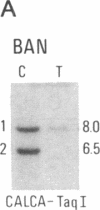
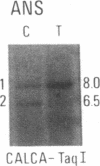
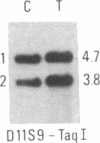
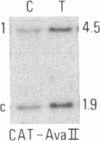
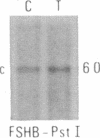
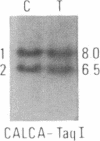
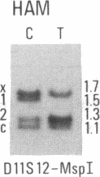
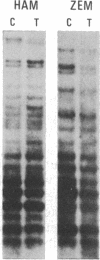
Abstract
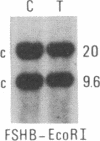
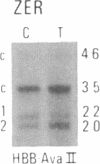
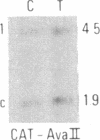
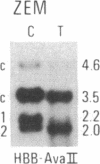
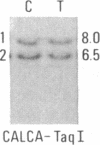
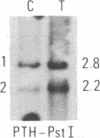
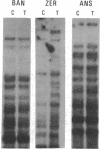
We have compared constitutional and tumor genotypes in nine cases of hereditary Wilms tumor (WT) and in three unrelated cases of familial adrenocortical carcinoma (ADCC). Since susceptibility to these tumors can be observed in malformation syndromes associated with a constitutional deletion of band 11p13 (WT) and with a constitutional duplication of band 11p15.5 (WT, ADCC), we investigated these two candidate regions by using 11p polymorphic markers. As expected, somatic chromosomal events, resulting in a loss of heterozygosity limited to region 11p15.5, were observed in the tumor of two familial cases of adrenocortical carcinoma. Surprisingly, however, analysis of the WT of two patients with a constitutional deletion of band 11p13, associated with aniridia, genitourinary abnormalities, and mental retardation (WAGR syndrome), revealed a loss of heterozygosity limited to region 11p15.5. These data therefore suggest that observation of a specific loss of heterozygosity may not necessarily point to the site of the initial germinal mutation. Together with previous similar observations of a loss of heterozygosity limited to 11p15.5 in breast cancer and in rhabdomyosarcoma, our data suggest that region 11p15.5 may carry a non-tissue-specific gene that could be involved in genetic predisposition, in tumor progression, or in both.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Lidereau R., Theillet C., Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- Benaily M., Schweisguth O., Job J. C. Les tumeurs cortico-surrenales de l'enfant. Etude retrospective de 34 cas observes de 1954 a 1973. Arch Fr Pediatr. 1975 May;32(5):441–453. [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Francke U., Holmes L. B., Atkins L., Riccardi V. M. Aniridia-Wilms' tumor association: evidence for specific deletion of 11p13. Cytogenet Cell Genet. 1979;24(3):185–192. doi: 10.1159/000131375. [DOI] [PubMed] [Google Scholar]
- Grundy P., Koufos A., Morgan K., Li F. P., Meadows A. T., Cavenee W. K. Familial predisposition to Wilms' tumour does not map to the short arm of chromosome 11. Nature. 1988 Nov 24;336(6197):374–376. doi: 10.1038/336374a0. [DOI] [PubMed] [Google Scholar]
- Habib R., Loirat C., Gubler M. C., Niaudet P., Bensman A., Levy M., Broyer M. The nephropathy associated with male pseudohermaphroditism and Wilms' tumor (Drash syndrome): a distinctive glomerular lesion--report of 10 cases. Clin Nephrol. 1985 Dec;24(6):269–278. [PubMed] [Google Scholar]
- Huff V., Compton D. A., Chao L. Y., Strong L. C., Geiser C. F., Saunders G. F. Lack of linkage of familial Wilms' tumour to chromosomal band 11p13. Nature. 1988 Nov 24;336(6197):377–378. doi: 10.1038/336377a0. [DOI] [PubMed] [Google Scholar]
- Humphries S. E., Jowett N. I., Williams L., Rees A., Vella M., Kessling A., Myklebost O., Lydon A., Seed M., Galton D. J. A DNA polymorphism adjacent to the human apolipoprotein CII gene. Mol Biol Med. 1983 Dec;1(5):463–471. [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Journel H., Lucas J., Allaire C., Le Mée F., Defawe G., Lecornu M., Jouan H., Roussey M., Le Marec B. Trisomy 11p15 and Beckwith-Wiedemann syndrome. Report of two new cases. Ann Genet. 1985;28(2):97–101. [PubMed] [Google Scholar]
- Kazazian H. H., Junien C. Report of the committee on the genetic constitution of chromosomes 10, 11, and 12. Cytogenet Cell Genet. 1987;46(1-4):188–212. doi: 10.1159/000132477. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Chin K. S., Easton D. F., Thorpe K., Carter C., Liou G. I., Fong S. L., Bridges C. D., Haak H., Kruseman A. C. A linked genetic marker for multiple endocrine neoplasia type 2A on chromosome 10. Nature. 1987 Aug 6;328(6130):527–528. doi: 10.1038/328527a0. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Matsunaga E. Genetics of Wilms' tumor. Hum Genet. 1981;57(3):231–246. doi: 10.1007/BF00278936. [DOI] [PubMed] [Google Scholar]
- Merrifield R. E., Simmons H. E. Topology of bonding in pi-electron systems. Proc Natl Acad Sci U S A. 1985 Jan;82(1):1–3. doi: 10.1073/pnas.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Parry D. M., Berg K., Mulvihill J. J., Carter C. L., Miller R. W. Strategies for controlling cancer through genetics: report of a workshop. Am J Hum Genet. 1987 Jul;41(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Pearson P. L., Kidd K. K., Willard H. F. Report of the committee on human gene mapping by recombinant DNA techniques. Cytogenet Cell Genet. 1987;46(1-4):390–566. doi: 10.1159/000132487. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Simpson N. E., Kidd K. K., Goodfellow P. J., McDermid H., Myers S., Kidd J. R., Jackson C. E., Duncan A. M., Farrer L. A., Brasch K. Assignment of multiple endocrine neoplasia type 2A to chromosome 10 by linkage. Nature. 1987 Aug 6;328(6130):528–530. doi: 10.1038/328528a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Turleau C., de Grouchy J. Beckwith-Wiedemann syndrome. Clinical comparison between patients with and without 11p15 trisomy. Ann Genet. 1985;28(2):93–96. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- WIEDEMANN H. R. COMPLEXE MALFORMATIF FAMILIAL AVEC HERNIE OMBILICALE ET MACROGLOSSIE--UN "SYNDROME NOUVEAU"? J Genet Hum. 1964 Sep;13:223–232. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]