%) were classified at high risk (mean ILI-score = 19), 725 (39.4%) at intermediate risk (median ILI-score = 10), and 945 (51.4%) at low risk (median ILI-score = 3) of developing complications of presumptive 2009 pandemic influenza A (H1N1) disease (Table 1). Two-hundred and thirty (12.5%) were admitted to hospital (median ILI-score = 15 [IQR = 11–19]) (Figure 3). Of 286 ambulatory patients who were prescribed oseltamivir (median ILI-score = 11, IQR = 7–15), none required subsequent medical evaluation. Of 1324 ambulatory patients who were not treated with oseltamivir (median ILI-score = 5, IQR = 1–8), 14 (0.8%) returned a median of 8 days after their initial visit. Three (21%) of the 14 returning patients (i.e. one pregnant and two with a history of tobacco abuse), were hospitalized and treated with oseltamivir (with a median ILI-score = 19). Two of these 3 returning patients who were subsequently hospitalized tested positive for 2009 pandemic influenza (H1N1). One (7%) of the 14 returning patients was prescribed oseltamivir and discharged from the ED, and 10 (71%) were discharged home without oseltamivir. One patient visited triage three times, but was not treated with oseltamivir. Three deaths occurred in hospitalized patients (aged 18, 37, and 54 years). Decedents presented to the ER a mean of 4 days after symptom onset with a mean ILI score of 16. One decedent was confirmed with pandemic H1N1, one had seasonal influenza A, and one was not tested. All other hospitalized patients improved and were discharged home.
Figure 2. Histogram of patients seeking care for acute respiratory infections at Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico.
Table 1. Demographic Characteristics of Patients Seeking Care for acute respiratory infection at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico.
| Demographics N (%) | All initially triaged patients (N = 1840) | All hospitalized patients treated with oseltamivir (N = 233)‡ | All ambulatory patients treated with oseltamivir as outpatients (N = 286) ‡ | Patients discharged from triage without oseltamivir (N = 1324) | All patients treated with oseltamivir with seasonal influenza A cases (N = 42)∞ | All patients treated with oseltamivir with pandemic (H1N1) 2009 cases (N = 104) ∞ |
| Median age | 29 | 28 | 29 | 29 | 31 | 23* |
| Females | 1017 (55%) | 134 (58%) | 154 (54%) | 741 (55%) | 20 (48%) | 45 (43%) |
| Most Frequently Reported Occupations | ||||||
| Home makers | 376 (20%) | 62 (27%) | 32 (11%) | 287 (22%) | 8 (19%) | 12 (12%) |
| Students | 288 (16%) | 40 (17%) | 51 (18%) | 198 (15%) | 3 (7%) | 28 (28%) |
| Health care workers | 230 (13%) | 17 (7%) | 88 (31%) | 126 (10%) | 6 (14%) | 13 (12%) |
| Retail workers | 163 (9%) | 18 (8%) | 14 (5%) | 132 (10%) | 4 (10%) | 3 (3%) |
| Construction workers | 121 (7%) | 8 (3%) | 4 (1%) | 111 (8%) | 2 (5%) | 5 (4%) |
| Unemployed | 74 (4%) | 11 (5%) | 4 (1%) | 60 (5%) | 3 (7%) | 1 (1%) |
| Assessment of risk | ||||||
| High risk | 167 (9%) | 114 (49%) | 52 (18%) | 4 (0.3%) | 14 (33%) | 38 (37%) |
| Intermediate risk | 725 (39%) | 104 (45%) | 173 (60%) | 451 (34%) | 18 (43%) | 49 (47%) |
| Low risk | 945 (51%) | 15 (6%) | 59 (21%) | 880 (66%) | 10 (24%) | 14 (16%)¥ |
| Median ILI-score | 6 | 15 | 11 | 5 | 14 | 13 |
*Difference between seasonal influenza and pandemic (H1N1), 2009, p = 0.0007.
¥2% of pandemic (H1N1) 2009 missing risk assessment information.
‡Includes all hospitalized cases regardless of influenza RT-PCR test results.
∞Includes all hospitalized cases and ambulatory patients treated with oseltamivir who tested positive for influenza A.
Figure 3. Patients seeking care with acute respiratory infections at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico.
Characteristics of hospitalized patients
Hospitalized patients presented within a median of 2 days after symptom onset with dyspnea and abnormal findings on chest imaging. Sixty-seven (30%) of the 230 hospitalized patients smoked tobacco (for a mean duration of 8 years), 45 (20%) had a history of alcohol abuse (i.e. using CAGE questionnaire), and 22 (10%) had a history of other drug use (Table 2). Ninety-one percent of hospitalized patients reported fatigue, 90% headache, 88% myalgias, 86% fever, 82% chills, and 63% dry cough (Table 3). During triage, fever (i.e. measured temperature ≥38°C) was documented in 184 (79%) of the 233 hospitalized patients (Table 4). Sixteen (33%) of the 49 hospitalized patients who were afebrile at triage reported using paracetamol, non-steroidal anti-inflammatory medications or oral corticosteroids prior to their ER visit. Nine (4%) of the 233 hospitalized patients had hypoxia (i.e. PO2 <70), 4 had hypotension (blood pressure <90/60), and 3 required invasive mechanical ventilation. One-hundred and fifty-six (69%) of 233 hospitalized patients had lymphopenia compared to 117 (41%) of 286 ambulatory patients treated with oseltamivir (p<0.0001). Similarly, 35 (15%) of 233 hospitalized patients had thrombocytopenia compared to 19 (7%) of 286 ambulatory patients treated with oseltamivir (p<0.001). Out of the 181 hospitalized patients tested, 36 (20%) were positive for 2009 pandemic influenza A (H1N1) and 24 (13%) were positive for seasonal influenza A. Similarly, out of the 187 hospitalized patients tested, 68 (36%) were positive for 2009 pandemic influenza A (H1N1) and 18 (10%) were positive for seasonal influenza A.
Table 2. Symptoms of Patients Seeking Care for Acute Respiratory Infections at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico◊.
| All initially triaged patients (N = 1239) | All hospitalized patients treated with oseltamivir (N = 233)‡ | All ambulatory patients treated with oseltamivir as outpatients (N = 286) ‡ | All patients treated with oseltamivir with seasonal influenza A cases (N = 42) ∞ | All patients treated with oseltamivir with pandemic (H1N1) 2009 cases (N = 104) ∞ |
| Past medical history N (%) | ||||
| Smoking | 67 (30%) | 4 (1%) | 8 (19%) | 13 (12%) |
| Alcoholism | 45 (20%) | 6 (2%) | 6 (14%) | 10 (10%) |
| Drug abuse | 22 (10%) | 0 (0%) | 4 (10%) | 3 (3%) |
| Hypertension | 20 (9%) | 5 (2%) | 3 (7%) | 4 (4%) |
| Diabetes | 13 (6%) | 7 (2%) | 4 (10%) | 2 (2%) |
| Tuberculosis | 11 (5%) | 1 (0.3%) | 2 (5%) | 1 (1%) |
| Asthma | 9 (4%) | 5 (2%) | 1 (2%) | 1 (1%) |
| Other lung disease | 9 (4%) | 3 (1%) | 2 (5%) | 1 (1%) |
| Other immune suppression | 8 (4%) | 5 (2%) | 1 (2%) | 2 (2%) |
| Neurological disease | 5 (2%) | 1 (0.3%) | 1 (2%) | 1 (1%) |
| Chronic renal problems | 5 (2%) | 2 (1%) | 2 (5%) | 2 (2%) |
| HIV | 4 (2%) | 1 (0.3%) | 0 (0%) | 0 (0%) |
| Pregnancy | 3 (2%) | 1 (0.3%) | 0 (0%) | 0 (0%) |
| Obesity | 3 (1%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Malnutrition | 2 (1%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Transplant | 2 (1%) | 1 (0.3%) | 0 (0%) | 1 (1%) |
| Influenza vaccine | 31 (13%) | 52 (18%) | 8 (19%) | 13 (12%) |
‡Includes all hospitalized cases regardless of influenza RT-PCR test results.
∞Includes all hospitalized cases and ambulatory patients treated with oseltamivir who tested positive for influenza A.
◊Insufficient data available from patients discharged from triage without oseltamivir to include in table.
Table 3. Presenting Symptoms of Patients Seeking Care for Acute Respiratory Infections at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico.
| All initially triaged patients (N = 1840) | All hospitalized patients treated with oseltamivir (N = 233)‡ | All ambulatory patients treated with oseltamivir as outpatients (N = 286) ‡ | Patients discharged from triage without oseltamivir (N = 1324) | All patients treated with oseltamivir with seasonal influenza A cases (N = 42) ∞ | All patients treated with oseltamivir with pandemic (H1N1) 2009 cases (N = 104) ∞ | |
| Symptoms N (%) | ||||||
| Median symptom onset before presentation | 2d | 2d | 2d | 2d | 2d | 2d |
| Headache | 1460 (79%) | 210 (90%) | 249 (87%) | 10111 (76%) | 32 (76%) | 93 (88%)* |
| Myalgia | 1336 (73%) | 204 (88%) | 224 (78%) | 919 (69%) | 31 (74%) | 85 (81%) |
| Fatigue | 1254 (68%) | 212 (91%) | 228 (79%) | 829 (62%) | 33 (79%) | 88 (83%) |
| Sore throat | 1251 (68%) | 163 (70%) | 192 (67%) | 906 (68%) | 28 (67%) | 75 (70%) |
| Chills | 1087 (59%) | 190 (82%) | 203 (71%) | 704 (53%) | 32 (76%) | 76 (74%) |
| Dry cough | 951 (52%) | 147 (63%) | 172 (60%) | 637 (48%) | 23 (55%) | 69 (64%) |
| Subjective Fever | 888 (48%) | 201 (86%) | 203 (71%) | 492 (37%) | 33 (79%) | 90 (85%) |
| Conjunctivitis | 791 (43%) | 127 (55%) | 115 (40%) | 556 (42%) | 22 (52%) | 48 (46%) |
| Rhinorrhea | 637 (35%) | 100 (43%) | 153 (53%) | 387 (29%) | 17 (40%) | 53 (51%) |
| Thoracic pain | 561 (30%) | 130 (56%) | 109 (38%) | 329 (24%) | 24 (57%) | 49 (45%) |
| Productive cough | 492 (27%) | 47 (20%) | 66 (23%) | 381 (29%) | 8 (19%) | 32 (31%) |
| Dyspnea | 438 (24%) | 120 (52%) | 90 (31%) | 230 (17%) | 13 (31%) | 42 (40%) |
| Diarrhea | 244 (13%) | 56 (24%) | 57 (20%) | 132 (10%) | 7 (17%) | 21 (20%) |
| Abdominal pain | 240 (13%) | 52 (23%) | 56 (20%) | 132 (10%) | 7 (17%) | 21 (20%) |
| Rales | 37 (2%) | 33 (14%) | 4 (1%) | 0 (0%) | 4 (10%) | 3 (3%) |
| Wheezing | 14 (1%) | 13 (6%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 1 (1%) |
*p = 0.04 when comparing pandemic (H1N1) 2009 test positives to seasonal influenza A test positives.
‡Includes all hospitalized cases regardless of influenza RT-PCR test results.
∞Includes all hospitalized cases and ambulatory patients treated with oseltamivir who tested positive for influenza A.
Table 4. Findings of patients seeking care for acute respiratory infections at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico ◊.
| All hospitalized patients treated with oseltamivir (N = 233)‡ | All ambulatory patients treated with oseltamivir as outpatients (N = 286) ‡ | All patients treated with oseltamivir with seasonal influenza A cases (N = 42) ∞ | All patients treated with oseltamivir with pandemic (H1N1) 2009 cases (N = 104) ∞ | |
| Findings | ||||
| Median temperature (°C) | 38.5 | 37.7 | 38.5 | 38 |
| Hypoxia N (%) | 9 (5%) | 0 (0%) | 1 (2%) | 1 (1%) |
| Lymphopenia | 156 (69%) | 117 (41%) | 27 (64%) | 66 (63%) |
| Thrombocytopenia | 35 (15%) | 19 (7%) | 2 (5%) | 9 (9%) |
| Radiology N (%) | Of 205 hospitalized patients who had chest X-ray [of which 83 had chest CT] | Of 258 ambulatory patients who had chest X-ray [of which 35 had chest CT] | Of 36 patients who tested positive for seasonal influenza A and who had chest X-ray s[of which 16 had chest CT] | Of 95 patients who tested positive for pandemic (H1N1) and who had chest X-rays [of which 30 had chest CT] |
| Abnormal chest X-ray | 79 (39%) | 112 (43%) | 14 (39%) | 59 (62%)¥ |
| Abnormal lung CT | 91 (97%) | 30 (86%) | 16 (100%) | 30 (100%) |
| Bilateral infiltrates | 49 (53%) | 23 (64%) | 12 (75%) | 28 (93%)* |
| Tree-in bud appearance | 69 (73%) | 26 (72%) | 15 (94%) | 28 (93%)* |
| Involvement of basal zone | 62 (66%) | 24 (67%) | 16 (100%) | 30 (100%)* |
| Air trapping | 52 (55%) | 23 (64%) | 15 (94%) | 27 (90%)* |
| Centrilobular nodules | 49 (52%) | 21 (58%) | 13 (81%) | 24 (80%)* |
| Thickened interlobar septa | 48 (51%) | 21 (58%) | 12 (75%) | 28 (93%)* |
| Multifocal distribution | 38 (40%) | 13 (36%) | 11 (69%) | 19 (63%)* |
| Involvement of middle zone | 27 (29%) | 9 (25%) | 6 (37%) | 18 (60%)* |
| Segmental consolidation | 15 (16%) | 1 (3%) | 1 (6%) | 5 (17%) |
| Segmental distribution | 14 (15%) | 0 (0%) | 1 (6%) | 3 (10%) |
| Involvement of apical zone | 10 (11%) | 4 (11%) | 1 (6%) | 3 (10%) |
| Peribronchial ground glass | 7 (7%) | 4 (11%) | 1 (6%) | 7 (23%)* |
¥p = 0.01 when comparing patients who tested positive for seasonal influenza A with those who tested positive for pandemic (H1N1) 2009.
*p≤0.009 when comparing patients who tested positive for pandemic (H1N1) 2009 to those who tested negative.
‡Includes all hospitalized cases regardless of influenza RT-PCR test results.
∞Includes all hospitalized cases and ambulatory patients treated with oseltamivir who tested positive for influenza A.
◊Insufficient data available from patients discharged from triage without oseltamivir to include in table.
Clinical presentation of patients who tested positive for 2009 pandemic influenza A (H1N1) virus
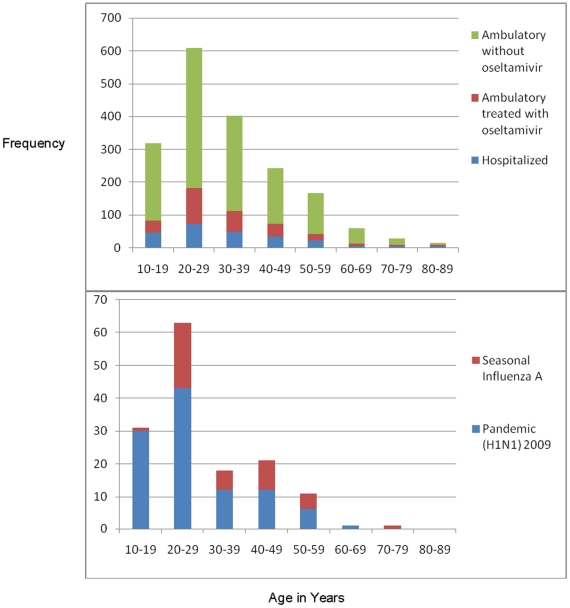
Of the 1840 persons triaged, 379 (21%) were tested for influenza (i.e. 371 (20%) by rRT-PCR, 112 (6%) by rapid diagnostic test, and 89 (5%) by immunofluorescence). Of the 371 patients tested by rRT-PCR, 104 (28%) had pandemic (H1N1) and 42 (11%) had seasonal influenza A detected. There was a 0.51 correlation between rRT-PCR and rapid diagnostic test results among the 85 patients who were tested by both methods (p<0.001). In contrast, there was a 0.15 correlation between rRT-PCR and immunofluorescence results among the 57 who were tested by both methods. In comparison to patients with seasonal influenza, patients whose rRT-PCR tested positive for 2009 pandemic influenza A (H1N1) were younger (Figure 4). The median age of patients who tested positive for 2009 pandemic influenza A (H1N1) was 23 years versus 31 years for patients who tested positive for seasonal influenza A (p = 0.0007)(Table 1). Patients whose rRT-PCR tested positive for 2009 pandemic influenza A (H1N1) were not more likely to be pregnant, report substance abuse, have other medical conditions (e.g. obesity), or require hospitalization within 2 days of developing symptoms than other patients (Table 2). At ER presentation, 69 (66%) of the 104 patients whose rRT-PCR tested positive for 2009 pandemic influenza A (H1N1) reported a dry cough (mean duration = 3 days) versus 145 (55%) of 264 test negative patients (p = 0.03). Thirty-two (31%) the 104 patients whose rRT-PCR tested positive for 2009 pandemic influenza A (H1N1) had a productive cough compared to 53 (20%) of 262 test negative patients (p = 0.03). Patients whose rRT-PCR tested positive for 2009 pandemic influenza A (H1N1) presented with a median temperature of 38.5°C which, on average, started 2 days before admission [IQR1–3]. There were no differences in WBC at ER presentation between patients whose rRT-PCR tested positive for 2009 pandemic influenza A (H1N1) and patients who tested negative for 2009 pandemic influenza A (H1N1).
Figure 4. Age distribution of patients triaged for acute respiratory infections at the Hospital Civil de Guadalajara during the (H1N1) pandemic 2009—Mexico.
Radiological findings of hospitalized patients
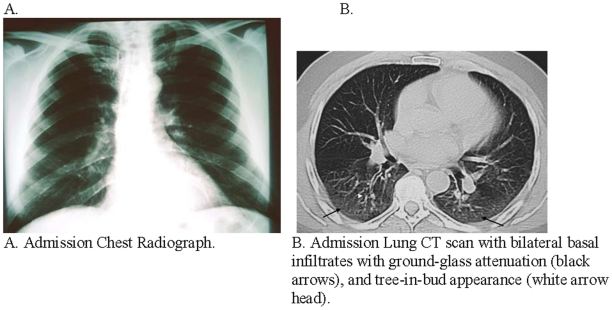
Eighteen (60%) of 30 hospitalized patients infected with 2009 pandemic influenza A (H1N1) with chest X-rays had abnormal findings while all 22 with chest CT scans had abnormal findings (Table 4). Similarly, 5 (25%) of 20 hospitalized patients infected with seasonal influenza A who had chest X-rays had abnormal findings while all 10 who had chest CT had abnormal findings. Hospitalized patients infected with 2009 pandemic influenza A (H1N1) were more likely to have abnormal chest X-rays than patients infected with seasonal influenza A (p = 0.02) (Table 4). Twenty (91%) of 22 imaged hospitalized patients infected with 2009 pandemic influenza A (H1N1) had bilateral infiltrates on chest X-ray or CT compared to 23 (38%) of 61 imaged patients who tested negative for 2009 pandemic influenza A (H1N1)(p<0.001). Similarly, more patients infected with 2009 pandemic influenza A (H1N1) had chest X-rays and CT scans with thickened interlobar septa (p<0.001), involvement of the middle zone (p<0.001), compared to imaged patients who tested negative for 2009 pandemic influenza A (H1N1) (Table 4) (Figure 5).
Figure 5. Typical radiological findings of Pandemic (H1N1) 2009 patient at the Hospital Civil de Guadalajara—Mexico.
Risk factors for increased length of hospitalization among 2009 pandemic influenza A (H1N1) cases
Testing positive for 2009 pandemic influenza A (H1N1) was not associated with prolonged stay. On average, 2009 pandemic influenza A (H1N1) patients were hospitalized for a median of 2 days [IQR 1–3days]. 2009 Pandemic influenza A (H1N1) infected patients with dyspnea on admission had a mean hospital stay of 2.1 days while those without dyspnea had a mean hospital stay of 1.3 days. The one decedent infected with pandemic influenza A (H1N1) presented 6 days after symptom onset with dyspnea and a 10 year history smoking history. There were no reported adverse events among patients associated with the use of oseltamivir.
Discussion
During 6 weeks when there was co-circulation of pandemic and seasonal influenza A viruses in the community, hospital staff triaged more than eighteen-hundred patients with respiratory complaints and identified 12% for inpatient care. The triage system was based on assumptions about who is at risk for developing complications from seasonal influenza (e.g. patients aged over 65 years). Our analyses, however, suggested that patients infected with 2009 pandemic influenza A (H1N1) tended to be younger than seasonal influenza A patients. Nevertheless, our data suggest that clinicians used the ILI-score to help them determine, with minimal misclassification, which patients needed hospitalization versus who could be managed as outpatients [18]. The ILI-score helped guide clinicians to decide who needed hospital care and antiviral treatment when timely laboratory confirmation of influenza was not available. Only 1% of patients triaged needed re-evaluation. Such a system could be readily used to efficiently triage patients during outbreaks and epidemics by adapting the system's scores to match the anticipated characteristics of patients who are at highest risk of developing complications.
While the triaging system led clinicians to hospitalize traditional groups at risk for complications from seasonal influenza (i.e. those with chronic medical illnesses), patients infected with 2009 pandemic influenza A (H1N1) were often young and had few pre-existing conditions [20]. These data are comparable with Mexican Directorate General of Epidemiology data that suggest 56% of pandemic (H1N1) confirmed deaths occurred among those aged 30–59 years, many of whom were previously healthy [21]. The age shift in 2009 pandemic influenza A (H1N1) cases may be caused by cross-reactive immunity from prior influenza infections in 33% of those aged more than 60 years [22], [23]. Health officials should adjust pandemic triaging tools to account for the younger age distribution of cases [24]. Pregnancy should also be included as a risk factor in triaging tools. Although there were too few pregnant women in our case series for subgroup analyses, other data suggest pregnant women are at high risk of developing severe complications from 2009 pandemic influenza A (H1N1) [25].
In this case series, hospitalized patients who tested positive for 2009 pandemic influenza A (H1N1) received oseltamivir within 2 days of symptom onset and appeared to recover quickly with a median hospital stay of two days. Similarly, no ambulatory patients treated with oseltamivir required further medical care. In contrast, 3 patients initially discharged from the ED without oseltamivir returned to triage and required hospital admission. Two of these 3 later tested positive for 2009 pandemic influenza A (H1N1). One additional patient who required mechanical ventilation and subsequently died had presented 6 days after symptom onset. Another 5 hospital decedent whose care was transferred to the infectious disease service and therefore not part of our triaged case-series presented a median of 15 days after symptom onset. These cases suggest the importance of early oseltamivir treatment.
Only one (1%) of 104 patients who tested positive for 2009 pandemic influenza A (H1N1) case-patients died. These findings contrast those of the National Institute of Respiratory Diseases in Mexico City where 12 (67%) of 18 patients required mechanical ventilation and 7 (39%) patients died [24], [26]. The discrepancy between these two case-series may be explained by when the populations served by these hospitals were affected by the pandemic. National Institute of Respiratory Diseases data were collected during March 24–April 24, 2009, when it was still unclear that a proportion of cases with severe acute respiratory infections had 2009 pandemic influenza A (H1N1). In Guadalajara, the outbreak started later. Hospitalized patients we described received earlier oseltamivir. Our patients were hospitalized during April 25–August 9. Seventy-five percent of our case-patients received oseltamivir within 72 hours of symptom onset. Patients in the Mexico City case-series presented with severe disease an average of 8 days after illness onset and received late oseltamivir.
Our findings have important limitations. A minority of all patients had respiratory specimens tested by RT-PCR, a large number of patients who were triaged were not confirmed with seasonal influenza or 2009 pandemic influenza A (H1N1) virus infection. No testing for other etiologies of acute respiratory illness was performed. Oseltamivir treatment among hospitalized patients was not randomized among cases and control. No comparison group was available to assess oseltamivir effectiveness for the treatment of 2009 pandemic influenza A (H1N1).
The triaging system with its ILI-score needs further validation. Nevertheless, such a triaging system can help guide the clinical management of patients presenting to the ED with acute respiratory illness in settings that lack timely diagnostic testing and have limited antivirals supplies. With some adaptation, the system may be especially useful in resource-poor countries, during the peak of pandemic influenza, or during other respiratory virus activity. Although no scoring system will replace clinical judgment, our experience suggests that the triaging system may have helped clinicians effectively triage patients and determine who needed hospital care and who could be managed as outpatients. The triaging system and the ILI-score should be modified to the local 2009 pandemic influenza A (H1N1) situation based upon hospital surge capacity, antiviral susceptibilities and supply, and the evolving epidemiology of this virus.
Acknowledgments
Joseph Bresee, Carolyn Bridges, Ann Moen, Dorothy Southern, Anthony Mounts, Kathryn Lafond, Joshua Mott, Nancy Cox, Alexander Klimov, Julie Harris, Jay McAuliffe, Abu Yushuf Sharker, Kelley Scanlon, Jo Grzelinska, and Stephen Luby.
Members of the Hospital Civil de Guadalajara, Fray Antonio Alcalde emerging respiratory infections response team were: M. Florez-Vaca, E. Jáuregui, S. Petersen, E. F. Acosta, V. Varela, C. A. Zaragoza, N. C. Becerra, H. A. Mendoza, M. M. Dominguez, M. Velasco, C. A. Albert, L. F. Miramontes, M. E. Angulo, S. Villanueva, R. Cruz (Instituto de Patologia Infecciosa y Experimental, Centro Universitario Ciencias de la Salud, Universidad de Guadalajara, Public Health Service), M. Vazquez, P. Gomez, (Infectious Diseases Unit Attendings), J. de la Cabada-Fauche, R. Zamora, A. Muñiz, V. H. Ahumada, P. G. Guevara (Infectious Diseases Unit Fellows), J.J. Rodriguez-Chagollan, G.Atilano, A. Gomez (Microbiology), C. Torres, H. I. Cruz, G. I. Ruiz, E. H. Muñoz, J. C. Vazquez, C. Gonzalez, (Internal Medicine, Residents), J. Robles, S. Esparza (Epidemiology), Jaime Agustin Alvarez (OPD, Hospital Civil de Guadalajara).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The U.S. Centers for Disease Control and Prevention (CDC) who provided technical assistance during the outbreak investigations in Mexico had a role designing the study, guiding data collection, analyzing data, manuscript development, and decision to submit to PLoS ONE. Specifically, CDC paid for U.S. Government staff to travel and provide technical assistance during the investigation. The authors are unaware of any further funding than that provided by coverage of per diem and travel for U.S. Government employees deployed in Mexico.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Glaser CA, Gilliam S, Thompson WW, Dassey DE, Waterman SH, et al. Medical care capacity for influenza outbreaks, Los Angeles. Emerg Infect Dis. 2002;8:569–574. doi: 10.3201/eid0806.010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, et al. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4:e247. doi: 10.1371/journal.pmed.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen-Van-Tam JS, Hampson AW. The epidemiology and clinical impact of pandemic influenza. Vaccine. 2003;2:1762–1768. doi: 10.1016/s0264-410x(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 5.World Health OrganizationGlobal Influenza Programme. 2009. Pandemic influenza preparedness and response: a WHO guidance document. Accessed at: http://www.who.int/csr/disease/influenza/PIPGuidance09.pdf.
- 6.Schull MJ, Stukel TA, Vermeulen MJ, Guttmann A, Zwarenstein M. Surge capacity associated with restrictions on nonurgent hospital utilization and expected admissions during an influenza pandemic: lessons from the Toronto severe acute respiratory syndrome outbreak. Acad Emerg Med. 2006;13:1228–1231. doi: 10.1197/j.aem.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Christian MD, Hawryluck L, Wax RS, Cook T, Lazar NM, et al. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175:1377–1381. doi: 10.1503/cmaj.060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinson L, Hick JL, Curtis JR, Branson RD, Burns S, et al. Definitive care for the critically ill during a disaster: a framework for optimizing critical care surge capacity: from a Task Force for Mass Critical Care summit meeting, January 26-27, 2007, Chicago, IL. Chest. 2008;133:32S–50S. doi: 10.1378/chest.07-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell T, Christ KC, Birkhead GS. Allocation of ventilators in a public health disaster. Disaster Med Public Health Prep. 2008;2:20–26. doi: 10.1097/DMP.0b013e3181620794. [DOI] [PubMed] [Google Scholar]
- 10.British Infection Society, British Thoracic Society and Health Protection Agency and Department of Health. Pandemic flu: clinical management of patients with an influenza-like illness during an influenza pandemic. Thorax. 2007;62:S1–S58. doi: 10.1016/S0163-4453(07)60001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health Agency of Canada. 2008. The Canadian Influenza Plan for the Health Sector. Annex G. Clinical Care Guidelines and Tools. Accessed at: http://www.phac-aspc.gc.ca/cpip-pclcpi/pdf-e/annex_g-eng.pdf.
- 12.U.S. Department of Health and Human Services. HHS Pandemic Influenza Plan. Supplement 5. Clinical Guidelines. Accessed at: http://www.hhs.gov/pandemicflu/plan/sup5.html. [DOI] [PubMed]
- 13.Tang CH, Yang CM, Chuang CY, Chang ML, Huang YC, et al. A comparative Study of Clinical Severity Scoring Systems in ICUs in Taiwan. Tzu Chi Med J. 2005;17:239–245. [Google Scholar]
- 14.Singanayagam A, Chalmers JD, Hill AT. Severity assessment in community-acquired pneumonia: a review. QJ Med. 2009;102:379–388. doi: 10.1093/qjmed/hcp027. [DOI] [PubMed] [Google Scholar]
- 15.Ho Y-C, Wang J-L, Wang J-T, Wu U-I, Chang C-W, et al. Prognostic factors for fatal adult influenza pneumonia. J of Inf. 2009;3:1–7. doi: 10.1016/j.jinf.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 17.CDC. 2009. Interim Guidance on Antiviral Recommendations for Patients with Novel Influenza A (H1N1) Virus Infection and Their Close Contacts. (Accessed July 14, 2009 at http://www.cdc.gov/h1n1flu/recommendations.htm)
- 18.Hak E, Wei F, Nordin J, Mullooly J, Poblete S, et al. Development and Validation of a Clinical Prediction Rule for Hospitalization Due to Pneumonia or Influenza or Death during Influenza Epidemics among Community-Dwelling Elderly Persons. JID. 2004;189:450–458. doi: 10.1086/381165. [DOI] [PubMed] [Google Scholar]
- 19.WHO. 2009. (Accessed July 14, 2009 at http://www.euro.who.int/Document/INF/CDC_realtime_RTPCR_H1N1.pdf)
- 20.Thompson WW, Shay DK, Weintraub E, Baumen L, Cox N, et al. Mortality Associated with Influenza & Respiratory Syncytial Virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 21.CDC. Novel Influenza A (H1N1) Virus Infection — Mexico, March–May, 2009. MMWR. 2009;58:585–589. [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, et al. Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med. 2009;361:1–8. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 23.Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;Aug 13;361(7):674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 24.Azziz-Baumgartner E, Smith N, Gonzalez-Alvarez R, Daves S, Layton M, et al. National pandemic influenza preparedness planning. Influenza and Other Respiratory Viruses. 2009;3:189–196. doi: 10.1111/j.1750-2659.2009.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, et al. Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]