Summary
Mammalian cells generally require both mitogens and anchorage signals in order to proliferate. An important characteristic of many tumour cells is that they have lost this anchorage-dependent cell-cycle checkpoint, allowing them to proliferate without signals provided by their normal microenvironment. In the absence of anchorage signals from the extracellular matrix, many cell types arrest cell-cycle progression in G1 phase as a result of Rb-dependent checkpoints. However, despite inactivation of p53 and Rb proteins, SV40LT-expressing cells retain anchorage dependency, suggesting the presence of an uncharacterised cell-cycle checkpoint, which can be overridden by coexpression of oncogenic Ras. We report here that, although cyclin-CDK complexes persisted in suspension, proliferation was inhibited in LT-expressing cells by the CDK inhibitor p27Kip1 (p27). Interestingly, this did not induce a stable arrest, but aberrant cell-cycle progression associated with stalled DNA replication, rereplication and chromosomal instability, which was sufficient to increase the frequency of oncogenic transformation. These results firstly indicate loss of anchorage in Rb- and p53-deficient cells as a novel mechanism for promotion of genomic instability; secondly suggest that anchorage checkpoints that protect normal cells from inappropriate proliferation act deleteriously in Rb- and p53-deficient cells to promote tumourigenesis; and thirdly indicate caution in the use of CDK inhibitors for cancer treatment.
Keywords: Anchorage, LT, p27, Genomic stability
Introduction
Mammalian cells generally require both mitogens and anchorage signals from a substratum to proliferate (Assoian and Schwartz, 2001). In the absence of anchorage, some cell types, such as fibroblasts, arrest in the cell cycle, whereas others, including epithelial cells, undergo anoikis (Frisch and Francis, 1994). Continued survival and proliferation following loss of contact with the extracellular matrix is dependent on the maintenance of intracellular signals in the absence of extracellular cues – a characteristic of transformed cells. Consistent with this, anchorage-independent proliferation is closely linked to tumourigenic potential (Freedman and Shin, 1974). Moreover, the ability of metastatic cells to colonise new microenvironments in vivo also relies on cell-cycle progression in the absence of normal extracellular stimuli. This dependency of normal cells on specific anchorage signals for proliferation means that the checkpoints responsible for cell-cycle arrest in the absence of anchorage are likely to act as important tumour-suppressive mechanisms.
Following loss of anchorage, normal cells arrest in G1 phase as a result of several checkpoint processes. Normally, attachment signals from the extracellular matrix via integrins synergise with mitogenic signals to promote sustained activation of the ERK pathway (Schwartz and Assoian, 2001). This sustained anchorage-dependent ERK response is required for the robust induction of cyclin D1 (CCND1) transcription, which is necessary to drive cells into the cell cycle, past the Rb-E2F restriction point and into S phase (Assoian and Klein, 2008; Roovers et al., 1999). In addition, the requirement for anchorage is reinforced by cell-cycle inhibitory signals that are induced upon detachment of the cells from the substratum. In particular, the cyclin-dependent kinase inhibitors p21 and p27 rapidly accumulate in detached cells, and inhibit cyclin-CDK activity (Fang et al., 1996; Zhu et al., 1996). Anchorage signalling via the focal adhesion kinase (FAK) appears to be important in the regulation of these inhibitors (Bryant et al., 2006). Other evidence, although less well understood, suggests that anchorage signals are also required for the induction of cyclin A gene (CCNA2) expression (Guadagno et al., 1993). Together, these mechanisms act to prevent cell-cycle progression in the absence of the appropriate anchorage signals and, as such, act as important regulatory mechanisms of cell-cycle control while also providing a safeguard against inappropriate proliferation.
Cancer cells are typically anchorage independent as a result of the disruption of these checkpoint mechanisms. However, consistent with the complexity and redundancy of the anchorage-checkpoint process, several oncogenic changes are required to confer anchorage independence on normal cells. In several mammalian cell types, it has been shown that abrogation of the Rb-family checkpoint, p53 inactivation and activation of Ras signalling pathways are all required to permit anchorage-independent proliferation, whereas immortalisation by TERT is an additional requirement in human cells (Hahn et al., 1999; Hanahan and Weinberg, 2000; Mitchell et al., 2003). Importantly, this combination of defects is frequently found in human tumours (Hanahan and Weinberg, 2000; Sherr and McCormick, 2002), thus validating the use of cells with defined oncogenic changes as models for stepwise transformation in cancer.
Cells expressing SV40 large T antigen (SV40LT) have non-functioning p53 and Rb-family pathways and can proliferate in the absence of mitogens, yet retain anchorage dependency. To explore the mechanisms responsible for this anchorage checkpoint, we have used SV40LT-expressing primary rat Schwann cells, in the absence or presence of oncogenic Ras, to model the barrier between anchorage dependent and independent proliferation. We found that although SV40LT-expressing cells maintained elevated expression of cyclin-CDK complexes in suspension, the expansion of these cells was inhibited by the induction of the cyclin-dependent kinase inhibitor p27. Unexpectedly, we found that although cell division was blocked in suspension, this was not the result of a stable cell-cycle arrest. Instead, cell-cycle progression was only partially inhibited, resulting in DNA replication stalling, rereplication and increased variability in chromosome number. Importantly, this increase in chromosomal instability was sufficient to lead to the emergence of fully transformed cells. Moreover, we made similar findings in a human cell model of oncogenic transformation. Chromosomal instability is a well-known hallmark of many cancer cells, and might contribute to the initiation and progression of tumours (Rajagopalan and Lengauer, 2004). Here, we describe a new link between loss of anchorage, the development of chromosomal instability and cellular transformation. These results indicate that anchorage checkpoints that suppress inappropriate proliferation in normal cells are imperfect in Rb- and p53-deficient cells and can promote tumourigenesis in these cells upon loss of anchorage. Moreover, these results suggest that caution should be taken in the use of CDK inhibitors for cancer treatment, because a partial loss of CDK activity might promote cancer.
Results
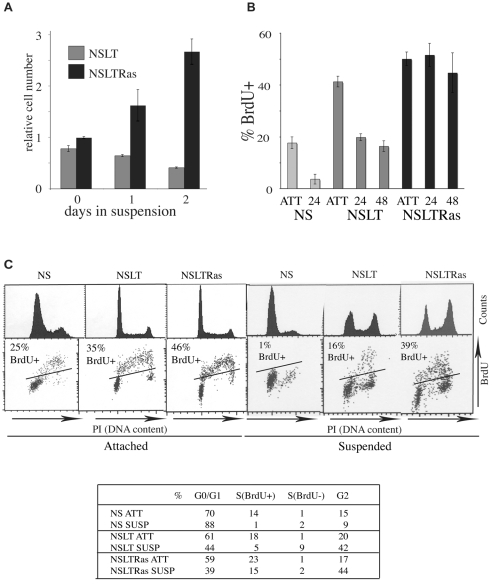
Soft agar assays are commonly used to assess the ability of cells to proliferate in the absence of anchorage signals. In soft agar, neither normal Schwann cells (NS) nor pools of NS cells constructed to express SV40LT (NSLT) form colonies; instead they persist as single cells within the agar. By contrast, pools of cells coexpressing SV40LT and an oncogenic form of Ras (NSLTRas) form colonies at high efficiency, indicating that these genetic changes are sufficient for anchorage-independent proliferation (Mitchell et al., 2003). To investigate biochemically why NSLT cells, despite being mitogen independent, are unable to proliferate in the absence of anchorage we used a modified suspension culture system in which cells were seeded into medium containing 1.8% methylcellulose. This forms a water-soluble gel that can be diluted and the cells efficiently retrieved by centrifugation (Hauser et al., 2004). By counting cells retrieved from this culture, we found that NSLTRas cells proliferated efficiently in the absence of anchorage, albeit with an increased cell-cycle time of 24 hours, compared with 18 hours in attached cells (Fig. 1A; supplementary material Fig. S1A). By contrast, and in agreement with the soft agar assays, the number of NSLT cells did not increase over the course of the experiment, indicating that NSLT cells require anchorage signals to proliferate. This effect was mostly independent of survival, because only slightly more apoptotic cells (∼20%) were seen in the NSLT compared with the NSLTRas cultures (∼10%) as measured by live-dead staining in methylcellulose at 24 hours and low levels of trypan blue staining at both 24 and 48 hours in suspension (supplementary material Fig. S1B).
Fig. 1.
NSLT cells do not proliferate in suspension, but show limited S-phase progression. (A) Cell counts from suspended NSLT and NSLTRas methylcellulose cultures. (B) Proportion of BrdU-incorporating cells attached (ATT) and in suspension at 24 hours (24) and 48 hours (48), measured by FACS. Graphs shows the mean ± s.e.m. of four separate experiments. (C) Representative FACS profiles of cells from 24 hour attached and suspension cultures, showing DNA content by propidium iodide (PI) staining (histograms) and BrdU incorporation after a 1 hour pulse (dot plots). Black lines on dot plots indicate the threshold above which cells are considered BrdU positive, defined by running similar samples without BrdU. FACS profiles at 48 hours showed identical profiles. Table shows quantitative data taken from these profiles.
To investigate how loss of anchorage inhibits the expansion of NSLT cells, we initially performed FACS analysis on attached cells and cells 24 and 48 hours after seeding in methylcellulose to determine the cell-cycle profile, and compared it with those of NS and NSLTRas cells. To monitor DNA replication, BrdU was added for 1 hour before cell retrieval and staining. Consistent with the increase in cell number, NSLTRas cells incorporated BrdU to similarly high levels whether the cells were attached or in suspension (Fig. 1B). NS cells, as expected from their inability to proliferate in suspension, exhibited a strong cell-cycle arrest, with suspended cells mostly in the G1 phase of the cell cycle. By contrast, NSLT cells behaved very differently to both the NS and NSLTRas cells. Unlike the NS cells, a significant proportion of NSLT cells continued to incorporate BrdU even after 48 hours in suspension (Fig. 1B), and this was confirmed by BrdU immunofluorescence in replated cells (not shown). NSLT cells also appeared in all phases of the cell cycle after 24 hours in suspension, with a DNA content similar to that of proliferating NSLTRas cells (Fig. 1C). In contrast to the NSLTRas, however, there was a sharp decrease in the proportion of NSLT cells incorporating BrdU in suspension. These results suggest a slower cell-cycle progression rather than a phase-specific arrest. Moreover, unlike either the NS or NSLTRas cells or the attached NSLT cells, a large proportion of the cells in S phase were BrdU negative. This demonstrates that progress through S phase was not simply slow but erratic, sometimes stalling for periods of an hour or more.
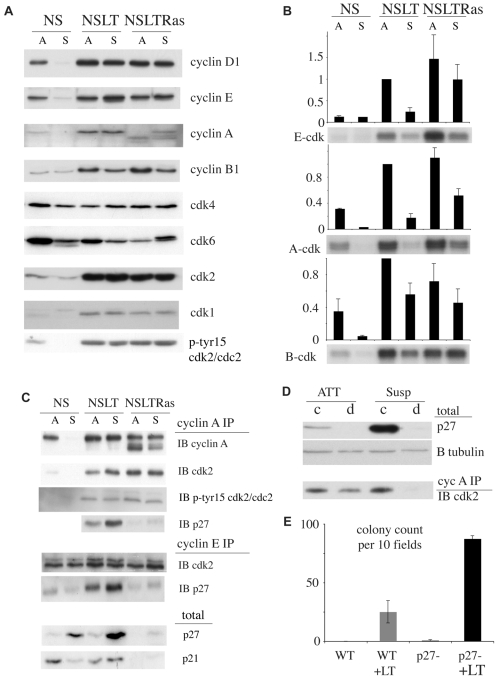
The inefficient and haphazard cell-cycle progression of NSLT cells in suspension suggested that the normal programme of cyclin-CDK activation had been disrupted. Analysis of the levels of cyclins and CDKs in attached and suspended cells showed that, similarly to the NSLTRas cells, cyclin-CDK levels remained high in the suspended NSLT cells (Fig. 2A). This was in contrast to NS cells, where levels of CDKs were notably decreased and cyclins were barely detectable in suspension, consistent with an Rb-mediated cell-cycle arrest. Moreover, levels of many cyclin and CDK proteins were several times higher in both the NSLT and NSLTRas attached cells compared with NS cells, consistent with the derepression of their gene promoters upon inactivation of the Rb family (Classon and Harlow, 2002; Markey et al., 2007). The levels of these proteins remained high in suspension, and were comparable between the NSLT and NSLTRas cells. However, when we measured cyclin-E- and cyclin-A-dependent kinase activity, we found that both kinases were strongly inhibited in the NSLT suspended cells (Fig. 2B). This difference in activity was not due to differences in the levels of cyclin-CDK complexes, because comparable amounts of cyclin-A-CDK2 and cyclin-E-CDK2 were detectable in the NSLT attached and suspended cells, and these levels were similar to those isolated from NSLTRas (Fig. 2C). In NS cells, however, levels of both complexes were very low in the suspended cells, which was consistent with the low levels of the constituent proteins.
Fig. 2.
Cyclin-CDK activity is inhibited in suspended NSLT cells by p27. (A) Western blot analysis of total lysate from attached (A) and suspended (S) cells. (B) Cyclin-dependent kinase activity in attached and suspended cells. Autoradiograms show representative results for cyclin E, cyclin A and cyclin B kinase assays. Graphs show mean ± s.e.m. from four similar experiments. (C) Western blots of immunoprecipitated (IP) cyclin A and cyclin E complexes, showing levels of bound CDK2 and p27 (IB). (D) p27 depletion of NSLT lysates, showing control (c) and p27-depleted (d) samples from attached (ATT) and suspended (Susp) cells. β-tubulin (B tubulin) was detected as a loading control. Immunoblot (IB) shows CDK2 from cyclin A immunoprecipitation (IP) of depleted lysates. (E) Colony formation in wild-type (WT) and p27-deficient (p27-) MEFs, expressing either SV40LT (+LT) or empty vector. p27-deficient MEFs express a truncation mutant (p27Δ51) unable to bind and inhibit CDKs. Cells were seeded in soft agar and colonies were counted after 1 week in ten microscope fields per well and at least two wells per cell type. Graph shows counts (± s.d.) from one of three similar experiments.
The activity of cyclin-CDK complexes can be regulated by the binding of inhibitors and by regulatory phosphorylations. We could not detect significant differences in known CDK2 regulatory phosphorylations in attached and suspended conditions by western blot or bound to cyclin A complexes (Fig. 2A,C; and data not shown). However, total levels of the CDK inhibitor p27 were several times higher in suspended NSLT cells compared with the attached cells (Fig. 2C), whereas p27 levels remained low in NSLTRas cells. Moreover, more p27 was bound to cyclin-CDK complexes in the suspended cells compared with levels in attached cells (Fig. 2C). To address whether this increase in p27 levels was sufficient to inhibit cyclin-CDK activity, we depleted p27-bound complexes from attached and suspended NSLT lysates and determined the levels of unbound cyclin-A-CDK left after depletion. Fig. 2D shows that p27 was efficiently depleted from lysates from both suspended and attached cells, but that unbound cyclin-A-CDK2 complexes were only detected in lysates from the attached cells. This indicated that essentially all cyclin-A-CDK2 complexes were bound by p27 when the cells were in suspension. To confirm the role of p27 in inhibiting anchorage-independent proliferation, we attempted to knock down p27 levels in NSLT cells using both siRNA and shRNA approaches. Although p27 protein was efficiently depleted from attached cells, once these were seeded into suspension, p27 levels increased and there was very little decrease in the amount of p27 bound to cyclin-CDK complexes compared with scrambled siRNA controls (not shown). As an alternative approach, we made use of mouse embryo fibroblasts (MEFs) engineered to express a mutant form of p27 (p27Δ51) that is unable to bind and inhibit cyclin-CDK complexes, and which has been shown in vivo to be phenotypically equivalent to a p27-null mutant (Kiyokawa et al., 1996). We infected these cells and corresponding wild-type MEFs with LT and seeded them into soft agar. Strikingly, infection of the p27Δ51 cells resulted in a high rate of colony formation, whereas control cells largely retained their anchorage dependence (Fig. 2E). These results confirm that CDK inhibition by p27 is a key factor limiting the anchorage-independent proliferation of checkpoint-deficient cells.
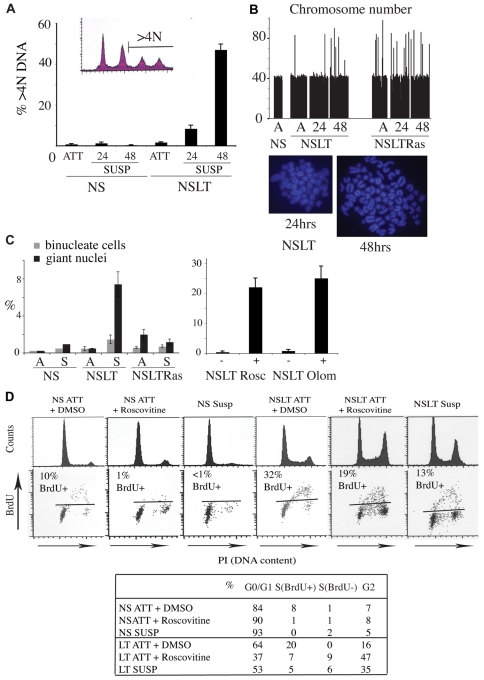
The FACS data showed that DNA replication was still taking place, to some extent, in suspended NSLT cells, but without a resultant increase in cell number. To test whether rereplication was occurring, we extended the gated population on profiles of cells stained with PI to include cells with greater than 4N DNA content. Fig. 3A showed that this was indeed the case, with the number of NSLT cells with a DNA content greater than 4N increasing dramatically to close to 50% at 48 hours. Metaphase spreads of NSLT cells retrieved from suspension also showed that many nuclei had more than the usual 42 chromosomes (Fig. 3B). Co-staining of whole reattached cells with Hoechst 33342 and CellTracker confirmed that most of these cells had single giant nuclei rather than being binucleate (Fig. 3C). By contrast, the NSLTRas attached cells had a significant proportion of cells with a greater than diploid chromosome number, but this did not change with time in suspension. The metaphase spread analysis also showed that the abnormal NSLT cells were not always tetraploid, because the total number of chromosomes varied significantly between cells. This indicates an underlying chromosomal instability, presumably because of an inability to accurately replicate the genome in suspension. Moreover, this analysis is likely to reflect an underestimation of the extent of chromosomal abnormalities, because only cells able to successfully enter mitosis upon reattachment would be scored using this assay.
Fig. 3.
Incomplete cell-cycle arrest in suspended NSLT cells results in genomic instability. (A) Quantification of >4N DNA content from FACS profiles of attached (ATT) and suspended (SUSP 24 or 48 hours) cells. Inset is the extended PI profile from NSLT cells after 48 hours in suspension. (B) Chromosome counts from metaphase spreads. Diploid chromosome complement is 42. Micrographs show diploid (left) and tetraploid (right) NSLT spreads, after 24 and 48 hours in suspension, respectively. (C) Percentage of giant nuclei and binucleate cells in attached (A) and recently replated suspension (S) cultures, fixed and stained with CellTracker and Hoechst 33342 (left). Percentage of giant nuclei in NSLT cells treated for 72 hours with 35 μM roscovitine (+), 125 μM olomoucine (+) or with DMSO (–) is shown on the right. (D) FACS profiles of attached DMSO and roscovitine-treated cells compared with untreated cells from 24 hour suspension culture for NS (left three panels) and NSLT (right three panels) cells. Table shows quantification of this analysis.
To address whether inhibition of cyclin-CDK activity in NSLT cells was sufficient to induce genomic instability, we treated NS and NSLT cells with the CDK inhibitors roscovitine and olomoucine. These treatments, similarly to loss of anchorage, abolished BrdU incorporation in attached NS cells (Fig. 3D; supplementary material Fig. S1D). Treated cells arrested mainly in G1, as in suspension, but also remained to a minor extent in G2 phase, indicating that not all cells in G2 were able to complete the cell cycle and return to G1. Importantly, inhibition of cyclin-CDK activity by roscovitine and olomoucine in attached NSLT cells also resulted in similar FACS profiles to those seen in cells in the absence of anchorage (Fig. 3D; supplementary material Fig. S1D): cells stalled in S phase, with a proportion still incorporating BrdU (although olomoucine-treated cells mostly accumulated in G1 rather than in G2-M). Moreover, this resulted in an increase in genomic abnormalities as judged by the large increase in cells with giant nuclei (Fig. 3C, right panel).
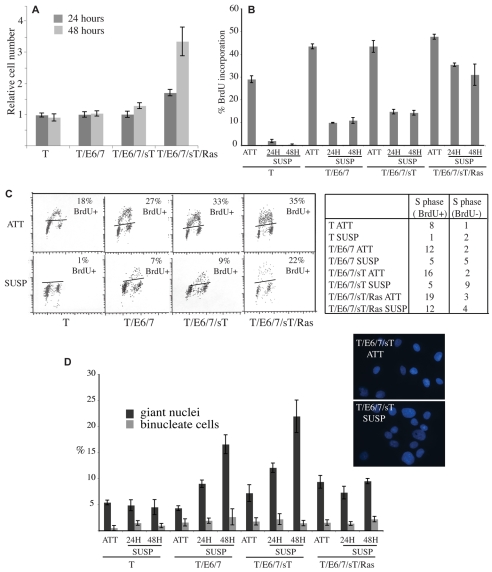
To confirm these findings, we performed the experiments in a second cell type, human mesenchymal stem cells (MSCs). For this type of analysis it is a requisite to use primary cells to which genetic changes can be added in a defined, sequential manner. Moreover, human stem cells have additional significance in that the transformation of stem cells is considered a prerequisite for the development of many human malignancies. As has been described for many human cell types, many human cells require additional genetic changes compared with rodent cells for full oncogenic transformation (Hahn et al., 1999; Hahn and Weinberg, 2002). These additional changes are the expression of TERT [which is expressed by rat Schwann cells (Mathon et al., 2001)] and SV40 small T. We used four populations of cells that have been previously characterised (Funes et al., 2007): (1) MSCs expressing TERT (T); (2) MSCs expressing TERT and HPV-16 E6 and E7 (which disrupt p53 and Rb function, respectively) (T/E6/7); (3) MSCs expressing TERT, E6 and E7 and SV40 small T antigen (T/E6/7/sT); (4) MSCs expressing TERT, E6 and E7, SV40 small T antigen and oncogenic Ras (T/E6/7/sT/Ras). Previous characterisation of these cells showed that only the Ras-expressing cells (T/E6/7/sT/Ras) were able to efficiently form colonies in soft agar. Consistent with this finding, only the Ras-expressing cells were able to proliferate efficiently in methylcellulose (Fig. 4A). BrdU and FACS analysis showed that the MSCs expressing only telomerase were strongly inhibited in suspension, with only 1-2% of cells incorporating BrdU (Fig. 4B,C). The efficient retrieval of the cells from suspension at 24 and 48 hours coupled with the absence of proliferation indicated that very few of these cells died in suspension. By contrast, and similarly to the Schwann cells, T/E6/7 and t/E6/7/sT cells did not increase in number in suspension but did show aberrant cell-cycle progression, including low levels of BrdU incorporation and a large increase in S phase cells that were BrdU negative, which indicates replication stalling. Moreover, upon fixation, these cells showed an increase in genomic abnormalities over time, with a large increase in the number of cells with giant nuclei after 48 hours in suspension (Fig. 4D).
Fig. 4.
Checkpoint-deficient mesenchymal stem cells undergo aberrant proliferation in suspension. (A) Cell counts for suspended T, T/E6/7, T/E6/7sT and T/E6/7/sT/Ras cells 24 and 48 hours after seeding. Numbers are expressed relative to cells seeded. (B) Proportion of BrdU-incorporating cells attached (ATT) and in suspension (Susp) measured by immunofluorescence following a 1 hour pulse of BrdU. Graph shows the mean of three coverslips of a representative experiment. (C) Representative FACS analysis of cells from attached (ATT) and cells in suspension for 24 hours (SUSP) following a 1 hour pulse. Black lines on dot plots indicate the threshold above which cells are considered BrdU positive, defined by running similar samples without BrdU. Table shows quantitative data taken from these profiles. (D) Graph showing percentage of giant nuclei and binucleate cells in attached (ATT) and suspended (SUSP) cultures 24 and 48 hours after seeding. Inset shows representative images of Hoechst-33342-stained T/E6/7sT cells showing an increase in binucleate cells following 48 hours in suspension. All graphs show mean ± s.d.
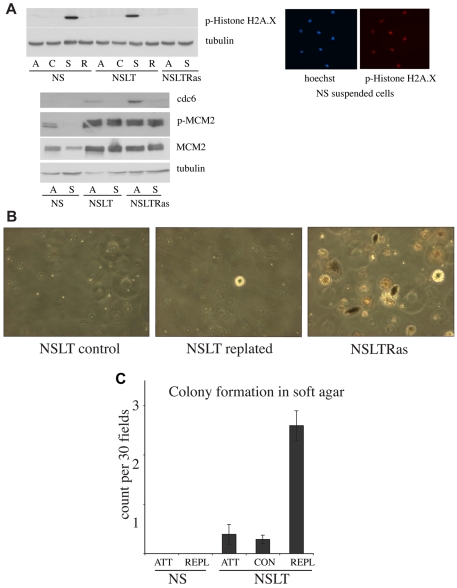
It has previously been reported that primary cells expressing oncogenes can undergo replication stress and endoreduplication both when proliferating in adherent conditions and in vivo (Bartkova et al., 2005; Bartkova et al., 2006; Blow and Gillespie, 2008; Di Micco et al., 2006; Gorgoulis et al., 2005; Halazonetis et al., 2008; Tort et al., 2006). In particular this `oncogenic stress' was associated with activation of the DNA damage checkpoint. To determine the role of this checkpoint in our system, we analysed the levels of phosphorylated histone H2A.X in NS, NSLT and NSLTRas cells in attached and suspension cultures by western blot. In all the attached cultures there were very low levels of H2A.X-P. This might be the result of our culture conditions, which have previously been shown to avoid the `culture shock' associated with the culture of many primary cells (Mathon et al., 2001). Interestingly, however, both the NS and NSLT cells showed a strong increase in the levels of H2A.X-P when the cells were in suspension and this was lost within 12 hours of replating (Fig. 5A). By contrast, the NSLTRas cells did not show any increase in suspension. However, the increase in H2A.X phosphorylation in suspended NS and NSLT cells was unlikely to be in response to replication stress, because immunofluorescence analysis indicated that the vast majority (>90%) of these cells expressed high levels of H2A.X-P in suspension (Fig. 5A; and data not shown) despite the fact that very few of the NS cells were in S phase. We also analysed levels of components of the pre-replication complexes and found high levels of active MCM2 and cdc6 in both NSLT and NSLTRas cells (Fig. 5A). Interestingly, the levels of active MCM2 remained high in suspension and might contribute to the ability of both these cell types to replicate their DNA in suspension. Cells with oncogenic changes and genomic instability are reported to be more susceptible to mitotic death. To assess whether mitotic death was contributing to our results, we determined the number of mitotic cells in attached and suspended NSLT and NSLTRas cultures by staining for the mitotic marker histone H3-Ser10. However, we found that in contrast to NSLTRas cells, very few of the NSLT cells were in mitosis (supplementary material Fig. S1C). Moreover, we could not detect any H3-Ser10 in the sub-G1 population by FACS (not shown). This analysis makes it unlikely that mitotic death contributes to inhibition of NSLT proliferation in suspension.
Fig. 5.
The genomic instability resulting from a period of suspension culture is sufficient to trigger an increased rate of cellular transformation. (A) Western blot analysis of total lysate from attached (A), control cells seeded in methylcellulose for 1 hour (C), suspended (S) and replated cells (R). Representative images of NS cells from suspended cultures immunostained for phosphorylated histone H2A.X and Hoechst 33342 are shown on the right. Attached cells showed no detectable staining at this exposure. Note that all cells are positively stained. (B) Soft agar assays of NSLT cells after 72 hours in suspension, followed by replating (centre), compared with NSLT passaged normally (left), and NSLTRas (right) as a positive control. (C) Quantification of combined results from six similar soft agar experiments. ATT, attached cells passaged normally; CON, control cells passaged normally that have undergone the methylcellulose seeding and retrieval procedure; REPL, replated cells after 72 hour methylcellulose suspension, plus two additional passages on ordinary culture plates. Error bars indicate s.e.m.
To address whether the genomic instability resulting from suspension of NSLT cells was sufficient to trigger an increased frequency of cellular transformation, we analysed the ability of the cells to form colonies in soft agar following a period in suspension. NS, NSLT and NSLTRas cells were maintained in methylcellulose suspension for 72 hours, and then retrieved and replated. As a control for the procedures, cells were also seeded into methylcellulose for 1 hour before retrieval. Two passages after replating, the cells were seeded in soft agar to assay colony formation. Remarkably, NSLT cells following this period in suspension had a significantly increased frequency of colony formation compared with both the NS and the NSLT control populations (Fig. 4A,B), thus demonstrating that the degree of genomic instability induced by loss of anchorage is sufficient to increase the frequency of cellular transformation.
Discussion
Here, we have shown that the failure of NSLT cells to proliferate in suspension is due to inhibition of cyclin-CDK activity resulting from the upregulation of p27. NSLTRas cells are immune to this, because Ras signalling pathways trigger the degradation of p27 (Kawada et al., 1997). Previous studies have indicated that SV40LT-expressing cells are refractory to the effects of p27 (Mann and Jones, 1996). Our results at first glance appeared to contradict these studies, because NSLT cell proliferation is inhibited in suspension as a result of p27 induction. However, detailed analysis showed that although these cells did not proliferate in suspension, the cell cycle was only partially arrested. Critically, this partial blockage, resulting from dysfunctional checkpoints, had deleterious consequences, resulting in DNA rereplication, genomic instability and an increased frequency of oncogenic transformation.
This work has several interesting implications. On the one hand, our results reaffirm the importance of p27 as a tumour suppressor: it acts as a barrier to anchorage-independent proliferation both in normal cells and in cells that have lost other checkpoint mechanisms such as p53-Rb. In agreement with this, p27 levels are inversely correlated with anchorage independence in human cancer cell lines (Kawada et al., 1998). In addition, oncogenic activity has been directly linked to p27 inactivation and turnover (Grimmler et al., 2007; Kawada et al., 1997), and either downregulation of p27 itself, its mislocalisation or increased degradation via Skp2, are all correlated with poor prognosis in a range of cancers (reviewed by Chu et al., 2008). On the other hand, our results clearly demonstrate how p27 induction, by inhibiting cyclin-CDK activity in checkpoint-deficient cells, whilst protecting against immediate outgrowth (and thus still acting to protect against cancer) does so imperfectly, resulting in an increase in genomic instability, which increases the rate of oncogenic transformation as a result of further genetic changes (examples of which could be p27 loss or Ras activation). This might be partly explained by the fact that CDK activity can prevent rereplication (Porter, 2008), which, combined with loss of cell-cycle checkpoints, might be expected to promote genomic instability (Srinivasan et al., 2007; Vaziri et al., 2003).
Previous work has reported that oncogenic changes can result in a DNA-damage response and endoreduplication (Bartkova et al., 2005; Bartkova et al., 2006; Blow and Gillespie, 2008; Di Micco et al., 2006; Gorgoulis et al., 2005; Halazonetis et al., 2008; Tort et al., 2006). The DNA-damage response has been proposed to act as a barrier to early tumourigenesis, induced, at least in part, in response to replication stress as a result of oncogenic signaling. In our cell system, we saw little evidence of a DNA-damage response in attached cells. This may be because a `culture shock' environment is a necessary co-factor. However, we do see a large increase in a DNA-damage marker (H2A.X-P) when cells lose their normal attachment signals, suggesting that loss of normal anchorage signals can also induce a form of `culture shock', which is exhibited as a DNA-damage response. This is consistent with a previous report in which normal human epithelial cells undergo senescence when plated onto plastic rather than onto a more physiological stromal environment (Ramirez et al., 2001). It will be of great interest to determine the mechanism involved and address whether this mechanism contributes to the damage response seen in early tumourigenesis. Other studies have reported that oncogenic signaling can result in elevated expression of components of the pre-replication complex. We confirm these findings, by showing that MCM2 and CDC6 are highly expressed in cells expressing SV40LT. Moreover, we find that MCM2 expression is maintained in the absence of anchorage and is likely to contribute to the endoreduplication that is seen in these cells.
To our knowledge, this is the first report demonstrating that loss of normal anchorage signals can lead to genomic instability and promote tumourigenesis. Human cells require additional genetic changes compared with rodent cells to elicit full transformation, and it is thought that an increase in genetic instability is required for sufficient changes to occur. Our findings in human stem cells, which show that loss of anchorage in Rb- and p53-deficient cells results in increased genomic instability, might thus have additional significance. Correct sensing of and response to cellular attachment signals are recognised as important tumour suppressive mechanisms, because of their links to cell-cycle control pathways. However, we find that the mechanisms designed to protect against inappropriate proliferation can have tumour-promoting effects in checkpoint-deficient cells. It is conceivable that the detachment of a checkpoint-deficient but noncancerous cell from its normal environment could precipitate tumourigenesis in a new location because of development of genomic instability upon loss of anchorage. This concept is supported by the finding that metastasis of single cells is not always a late event in tumour progression and can occur before the cell has fully transformed (Husemann et al., 2008). However, it could also be envisaged that this process could be involved in tumourigenesis at an early stage at the site of initiation. It is becoming increasingly clear that changes to the microenvironment are key triggers for tumourigenesis (Elenbaas and Weinberg, 2001; Tlsty and Coussens, 2006). Some of these changes are likely to result in changes to the normal anchorage environment and to disruption of normal anchorage signaling to the cell cycle. In the case reported here, the effect on the cell cycle is triggered by the induction of a CDK inhibitor following loss of normal anchorage signals; however, it would be predicted that inhibiting CDK activity by other mechanisms would have a similar effect. Consistent with this, we found that the addition of moderate doses of the CDK inhibitors roscovitine or olomoucine to checkpoint-deficient cells produced a phenotype which was similar to that induced in our suspended cells. CDK inhibitors have been developed, in part, as potential cancer therapeutics and several are currently in clinical trials (Malumbres et al., 2008). Our results suggest that insufficient doses of CDK inhibitors, particularly in checkpoint-deficient cells, might have deleterious consequences by promoting gross chromosomal instability, which might increase the chances of tumour progression. These findings, coupled with previous reports of the importance of CDK activity for appropriate repair responses (Jazayeri et al., 2005), suggest that care should be taken with their clinical use.
Materials and Methods
Cells
Normal primary Schwann (NS) cells from rat sciatic nerve were routinely cultured on plastic dishes (Nunc) coated with poly-L-lysine (Sigma) in DMEM (Invitrogen) supplemented with 3% stripped foetal calf serum (Biosera), forskolin (Calbiochem) and glial growth factor, as described (Mathon et al., 2001). Schwann cells stably expressing SV40LT were infected with virus from Phoenix producer cells transfected with either LXSN empty vector (NSLT cells) or H-RasG12V LXSN (NSLTRas cells). Infected cells were selected with G418 to ensure stable vector expression. MEFs from wild-type and p27Δ51 knock-in mice (Kiyokawa et al., 1996) were a kind gift from Andrew Koff (Sloan Kettering Institute, New York, NY) and were cultured in 3% oxygen, in DMEM supplemented with 10% FBS (Sigma) plus antibiotic. Human primary mesenchymal stem cells (MSCs) expressing either TERT, TERT + HPV16 E6 + E7, TERT + E6 + E7 + SV40 small t, or TERT + E6 + E7 + small t + H-RasG12V, were a kind gift from Chris Boshoff (UCL Cancer Institute, London, UK). MSCs were cultured in MesenCult Basal medium plus 10% stimulatory supplements (Stemcell Technologies) and 1 ng/ml basic FGF (R&D Systems).
Suspension culture
Cells were seeded in centrifuge tubes in medium containing 1.8% dissolved methylcellulose (Sigma) to form a semisolid hydrogel. Tubes were incubated with loose lids to allow equilibration with the CO2-controlled atmosphere. Control experiments were carried out to ensure that cells remained suspended evenly throughout the gel (not shown). To retrieve cells, the suspension was diluted fivefold with DMEM containing 1% serum and centrifuged at 500 g for 10 minutes (Beckman Coulter J6-M). The pellet was then washed twice with ice-cold PBS.
Cell counts
Cell pellets retrieved from methylcellulose were resuspended and dissociated for 1-5 minutes in trypsin-EDTA followed by inactivation with medium. The resulting suspension was diluted in isotonic solution and cells counted using a Coulter Counter (Beckman Coulter).
Flow cytometry
BrdU was added to the medium or methylcellulose suspension 1 hour before harvesting cells. Ethanol-fixed samples were stained with anti-BrdU (Roche) and propidium iodide (Sigma) and analysed using Cellquest Pro software (Becton Dickinson). 10,000 events were collected for each sample. For roscovitine and olomoucine treatment, the drugs were added to the medium of attached cells 24 hours before harvesting. Roscovitine was used at 35 μM and olomoucine at 125 μM.
Western blot
Antibodies against the following proteins were used: cyclin D1 (sc450), cyclin E (sc481), cyclin A (sc596), cyclin B1 (sc595), CDK4 (sc260), CDK2 (sc163), CDK1 (sc747), p21 (sc397-G), p27 (sc1641) (all Santa Cruz Biotechnology); CDK6 (Neomarkers Ab-3), β-tubulin (Sigma T4026), Tyr15-P cdc2 and cdk2 (Cell Signaling Technology 9111), MCM2 (BD Biosciences BM-28), MCM2-P (Ser53) (Bethyl Laboratories A300-756a), H2A.X-P (Cell Signaling Technology 2577).
Immunoprecipitation and kinase assays
Total protein lysate (100-300 μg) was incubated with either protein G beads crosslinked to cyclin A antibody (Cancer Research UK clone E72), cyclin B antibody (Santa Cruz Biotechnology sc245), or protein A beads crosslinked to cyclin E antibody (Santa Cruz Biotechnology sc481). Bound complexes were assayed for kinase activity using purified histone H1 substrate (Sigma) and [γ-32P]ATP (GE Healthcare) at 37°C for 30 minutes, before gel electrophoresis and film exposure.
Immunodepletion
250 μg protein lysate from attached or suspended NSLT was rotated at 4°C for 1 hour with protein G beads either alone, or with p27 polyclonal antibody sc528 (Santa Cruz Biotechnology). Beads were discarded and the depletion was carried out twice more, then control and depleted samples were immunoprecipitated using E72 beads as above, to pull down cyclin A complexes.
Soft agar assay
Schwann cells (500,000 per 15 cm plate) were seeded in 0.5% agar on top of a bottom layer of 1% agar (SeaPlaque, Lonza). Colonies were counted after 4 weeks in 30 fields per plate. For MEFs, cells were seeded in six-well plates at 4000 cells per well. Colonies were counted after 1 week in 10 fields per well.
Viability assays
Live-dead staining (using kit L-3224, Invitrogen) was carried out according to the manufacturer's instructions, except that the reagent was incubated with a small aliquot of cells in methylcellulose suspension instead of adherent cells. Trypan blue staining was carried out on cells retrieved from suspension as for counting, incubated for 5 minutes at room temperature in 0.04% trypan blue solution (Sigma T8154) and scored for dye exclusion using a haemocytometer.
Microscopy
BrdU staining was carried out on attached MSCs and those from suspension culture, following a 1 hour BrdU pulse. Cells were retrieved from suspension, washing out the BrdU in the process, and allowed to reattach before fixing in 4% formaldehyde. Attached cells were fixed directly following the pulse. Fixed cells were acid-treated in 2 M HCl, permeabilised in 0.2% Triton X-100 and blocked in 3% BSA in PBS before staining with anti-BrdU (Roche) at 1:300, plus Hoechst 33342. To quantify giant nuclei and binucleate cells, samples from attached and suspension culture were stained with CellTracker (Invitrogen) and Hoechst 33342. Giant nuclei and binucleate cells were scored by eye, counting a total of at least 200 cells per coverslip. Cells were treated with 35 μM roscovitine or 125 μM olomoucine every 24 hours for a total of 72 hours before fixing. For histone H2A.X-P staining, cells were fixed in 95% ethanol, 5% acetic acid and blocked in 3% BSA before staining with primary antibody at 1:2000 (Millipore 05-636).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/18/3272/DC1
We thank Andrew Koff (Sloan Kettering Institute, New York, NY) for kindly donating p27Δ51 and WT MEFs. We thank Juan M. Funes and Chris Boshoff (The UCL Cancer Institute, University College London, London, UK) for the MSC cells and helpful advice on their culture and Kai Stoeber (Wolfson Institute for Biomedical Research, University College London, London, UK) for advice and gifts of antibodies. We thank Buzz Baum and Simona Parrinello for critical reading of the manuscript and Melissa Collins, Laura Rosenberg and Patrick Wingfield-Digby for help with some experiments. A.C.L. is a CRUK Senior Cancer Research Fellow. C.C. is supported by an MRC studentship. Deposited in PMC for release after 6 months.
References
- Assoian, R. K. and Schwartz, M. A. (2001). Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11, 48-53. [DOI] [PubMed] [Google Scholar]
- Assoian, R. K. and Klein, E. A. (2008). Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 18, 347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova, J., Horejsi, Z., Koed, K., Kramer, A., Tort, F., Zieger, K., Guldberg, P., Sehested, M., Nesland, J., Lukas, C. et al. (2005). DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864-870. [DOI] [PubMed] [Google Scholar]
- Bartkova, J., Rezaei, N., Liontos, M., Karakaidos, P., Kletsas, D., Issaeva, N., Vassiliou, L.-V., Kolettas, E., Niforou, K., Zoumpourlis, V. et al. (2006). Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633-637. [DOI] [PubMed] [Google Scholar]
- Blow, J. and Gillespie, P. (2008). Replication licensing and cancer, a fatal entanglement? Nat. Rev. Cancer 8, 799-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, P., Zheng, Q. and Pumiglia, K. (2006). Focal adhesion kinase controls cellular levels of p27/Kip1 and p21/Cip1 through Skp2-dependent and -independent mechanisms. Mol. Cell. Biol. 26, 4201-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, I., Hengst, L. and Slingerland, J. (2008). The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 8, 253-267. [DOI] [PubMed] [Google Scholar]
- Classon, M. and Harlow, E. (2002). The retinoblastoma tumour suppressor in development and cancer. Nat. Rev. Cancer 2, 910-917. [DOI] [PubMed] [Google Scholar]
- Di Micco, R., Fumagalli, M., Cicalese, A., Piccinin, S., Gasparini, P., Luise, C., Schurra, C., Garre', M., Nuciforo, P., Bensimon, A. et al. (2006). Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638-642. [DOI] [PubMed] [Google Scholar]
- Elenbaas, B. and Weinberg, R. A. (2001). Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp. Cell Res. 264, 169-184. [DOI] [PubMed] [Google Scholar]
- Fang, F., Orend, G., Watanabe, N., Hunter, T. and Ruoslahti, E. (1996). Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science 271, 499-502. [DOI] [PubMed] [Google Scholar]
- Freedman, V. H. and Shin, S. I. (1974). Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3, 355-359. [DOI] [PubMed] [Google Scholar]
- Frisch, S. and Francis, H. (1994). Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes, J. M., Quintero, M., Henderson, S., Martinez, D., Qureshi, U., Westwood, C., Clements, M. O., Bourboulia, D., Pedley, R. B., Moncada, S. et al. (2007). Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl. Acad. Sci. USA 104, 6223-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis, V., Vassiliou, L. V., Karakaidos, P., Zacharatos, P., Kotsinas, A., Liloglou, T., Venere, M., Ditullio, R., Kastrinakis, N., Levy, B. et al. (2005). Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907-913. [DOI] [PubMed] [Google Scholar]
- Grimmler, M., Wang, Y., Mund, T., Cilensek, Z., Keidel, E. M., Waddell, M. B., Jäkel, H., Kullmann, M., Kriwacki, R. W. and Hengst, L. (2007). Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128, 269-280. [DOI] [PubMed] [Google Scholar]
- Guadagno, T. M., Ohtsubo, M., Roberts, J. M. and Assoian, R. K. (1993). A link between cyclin A expression and adhesion-dependent cell cycle progression. Science 262, 1572-1575. [DOI] [PubMed] [Google Scholar]
- Hahn, W. C. and Weinberg, R. A. (2002). Rules for making human tumor cells. N. Engl. J. Med. 347, 1593-1603. [DOI] [PubMed] [Google Scholar]
- Hahn, W. C., Counter, C. M., Lundberg, A. S., Beijersbergen, R. L., Brooks, M. W. and Weinberg, R. A. (1999). Creation of human tumour cells with defined genetic elements. Nature 400, 464-468. [DOI] [PubMed] [Google Scholar]
- Halazonetis, T., Gorgoulis, V. and Bartek, J. (2008). An oncogene-induced DNA damage model for cancer development. Science 319, 1352-1355. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. and Weinberg, R. (2000). The hallmarks of cancer. Cell 100, 57-70. [DOI] [PubMed] [Google Scholar]
- Hauser, P., Ma, L., Agrawal, D., Haura, E., Cress, W. D. and Pledger, W. J. (2004). Efficient down-regulation of cyclin A-associated activity and expression in suspended primary keratinocytes requires p21(Cip1). Mol. Cancer Res. 2, 96-104. [PubMed] [Google Scholar]
- Husemann, Y., Geigl, J. B., Schubert, F., Musiani, P., Meyer, M., Burghart, E., Forni, G., Eils, R., Fehm, T., Riethmuller, G. et al. (2008). Systemic spread is an early step in breast cancer. Cancer Cell 13, 58-68. [DOI] [PubMed] [Google Scholar]
- Jazayeri, A., Falck, J., Lukas, C., Bartek, J., Smith, G., Lukas, J. and Jackson, S. (2005). ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8, 37-45. [DOI] [PubMed] [Google Scholar]
- Kawada, M., Yamagoe, S., Murakami, Y., Suzuki, K., Mizuno, S. and Uehara, Y. (1997). Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene 15, 629-637. [DOI] [PubMed] [Google Scholar]
- Kawada, M., Uehara, Y., Mizuno, S., Yamori, T. and Tsuruo, T. (1998). Up-regulation of p27Kip1 correlates inversely with anchorage-independent growth of human cancer cell lines. Jpn. J. Cancer Res. 89, 110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa, H., Kineman, R. D., Manova-Todorova, K. O., Soares, V. C., Hoffman, E. S., Ono, M., Khanam, D., Hayday, A. C., Frohman, L. A. and Koff, A. (1996). Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85, 721-732. [DOI] [PubMed] [Google Scholar]
- Malumbres, M., Pevarello, P., Barbacid, M. and Bischoff, J. R. (2008). CDK inhibitors in cancer therapy: what is next? Trends Pharmacol. Sci. 29, 16-21. [DOI] [PubMed] [Google Scholar]
- Mann, D. J. and Jones, N. C. (1996). E2F-1 but not E2F-4 can overcome p16-induced G1 cell-cycle arrest. Curr. Biol. 6, 474-483. [DOI] [PubMed] [Google Scholar]
- Markey, M. P., Bergseid, J., Bosco, E. E., Stengel, K., Xu, H., Mayhew, C. N., Schwemberger, S. J., Braden, W. A., Jiang, Y., Babcock, G. F. et al. (2007). Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene 26, 6307-6318. [DOI] [PubMed] [Google Scholar]
- Mathon, N. F., Malcolm, D. S., Harrisingh, M. C., Cheng, L. and Lloyd, A. C. (2001). Lack of replicative senescence in normal rodent glia. Science 291, 872-875. [DOI] [PubMed] [Google Scholar]
- Mitchell, P. J., Perez-Nadales, E., Malcolm, D. S. and Lloyd, A. C. (2003). Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype. Mol. Cell. Biol. 23, 2530-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, A. (2008). Preventing DNA over-replication: a Cdk perspective. Cell Div. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan, H. and Lengauer, C. (2004). Aneuploidy and cancer. Nature 432, 338-341. [DOI] [PubMed] [Google Scholar]
- Ramirez, R. D., Morales, C. P., Herbert, B. S., Rohde, J. M., Passons, C., Shay, J. W. and Wright, W. E. (2001). Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15, 398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers, K., Davey, G., Zhu, X., Bottazzi, M. E. and Assoian, R. K. (1999). Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol. Biol. Cell 10, 3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. A. and Assoian, R. K. (2001). Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553-2560. [DOI] [PubMed] [Google Scholar]
- Sherr, C. and McCormick, F. (2002). The RB and p53 pathways in cancer. Cancer Cell 2, 103-112. [DOI] [PubMed] [Google Scholar]
- Srinivasan, S. V., Mayhew, C. N., Schwemberger, S., Zagorski, W. and Knudsen, E. S. (2007). RB loss promotes aberrant ploidy by deregulating levels and activity of DNA replication factors. J. Biol. Chem. 282, 23867-23877. [DOI] [PubMed] [Google Scholar]
- Tlsty, T. D. and Coussens, L. M. (2006). Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 1, 119-150. [DOI] [PubMed] [Google Scholar]
- Tort, F., Bartkova, J., Sehested, M., Orntoft, T., Lukas, J. and Bartek, J. (2006). Retinoblastoma pathway defects show differential ability to activate the constitutive DNA damage response in human tumorigenesis. Cancer Res. 66, 10258-10263. [DOI] [PubMed] [Google Scholar]
- Vaziri, C., Saxena, S., Jeon, Y., Lee, C., Murata, K., Machida, Y., Wagle, N., Hwang, D. S. and Dutta, A. (2003). A p53-dependent checkpoint pathway prevents rereplication. Mol. Cell 11, 997-1008. [DOI] [PubMed] [Google Scholar]
- Zhu, X., Ohtsubo, M., Böhmer, R. M., Roberts, J. M. and Assoian, R. K. (1996). Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133, 391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.