Abstract
In the hippocampus, glucocorticoids bind to two types of receptors: the mineralocorticoid receptor, which binds corticosterone with high affinity and is tonically occupied; and the glucocorticoid receptor, which is occupied during stress and at certain phases in the circadian cycle. Diabetes mellitus increases levels of glucocorticoids in both humans and animal models. To explore the contributions of hippocampal corticosteroid receptors to the diabetes-induced suppression of neuroplasticity, we manipulated these receptors in hippocampal slices from streptozocin-diabetic rats, a model of Type 1 diabetes mellitus. STZ-diabetes reduced long-term potentiation (LTP) at medial perforant path synapses in the dentate gyrus, and induced a bias in favor of long-term depression following intermediate stimulation frequencies. Bath application of the mineralocorticoid receptor agonist aldosterone restored LTP in slices from diabetic animals. These results suggest additional mechanisms for diabetes-induced functional alterations and support a restorative role for dentate gyrus mineralocorticoid receptors.
Keywords: diabetes, glucocorticoid, corticosterone, aldosterone, hippocampus
Diabetics are more likely than non-diabetics to experience major depression and anxiety, both of which may alter activation of the hypothamic-pituitary-adrenal axis (HPA axis; Fenton and Stover, 2006). Alterations in HPA axis function and behavioral anxiety have also been reported in rodent models of diabetes (Magarinos and McEwen, 2000; Chan et al., 2001). In non-diabetic animals, prolonged increases in circulating corticosterone levels induce deficits in hippocampus-dependent memory and synaptic plasticity (Yamada et al., 2003, Pavlides et al., 2002). Diabetic rodents also exhibit glucocorticoid-dependent impairments in hippocampal synaptic plasticity (Stranahan et al., 2008). However, the contributions of corticosteroid receptors to the impairment of synaptic plasticity in diabetes have not been established.
Long-term potentiation (LTP) of synaptic transmission in the hippocampus occurs in response to brief high frequency stimulation and is a form of synaptic modification associated with learning and memory (Teyler and Discenna, 1984; Lynch, 2004). In rodents, chronic exposure to high levels of corticosterone impairs dentate gyrus LTP (Yamada et al., 2003, Pavlides et al., 2002) through the actions of corticosterone on the type 2 glucocorticoid receptor (Korz and Frey, 2003). Because diabetes results in levels of circulating adrenal glucocorticoids that are similar to those observed during chronic stress (Magarinos and McEwen, 2000), we manipulated corticosteroid receptors in hippocampal slices from diabetic and non-diabetic rats. We observed that activation of mineralocorticoid receptors (MR), but not blockade of glucocorticoid receptors (GR), restored perforant path LTP in diabetic animals. In addition to the reduction in LTP, diabetes induced a bias in favor of LTD following intermediate stimulation frequencies. These findings suggest that activation of MR in hippocampal neurons can restore synaptic function in insulin-deficient diabetes.
Materials and Methods
Animals and Glucose Measurements
Adult male Sprague-Dawley rats (>250g, Charles River) were used in these studies. Animals were housed individually for a minimum of 2 weeks prior to beginning experiments, with food and water available ad libitum. The vivarium was maintained on a 12 hr light/12 hr dark cycle (lights on at 6 am). Rats were treated with STZ (70 mg/kg, IV) or vehicle three to six weeks before being euthanized for slice preparation. Animals were considered diabetic when glucose levels were >200 mg/dL, as confirmed by analysis of blood obtained by tail-nick sampling after an overnight fast using a Therasense handheld analyzer (Therasense, Anaheim, CA). Procedures were approved by the National Institute on Aging IACUC and followed the recommendations in the NIH Guide for the Care and Use of Animals.
Corticosterone radioimmunoassay
To measure levels of corticosterone, animals were rapidly captured and restrained and trunk blood was collected following decapitation within 3 minutes from the initial time of cage disturbance. Corticosterone levels were measured using a commercially available kit (Diagnostic Products, Los Angeles, CA). To separate the serum from trunk blood, samples were centrifuged at 14,000 rpm for 2 minutes. Serum samples were stored at −20°C prior to analysis. Samples and corticosterone standards were brought to room temperature and added to antibody-coated tubes in duplicate. Next, 1.0 ml of I-125 labeled corticosterone was added to each tube before incubation for 2 hours at room temperature. Tubes were then decanted and counted using a gamma counter.
Electrophysiology
Animals were decapitated under light Isoflurane anesthesia and brains were dissected in cold artificial cerebrospinal fluid (ACSF; in mM: NaCl, 120; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 26; MgSO4, 1.3; CaCl2, 2.5; glucose, 10; osmolality=300). Transverse hippocampal slices (400 μm thickness) were prepared in cold oxygenated ACSF using a vibratome. Slices were allowed to recover for a minimum of one hour at room temperature before being transferred to a submerged recording chamber for experiments. During recording, slices were maintained at 30C while being perfused at 5–6 ml/min with ACSF containing 100 μM picrotoxin (Sigma). Where indicated, slices were pre-treated with the MR agonist aldosterone (30 nM; Sigma) or the GR antagonist RU486 (500 nM; Sigma) for at least two hours before recording. The doses used for drug application were based on previously published studies (Joels et al., 1991).
Stimuli were delivered through a square pulse stimulator (Grass model S48), isolated, and administered through bipolar twisted nichrome wire to the medial perforant path, defined anatomically by positioning electrodes in the middle molecular layer, and functionally by the presence of paired-pulse depression (Colino and Malenka, 1993). Stimulus intensity was adjusted to produce responses at 30% of maximum, and test stimuli were delivered at a rate of 0.05 Hz. Responses were recorded through a glass micropipette (1–2 MΩ) filled with ACSF, and amplified using an Axopatch 1D amplifier. The amplified response was digitized at 10 kHz using a Digidata 1320A attached to a Dell computer running Clampex v.8.1 (Molecular Devices Corp, Sunnyvale, CA). Field excitatory postsynaptic potentials (fEPSPs) were measured over 0.5 milliseconds of the initial slope of the response, excluding the fiber volley when possible. LTP was induced with a 1 second train delivered at 100 Hz; LTD was induced using 900 pulses delivered at 1 Hz. For experiments where we tested the threshold for LTP and LTD, slices were stimulated with 900 pulses delivered at either 5 or 10 Hz.
Statistics
Differences in the amount of potentiation during the last 10 minutes of LTP and LTD recordings were compared between diabetic and non-diabetic rats using separate t-tests for recordings made under different drug treatment conditions. For all analyses, significance was set at p<0.05. The n-number used for statistical analysis was derived from the number of slices; for all experiments, at least 12 slices from at least 6 different animals were used.
Results
Diabetes reduces LTP and alters the threshold for LTD at medial perforant path synapses in the dentate gyrus
Initially, we performed a series of experiments to characterize the effects of Type 1 diabetes on bidirectional synaptic plasticity at medial perforant path synapses in the hippocampal dentate gyrus. The medial perforant pathway was identified by its anatomical location in the dentate molecular layer, and by the presence of paired-pulse depression (Colino and Malenka, 1993). All experiments were conducted in the presence of 100 μM picrotoxin.
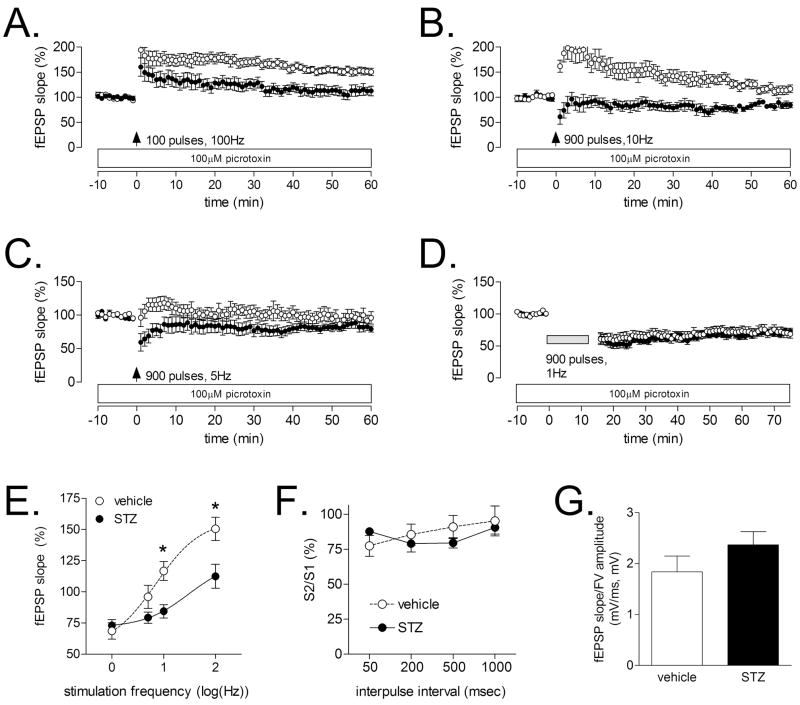
Following a single, 100 Hz stimulus train administered for one second, STZ-diabetic rats showed less LTP during the last ten minutes of the recording (Figure 1A, t20=2.274, p=0.03). These findings concur with previous reports (Kamal et al., 1999). In contrast with the robust suppression of LTP, there was no difference between STZ- and vehicle-treated rats following induction of LTD with 900 pulses administered at 1 Hz (t11=0.24, p=0.81, Figure 1D).
Figure 1. Diabetes impairs LTP at medial perforant path synapses in the dentate gyrus, and alters the threshold for induction of LTD.
(A), In agreement with previous reports (Kamal et al., 1999, Stranahan et al., 2008), diabetic rats showed reduced LTP at medial perforant path synapses on dentate granule neurons following a single, 1-second train delivered at 100 Hz. (B), Following a train delivered at 10 Hz, non-diabetic rats exhibit strong post-tetanic potentiation, with a gradual return to within 10–15% of baseline. In contrast, diabetic animals showed post-tetanic depression, followed by a return to baseline. (C), In response to a 5 Hz train, non-diabetic rats exhibit a slight posttetanic potentiation, followed by a return to baseline, while diabetic rats exhibit posttetanic depression and a return to baseline. (D), There was no difference in the magnitude of LTD following 900 pulses delivered at 1 Hz. (E), Summary graph showing the fEPSP slope as a percentage of pre-tetanus baseline, plotted against the log of the stimulation frequency. Asterisk (*) indicates significance at p<0.05 following ANOVA with Tukey’s post hoc. (E), Diabetes did not alter the paired-pulse depression that is characteristic of the medial perforant pathway (Colino and Malenka, 1993). (F), The ratio of the presynaptic fiber volley and the postsynaptic fEPSP was not influenced by diabetes, suggesting that there was no change in the input-output relationship.
Next, we used intermediate stimulation frequencies (5 and 10 Hz) to evaluate the possibility that there might be a shift in the threshold for LTP and LTD. Following 900 pulses at 10 Hz, non-diabetic rats showed a large post-tetanic potentiation, which gradually returned to within 10–15% of baseline (Figure 1B, 1E). In contrast, diabetic rats exhibited slight post-tetanic depression, followed by a return to baseline (Figure 1B, 1E). After 900 pulses at 5 Hz, non-diabetic animals showed mild short-term potentiation, followed by a return to baseline (Figure 1C, 1E). In diabetic animals, this same stimulus induced a slight depression in the slope of the fEPSP (Figure 1C, 1E). Taken together, these results indicate that, in addition to LTP deficits, diabetic animals also show a shift in the threshold for LTP and LTD.
Diabetes did not alter the strength of paired-pulse depression (F1,27=0.03, p=0.87, Figure 1F). Moreover, there was no effect of diabetes on the input-output relationship, measured by calculating the ratio of the presynaptic fiber volley and the postsynaptic fEPSP (t33=1.285, p=0.21, Figure 1G). The absence of any changes in presynaptic plasticity and the input-output relationship suggests that there is a primarily postsynaptic deficit at medial perforant path synapses on dentate granule neurons in diabetic rats.
Mineralocorticoid receptor activation restores medial perforant path LTP in diabetic animals
We used two experimental approaches to test the effect of manipulating GR and MR in slices from diabetic animals. Because diabetic animals have elevated levels of corticosterone, we reasoned that LTP impairment could be due to occupation of the GR, which has previously been shown to impair dentate gyrus LTP (Korz and Frey, 2003). We also evaluated the contribution of the MR, which facilitates perforant path LTP (Avital et al., 2006). Slices were prepared in the morning, when circulating corticosterone levels are low. We confirmed that non-diabetic animals used for slice preparation had low levels of corticosterone by analyzing serum collected immediately prior to slice preparation (ng/ml; vehicle=6.43 ± 0.95, STZ=378.40 ± 16.02, t7=20.45, p<0.0001).
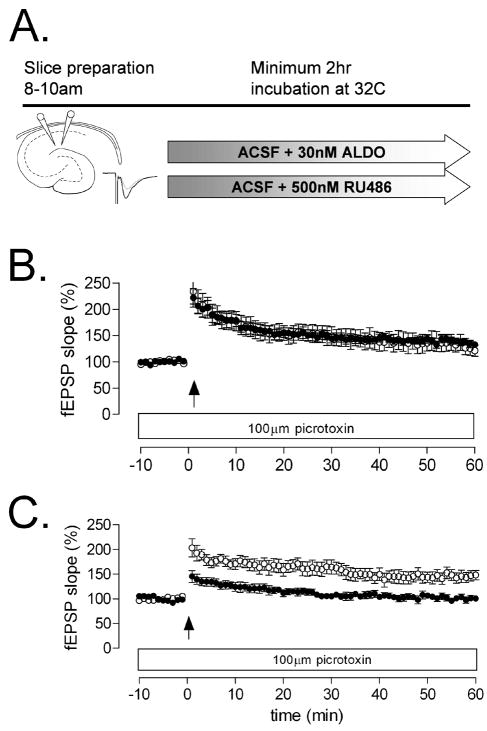
Inactivation of hippocampal GR had no effect on LTP deficits in diabetic animals (t17=4.12, p=.007, Figure 2C). In contrast, treatment with the MR agonist aldosterone restored medial perforant path LTP to control levels (t19=1.01, p=0.32, Figure 2B). This result is surprising, given the high levels of serum corticosterone in STZ-diabetic rats, which suggests that all central MR would be occupied by corticosterone. Further MR activation via application of aldosterone appears to rescue LTP in diabetic rats, despite high levels of circulating corticosterone.
Figure 2. Impairment of medial perforant path LTP is reversible by mineralocorticoid receptor activation in diabetic rats.
(A), Experimental design. (B), Preincubation with 30 nM aldosterone, with continued application of aldosterone in the perfusion medium during recording, restored LTP at medial perforant path synapses in the dentate gyrus. (C), There was no effect of pretreatment with 500 nM RU486, a glucocorticoid receptor antagonist.
Discussion
Diabetes induces a number of neurological changes that may render the hippocampus more susceptible to age-related structural and functional deficits. In hippocampal slices from diabetic and non-diabetic animals, application of the MR agonist aldosterone restored LTP at medial perforant path – dentate gyrus synapses, implicating this receptor population in corticosterone-mediated learning impairments (Stranahan et al., 2008). We have also replicated and extended previous studies demonstrating impairment of dentate gyrus LTP in STZ diabetic animals (Kamal et al., 1999; Stranahan et al., 2008). Our observation of a change in the threshold for LTD suggests that bidirectional synaptic plasticity may also be altered at medial perforant path synapses in the diabetic brain.
The effect of diabetes on bidirectional synaptic plasticity in the dentate gyrus is similar, but not identical to the literature on diabetes-induced regulation of Schaffer collateral LTD in hippocampal area CA1. A previously published study has shown that insulin deficient rats exhibit reduced LTP and enhanced LTD at Schaffer collateral-CA1 synapses (Kamal et al., 1999). However, we have observed reduced LTP, and a bias in favor of LTD following intermediate stimulus frequencies (5 and 10 Hz) in the dentate gyrus. We did not observe any differences in the magnitude of LTD following 900 pulses at 1 Hz. One possible explanation for this difference involves adult neurogenesis, which occurs throughout life in the dentate gyrus, but not in CA1. The number of adult-generated neurons is reduced in STZ-diabetic animals (Stranahan et al., 2008). Because newly generated neurons show increased excitability and lower thresholds for LTP induction (Schmidt-Hieber et al., 2004), it is possible that a reduction in their number could influence the threshold for LTP and LTD in the dentate gyrus. Taking into consideration the fact that these experiments were conducted in the presence of the GABAA receptor antagonist picrotoxin, which silences GABAergic excitation among new neurons during the first week after their birth (Ge et al., 2006), it is tempting to speculate that the current results might be due to reductions in the number of adult-generated neurons that reach maturity. Even after new neurons transition from GABAergic excitation to glutamatergic excitation, they retain greater morphological plasticity (Zhao et al., 2006), and the loss of this population could have an impact on synaptic plasticity.
In diabetic humans, pharmacological treatments that alter the availability of corticosterone can improve cognition (Sandeep et al., 2004). It remains to be seen whether central activation of MR alters cognition in individuals with diabetes. We have observed that aldosterone restores LTP at medial perforant path synapses on dentate granule neurons in diabetic animals. Future studies will be necessary to fully characterize the role of the MR system in diabetes models, and in models of chronic stress. Understanding the overlap between the mechanisms of diabetes and anxiety could help to develop population-specific treatment for individuals suffering from both disorders.
Acknowledgments
This research was supported by NIH NRSA F31 AG024690-03 through Princeton University, and by the National Institute on Aging Intramural Research Program.
References
- Avital A, Segal M, Richter-Levin G. Contrasting roles of corticosteroid receptors in hippocampal plasticity. J Neurosci. 2006;26:9130–4. doi: 10.1523/JNEUROSCI.1628-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–35. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Chan O, Chan S, Inouye K, Vranic M, Matthews SG. Molecular regulation of the hypothalamo-pituitary-adrenal axis in streptozotocin-induced diabetes: effects of insulin treatment. Endocrinology. 2001;142:4872–4879. doi: 10.1210/endo.142.11.8474. [DOI] [PubMed] [Google Scholar]
- Colino A, Malenka RC. Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. J Neurophysiol. 1993;69:1150–9. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Stover ES. Mood disorders: cardiovascular and diabetes comorbidity. Curr Opin Psychiatry. 2006;19:421–7. doi: 10.1097/01.yco.0000228765.33356.9f. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Hesen W, de Kloet ER. Mineralocorticoid hormones suppress serotonininduced hyperpolarization of rat hippocampal CA1 neurons. J Neurosci. 1991;11:2288–94. doi: 10.1523/JNEUROSCI.11-08-02288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Urban IJ, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90:737–45. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J Neurosci. 2003;23:7281–7. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci U S A. 2000;97:11056–61. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–57. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Sandeep TC, Yau JL, MacLullich AM, Noble J, Deary IJ, Walker BR, Seckl JR. 11Beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc Natl Acad Sci U S A. 2004;101:6734–9. doi: 10.1073/pnas.0306996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984;319:15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, McEwen BS, Pavlides C. Site and time dependent effects of acute stress on hippocampal long-term potentiation in freely behaving rats. Exp Brain Res. 2003;152:52–9. doi: 10.1007/s00221-003-1519-0. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]