Abstract
Central auditory function is commonly compromised in people with a diagnosis of Alzheimer's disease (AD) and may precede the onset of clinical dementia by several years. Given that screening for AD in its earliest stages might someday be useful for emerging therapies aimed at limiting progression, we inquired whether central auditory testing might be suitable for identifying people at risk for dementia.
To address this question, we performed a battery of behavioral central auditory tests in a cohort of 313 older people enrolled in a dementia surveillance research program. The cohort consisted of three groups: controls without memory loss (N=232), targets with mild memory impairment but without dementia (N=64), and targets with a dementia diagnosis (N=17). The auditory tests were the Synthetic Sentence Identification with Ipsilateral Competing Message (SSI), the Dichotic Sentence Identification test (DSI), the Dichotic Digits Test (DDT), and the Pitch Pattern Sequence (PPS) test. Additional control was provided by electrophysiologic testing to assess the integrity of the primary auditory pathways.
The mean score on each central auditory test worsened significantly across the three memory groups even after adjusting for age and peripheral hearing status, being poorest in the pAD group and moderately reduced in the memory-impaired group compared to the mean scores in the control group. Heterogeneity of results was noted in all three groups. The electrophysiologic tests did not differ across the three groups.
Central auditory function was affected by mild memory impairment. The Dichotic Sentence Identification in the free report mode appears to be the central auditory test most sensitive to the presence of memory impairment. Although central auditory testing requires specialized equipment and training, the objectivity of these tests is appealing. We recommend that comprehensive auditory testing be considered and further evaluated for its potential value as a baseline measure for a) new geriatric patients and b) people being evaluated because of memory impairment. Because central auditory dysfunction may have considerable impact on hearing rehabilitation, we also recommend including a central auditory test, such as the DSI, to the auditory assessment of older people who seek assistance for hearing difficulty.
BACKGROUND
Behavioral auditory testing provides a clinical opportunity to examine brain functions involved in sound perception and processing. By using standard test paradigms, the contributions of central auditory processing in understanding and interpreting speech may be delineated. Because poor peripheral auditory function may confound the interpretation of central auditory tests, basic audiometry is necessary to assure adequate sound perception ability. Our working definition of adequate peripheral function for this study is a pure-tone threshold average (PTA) of 48 dB HL or better (moderate hearing loss) in both ears, after adjusting for any conductive loss, and word recognition scores of 70 percent or higher at an optimal loudness level.
We have previously demonstrated that central auditory processing (CAP) dysfunction – as judged by competing message testing – is highly prevalent in people with a diagnosis of Alzheimer's dementia (AD) and adequate peripheral function.1 The present report extends those observations and provides additional evidence about the possible mechanism(s) involved.
CAP dysfunction is a general term applied to people whose hearing in quiet is normal or near normal yet who have substantial hearing difficulty in the presence of auditory stressors such as competing noise and other difficult listening situations. People with CAP dysfunction typically note difficulty hearing one conversation amidst several competing conversations (“cocktail party effect”). CAP dysfunction, per se, is not generally helped by hearing aids. The prevalence of CAP dysfunction increases with age.2 Because cochlear function declines with age, CAP tests are usually done in the presence of reduced cochlear function due to age-related hearing loss (presbycusis).
Common causes of CAP dysfunction are aging, dementia, stroke, head injury, neoplasm, and developmental disturbances.3 CAP dysfunction may be suspected by below-normal responses on one or more of a heterogeneous group of auditory tests. These tests stress the auditory system in one of several ways. Of the three types of CAP tests used in the present study, one involves the simultaneous presentation of speech in the presence of competing speech in the same ear. A second type involves listening to two different signals (words or numbers) presented simultaneously, one in each ear (dichotic presentation). These two types of CAP test paradigms involve managing competing signals, which requires short term memory, attention shifting, and competitive inhibition. A third type of central test used in the present study involves temporal sequencing of non-speech stimuli presented to each ear separately (tonal pitch patterns). This type of test involves both cerebral hemispheres.
In the present report we asked whether the abnormal central auditory results that have been previously demonstrated in people with Alzheimer's type dementia could also be observed in people with memory loss but none of the other criteria for a diagnosis of AD. If central auditory dysfunction should prove to be associated with early cognitive decline, then tests for central auditory dysfunction might have value in identifying people at risk for dementia.
METHODS
Overview
We report the findings from 313 members of a dementia surveillance cohort aged 71-99 years (mean age 80 years) obtained by comprehensive audiometry (behavioral thresholds and word recognition, central auditory tests, and electrophysiologic measures) to determine whether poor performance on central auditory tests was associated with memory deficits, with auditory pathway deficits (as measured electrophysiologically), or both.
Study Population
Participants were enrolled in the Adult Changes in Thought (ACT) study, a population-based longitudinal study of aging and dementia that began in 1994. The ACT study was designed to determine the incidence of Alzheimer's disease (AD), other types of dementia and cognitive impairment, and to determine risk factors for these conditions. The details of the ACT study population have been previously described.4 Briefly, study participants, age 65 years and older, were randomly sampled from Seattle-area members of Group Health Cooperative (GHC), a consumer-governed HMO serving approximately 550,000 enrollees in Washington State and northern Idaho. Participants were ineligible for the study if they had an existing diagnosis of dementia, were current residents of a nursing home, or were participating in other research studies. The original cohort of 2581 men and women was enrolled between 1994 and 1996, and an additional 811 participants - the expansion cohort - were enrolled between 2000 and 2002. Subsequent to 2002, ACT has continued to enroll study participants in order to maintain a sizeable cohort of dementia-free participants under observation.
ACT participants who volunteered were screened over the telephone by the hearing study staff to confirm eligibility, specifically that the participant's hearing was good enough to be considered potentially eligible for enrollment. After the step-by-step details of participation were explained, eligible participants were scheduled for a 3.5 hour test session or two shorter sessions on separate days. At the end of the test session, participants received a copy of their hearing test, counseling about their test results, and information about assistive listening devices and local hearing resources, as needed.
Informed consent was obtained for all participants using forms and procedures approved by the Human Studies Committee of the University of Washington and Institutional Review Board of the Group Health Cooperative. Proxy consent via a family member or friend was obtained in addition to the participant's consent for ACT subjects with an established diagnosis of cognitive impairment.
Eligibility Criteria
A total of 337 ACT participants was enrolled in the hearing study out of 445 people evaluated on-site and 90 people who were telephone-screened. The 198 ACT participants not enrolled after initial contact either refused to participate or were ineligible. Enrolled participants were subsequently excluded from the present analysis if their peripheral auditory function was inadequate, i.e.: a) pure-tone threshold average (PTA) for 0.5, 1.0, or 2.0 kHz differed by more than 25 between ears, b) a PTA greater than 48 dB HL in either ear, c) word recognition score (WRS) of less than 70% in either ear, or d) evidence of middle ear disease on examination. Twenty-four participants were excluded based on inadequate peripheral auditory function, resulting in a final sample size of 313 for analysis.
Cognitive Assessment
Cognitive function was evaluated using the Cognitive Ability Screening Instrument (CASI).5 The CASI consists of 25 items which cover nine cognitive domains (attention, mental manipulation, orientation, short-term memory, long-term memory, language ability, visual construction, list-generating fluency, and abstraction and judgment). Total scores range from 0 to 100, with higher scores indicating better cognitive performance. Follow-up examinations, including CASI screening, are conducted biennially with the ACT cohort to identify incident cases of dementia and AD. Participants scoring 87 or higher on the CASI are considered dementia free. Those scoring 86 or below undergo a standardized clinical and neuropsychological evaluation, which is reviewed at a consensus diagnosis meeting attended by a geriatric physician, neurologist, research nurse, and neuropsychologist. For this study we considered that CASI scores of 90 or lower may be indicative of possible cognitive decline, although that score did not trigger the diagnostic evaluation for dementia in the parent study
Group Classification
In the current hearing study, two groups of ACT participants were enrolled. The memory-impaired (target) group had a CASI total score < 86, or a total CASI < 90 with a CASI memory subscale score < 10. Clinical Dementia Rating (CDR)6 scores of 0.5 (questionable dementia) or 1.0 (mild dementia) also qualified participants for the target group. We refer to the target group as memory-impaired throughout this report. Participants who did not show signs of memory impairment significant enough to qualify for the target group were enrolled in the control (non-memory-impaired) group.
The memory-impaired group was further subdivided into no-AD and AD-positive subgroups. Participants who at consensus conference met National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria7 for possible or probable Alzheimer's disease were considered incident AD cases.
Other Measures
Other variables collected from study participants included age, gender, years of education, APOE 4 genotype, depression, physical exercise, and reports of coronary heart disease (CHD) and cerebrovascular disease (CVD). CHD was defined as a self-reported history of myocardial infarction, angina, coronary bypass surgery, or angioplasty. CVD was defined as a self-report of stroke, cerebral hemorrhage, and small strokes or transient ischemic attacks. Depression was measured by the 20-item Center for Epidemiologic Studies Depression Scale (CESD) with scores above 16 indicating the presence of depressive symptoms.8 Age was noted at the time of the hearing testing. Gender, education, and APOE 4 genotype were collected upon enrollment into the ACT study. All other variables were assessed at the ACT biennial visit that occurred closest in time to the hearing testing.
Auditory Testing
Otoscopy and Questionnaire
Otoscopy and cerumen removal (if necessary) were carried out. A brief hearing and noise history questionnaire was administered.
Tympanometry and Pure-Tone Thresholds
Conventional 226 Hz probe tone tympanometry was conducted to assess middle ear status. Standard audiometric measures were obtained with the participant seated in a double-walled sound treated room listening through ER-3A insert earphones to signals generated by a Grason-Stadler16 audiometer calibrated to S3.6-1996 specifications. Conventional pure-tone air conduction thresholds were obtained from 250 to 8000 Hz and pure-tone bone conduction thresholds were obtained at 500 and 1000 Hz.
Word Recognition Testing
The Auditec (St. Louis) compact disc recording of the Central Institute for the Deaf (CID) Auditory Test W-22 was used for word-recognition testing at a presentation level of 90 dB HL, or the upper limit of comfortable loudness if this was less than 90 dB HL. Twenty-five words were presented to each ear with speech-noise masking in the contralateral ear at a presentation level 20 dB lower than the word-recognition test level. A second 25-word list was presented at a 10 dB lower level if the participant scored less than 80% on the first trial to exclude auditory adaptation as a cause of the reduced word recognition.
Central Auditory Test Battery
Four behavioral central auditory tests were chosen for this study based on robustness, standardization, ease of use, likelihood of being affected by dementia, and testing of different central auditory skills. The tests were 1) the Synthetic Sentence Identification test with ipsilateral competing message (SSI-ICM), a monaural speech test with competing message 2) two dichotic tests involving speech, the Dichotic Sentence Identification test (DSI), and the Dichotic Digits test (DDT); and 3) the Pitch Pattern Sequence (PPS) test, a tonal test involving temporal ordering of pure tones. The order of test presentation was randomized to prevent an order effect. Auditec recorded materials on compact disc were used for these four tests. Completion time was 30 minutes or less for facile subjects and up to an hour for those needing extra time.
The Synthetic Sentence Identification test (SSI)
The SSI requires the listener to select which one of 10 nonsense sentences was presented against a background of an interesting narrative presented by the same talker. The competing narrative may be presented to the same ear as the sentences, referred to as Ipsilateral Competing Message (SSI-ICM) or to the opposite ear, referred to as Contralateral Competing Message (SSI-CCM). A practice presentation of 1 to 3 lists was completed at 50 dB above the pure-tone average with a +10 dB signal-to-noise ratio (SNR). For the actual test the stimulus was at 0 dB SNR at the same presentation level. Research has indicated that up to 30 presentations are necessary to reach an asymptote.9;10 Only one list of 10 sentences was presented for participants scoring 90% or better, two lists if the score was 80% or better, otherwise three lists were presented. Since raw SSI-ICM scores decrease with age and hearing level (peripheral presbycusis), we used a presentation level 50 dB above the pure-tone average and used insert earphones to enhance high-frequency audibility and avoid collapsing ear canals.11 To obtain optimal performance from the participants, pauses between presentations were taken as needed for slow responders. Correct identification of 80% or more of 10-30 sentence presentations is considered normal performance. Both the SSI tests are sensitive to cognitive decline12 and Alzheimer's disease.13
Dichotic Sentence
Identification (DSI). The DSI uses six of the same sentences as the SSI but presents one sentence to each ear simultaneously at 50 dB SL and the participant is asked to select from a printed list which 2 sentences were heard. Fifer et al, 14showed that the test is resistant to the effects of sensorineural hearing loss until the degree of loss exceeds 50 dB HL. The DSI was administered as outlined by Jerger et al. (1994) in both a free and directed mode. In the directed mode, only the sentence heard in test ear is noted whereas in the free mode the sentences heard in both ears are reported. Five presentations were used if the score was 100%, otherwise, another five sentences per ear, respectively, were administered. Twenty sentences were presented. Scores are improved in the directed mode compared to the free mode and in adults the right ear scores are normally higher than the left ear scores, presumably due to age-related corpus callosum dysfunction.15 Normal scores are 80% and above
Dichotic Digits Test (DDT)
The DDT is a widely used dichotic test to screen for central auditory dysfunction.16 The DDT was given at 50 dB above the PTA. Following the practice round, fifty presentations of the stimuli were administered. Forty numbers (1-10, excluding 7) were presented in pairs to each ear simultaneously. If all numbers are recognized correctly, a score of 100% (40 × 2.5) is given. The participant reports all digits heard for each presentation. The DDT is relatively easy to administer, is not greatly affected by mild to moderate hearing loss, and is commonly abnormal in people with a clinical diagnosis of AD.17 Strouse 18 demonstrated acceptable test-retest reliability of the DDT in 10 people with mild to moderate clinical AD. Normal scores for adults are 90% and above.19
Pitch Pattern Sequence test (PPS)
The PPS is a fairly easy measure of pitch perception and temporal ordering.20 It consists of a series of 3 tone bursts composed of low and high frequencies (1 kHz and 1.5 kHz) presented for 300 msec every 6 seconds. One out of every 3 tone bursts differs e.g., high, low, high). The PPS was administered at 50 dB above the 1000 Hz threshold beginning with the better ear. The participant was instructed to repeat back the order of the three tones, either “high” or “low” with a total of six combinations possible (e.g. high/low/high, etc). Twenty-five random presentations of the “high” or “low” patterns were given.21 If the participant had difficulty with differentiating the tones, a practice list of five to nine patterns with only two tone presentations was given and then the actual test was attempted again. The PPS is a monaural test that evaluates both pattern perception and temporal sequencing ability while excluding auditory verbal cues. Subjects who score poorly (<80%) with a verbal response are asked to hum the sounds, which permits a judgment of the separate tasks of perceiving and of naming the sounds which discriminates between right hemisphere and corpus callosum or deep left hemisphere lesions.22
Electrophysiologic Tests
A battery of electrophysiologic tests was administered to assess auditory-evoked potentials. These were the Auditory Brainstem Responses, Middle Latency Responses and the Late Latency Responses. These results will be reported separately.
Cognitive Testing
Additional neuropsychological tests were also conducted with enrolled subjects, including the Trail Making Test, Stroop Test, and Clock Drawing. The results of these tests will be reported separately.
Statistical Methods
The peripheral auditory function was assessed, and the behavioral central auditory processing (CAP) tests were scored on both the left and right ear of each study participant. For each CAP test, the analysis used the lower (poorer) of the two scores from the left and right ears. In multivariate analyses, which adjusted for peripheral auditory function, we adjusted for the pure tone threshold measured in the ear with the lower CAP score, even if that was not the ear with the lowest peripheral auditory function.
Chi-square tests and analysis of variance models were used to assess differences in demographic characteristics and peripheral audiometric measures between memory impairment groups. When the overall test for group differences was significant, post-hoc analyses assessed differences between each of the target groups compared to the control group. Linear regression models were used to assess differences in mean CAP scores across memory impairment groups. Models adjusted for age at hearing testing, and pure tone threshold. To assess the ability of the CAP scores to identify participants with memory impairment, we computed the receiver operating characteristic (ROC) curve associated with each of the CAP tests. The ROC curve illustrates the tradeoff between the sensitivity and specificity for a test, as the test threshold is varied. CAP test scores less than 80% are typically considered abnormal. However, the ROC curve analysis allows us to consider other cut-points, and to examine the overall discriminating ability of the four CAP tests across all cut-points. Additionally, we considered the ROC results for a composite test, defined as the lowest score on any of the four CAP tests, to see if this simple combination of test results would significantly increase the ability of the CAP tests to identify participants with memory impairment.
STATA version 7.0 for Windows statistical software was used for all data analyses
RESULTS
Table 1 summarizes the demographic characteristics and peripheral audiometric measures of the study sample by memory impairment group. As expected, the mean age in the two groups with memory impairment was greater than that of the controls. In addition, the number of years of education was greater in the control group compared to the target groups (significant result only when compared to targets without AD). There were no statistically significant differences between groups by gender, race, cardiovascular disease or cerebrovascular disease, or frequency of exercise. The results of the electrophysiologic tests (data not shown) did not vary by memory group. The memory impaired groups had significantly worse peripheral hearing when compared to the control group, with the pure tone average significantly poorer in both the better and worse ear, and the word recognition score significantly poorer in the worse ear.
Table 1.
Comparison Of Demographics Across Study Groups
|
|
Control n=232 |
Target - no AD n=64 |
Target - AD n=17 |
|---|---|---|---|
| Age | 78.8 (4.7) | 82.3 (6.1) + | 84.0 (5.1) + |
| Male (%) | 37.1 | 35.9 | 58.8 |
| Caucasian (%) | 89.7 | 82.8 | 76.5 |
| Education, years | 15.2 (2.9) | 13.6 (2.7) + | 13.8 (3.0) |
| APOE4 (%) | 24.3 | 24.1 | 60.0+ |
| Depressive symptoms (%) | 4.1 | 6.7 | 6.7 |
| Exercise ≥ 3 times/week (%) | 71.9 | 67.2 | 64.7 |
| Cardiovascular disease (%) | 22.4 | 32.8 | 29.4 |
| Cerebrovascular disease (%) | 11.2 | 9.4 | 5.9 |
| PTA, better ear | 22.9 (8.7) | 27.5 (8.3) + | 28.6 (8.8) + |
| PTA, worse ear | 27.1 (9.3) | 32.4 (8.5) + | 34.3 (9.8) + |
| WRS, better ear | 96.1 (4.4) | 95.1 (5.4) | 93.9 (4.3) * |
| WRS, worse ear | 91.7 (6.0) | 90.2 (6.4) | 86.1 (6.9) + |
| CASI Total Score | 96.6 (2.7) | 85.5 (4.1) + | 80.1 (8.0) + |
| CASI STM Subscore | 11.4 (0.8) | 8.5 (2.0) + | 6.4 (2.2) + |
p<.05
p<.01: Comparing group to controls
AD = Alzheimer's Diease
PTA = pure-tone average of thresholds at 0.5, 1.0, and 2.0 kHz.
WRS = word recognition score using CID lists at most tolerable loudness.
CASI = Cognitive Abilities Screening Instrument.
STM = short-term memory subscale.
There were no ears with otoscopic or tympanometric evidence of middle ear effusion, perforation, or otorrhea. The vast majority of audiograms displayed the typical presbycusis pattern with a gradually sloping, monotonic, high-frequency threshold elevation.
Table 2 illustrates the mean scores on each CAP test for the memory impairment groups. Differences in group means, both unadjusted, and adjusted for age and pure tone threshold, are also reported in table 2. The mean CAP scores are significantly lower in both of the target groups, compared to the control group for all four of the CAP tests. The DSI showed the largest difference between groups, with the adjusted mean 31.9 points lower in the target group without AD, and 38.8 points lower in the AD group compared to the mean in the control group The SSI test had the largest difference in means between the two target groups (adjusted mean difference 17.1), suggesting that the SSI may be the test most sensitive to change when memory impairment has progressed to a diagnosis of AD.
Table 2.
Mean Central Auditory Processing (CAP) Scores, With Unadjusted And Adjusted Differences Between Groups.
| Unadjusted difference | Adjusted differences* | ||||||
|---|---|---|---|---|---|---|---|
| Control | Target, no AD+ |
Target, with AD+ |
Target, no AD vs. Control |
Target, with AD+ vs. Control |
Target, no AD+ vs. Control |
Target, with AD+ vs. Control |
|
| Synthetic Sentence Identification (SSI) | 76.5 (22.7) |
50.4 (28.5) |
29.8 (30.5) |
−26.1 (−32.9, −19.3) |
−46.7 (−59.4, −33.9) |
−16.0 (−21.7, −10.2) |
−29.0 (−39.7, −18.3) |
| Dichotic Sentence Identification (DSI) | 76.9 (19.1) |
41.7 (31.9) |
33.8 (26.8) |
−35.2 (−41.5, −28.8) |
−43.1 (−54.7, −31.6) |
−31.8 (−38.3, −25.3) |
−38.5 (−50.2, −26.8) |
| Dichotic Digits Test (DDT) | 76.7 (15.6) |
61.4 (17.7) |
55.2 (19.1) |
−15.3 (−19.8, −10.8) |
−21.5 (−29.5, −13.5) |
−12.1 (−16.7, −7.6) |
−15.8 (−23.9, −7.8) |
Adjusted for age, pure-tone average, and word recognition score
Alzheimer's Disease (AD)
Even though the group means showed a robust and consistent degradation of test scores across the three cognitive groups, there was considerable heterogeneity among the test results with overlap of outcomes among comparable participants: some memory impaired people were normal on all tests, some were abnormal on one but not the others, and some were abnormal on all tests (data not shown).
Scores below 80 are typically considered abnormal for the CAP tests. The percentages of target/control participants with abnormal test results (i.e. <80%) were 79%/42%, 84%/42%, 84%/47% and 37%/10% for the SSI, DSI, DDT, and PPS respectively. Table 3 reports the sensitivity and specificity of each of the CAP tests and for the composite test (defined as the minimum of the four test scores) for discriminating between targets and controls using this threshold. The sensitivity of the DSI test is 83.8%, meaning that 83.8% of the participants with memory impairment (target group) score below the 80% threshold. Among those without memory impairment (control group), 58.6% score above the 80% threshold (specificity=58.6%). Results for the SI and DDT tests are similar to the DSI test. Table 3 also reports the sensitivity and specificity for each test using the cut-point of 50% to define an abnormal test. At this threshold, the SSI and DSI tests have similar sensitivity (55.7 and 52.5 respectively) and specificity (85.8 and 93.5 respectively), whereas the DDT test has similar specificity (92.6) but at the expense of greatly reduced sensitivity (25.9).
Table 3.
Sensitivity and Specificity for Two Testing Thresholds
| Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|
| Score < 80% | |||
| Synthetic Sentence | 78.5 | 60.8 | |
| Identification (SSI) | |||
| Dichotic Sentence | 83.8 | 58.6 | |
| Identification (DSI) | |||
| Dichotic Digits Test (DDT) | 84.0 | 54.1 | |
| Composite test (lowest score) | 97.5 | 28.9 | |
|
| |||
| Score < 50% | |||
| Synthetic Sentence | 55.7 | 85.8 | |
| Identification (SSI) | |||
| Dichotic Sentence | 52.5 | 93.5 | |
| Identification (DSI) | |||
| Dichotic Digits Test (DDT) | 25.9 | 92.6 | |
| Composite test (lowest score) | 80.2 | 78.4 | |
Figure 1 illustrates the Receiver Operator Characteristic (ROC) curves for the four CAP tests and the composite test, for differentiating between controls and targets. The ROC curve plots the sensitivity and specificity for each test for all possible choices for the threshold value. The ROC curve for the DSI test is above the curve for each of the other individual tests, indicating that for any given value of specificity, the DSI test is going to result in greater sensitivity. The area under the ROC curve (AUC) is a summary measure of diagnostic ability. The AUC was largest for the DSI test (AUC=0.83), followed by the SSI (0.78), DDT (0.75), and PPS (0.71). The curve for the composite test was slightly above the curve for DSI, however the marginal improvement must be weighed against the added test complexity, and the additional time required to administer all four tests relative to the time required for the DSI test alone.
Fig. 1. ROC curves comparing the area under the curve for each CAP test.
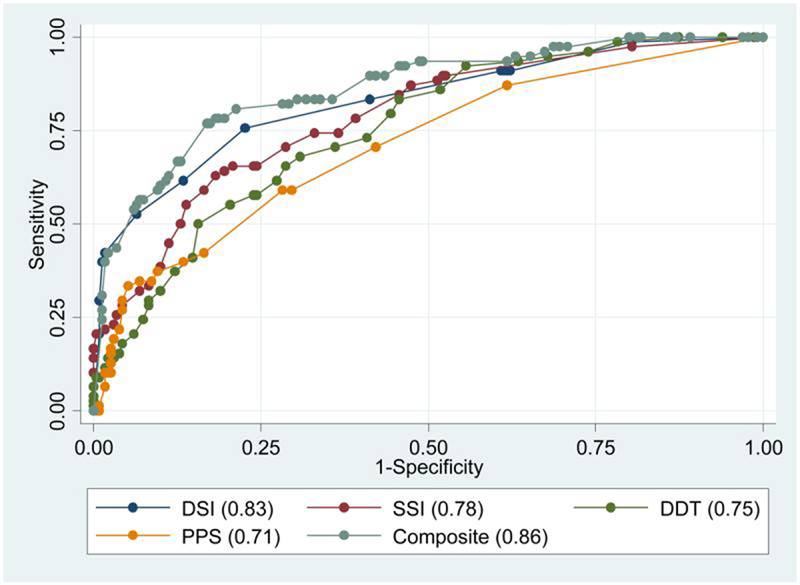
The area under the curve (AUC) is shown for each test: DSI (area 0.83), SSI (area 0.78), DDT (area 0.75), PPS (area 0.71), and the Composite (lowest score on any of the 4 tests) score (area 0.86).
DISCUSSION
This study sought to evaluate whether abnormal central auditory processing test results, which have been previously demonstrated in persons with Alzheimer's type dementia1, could also be observed in people with memory loss but none of the other criteria for a diagnosis of AD. Our hypotheses were confirmed. Test performance was consistently worst in memory-impaired subjects with dementia, but each of the four CAP tests also showed poorer performance in the target subjects without dementia than in the controls, even after adjustment for age, hearing threshold, and word recognition score. This illustrates the robust relationship of even early memory loss and tests of central auditory function.
The recent emergence of therapies aimed at delaying the progression of AD23 has generated interest in new methods for early diagnosis of AD, such as imaging24 and cognitive screening25. We suggested previously that central auditory testing might warrant consideration as a screening test based on the observations from the Framingham Dementia Cohort where very poor performance on the SSI–ICM preceded the diagnosis of AD by several years.26
The present study shows that central auditory tests are also frequently abnormal in memory-impaired non-demented older people. Given that about half of older adults with isolated memory loss progress to frank dementia27, identifying early cases has considerable merit. While adding central auditory tests to a screening cognitive battery might have value, the logistical implications of such an approach have not been established or evaluated. However, adding central auditory testing to the initial evaluation of older people seeking hearing assistance, is a simple addition to existing services that would have also have value for auditory rehabilitation.28 Thus, alerting audiologists, otologists, and geriatricians about the potential value of central auditory testing is one of the goals of this report.
Although memory decline and age-related hearing loss often coexist, a causal relationship is not established by this report. Our working hypothesis that memory impairment and central auditory dysfunction have a common cause – frontal lobe dysfunction – is supported but not proven by the present findings. We do not yet know whether the combined presence of memory impairment and central auditory dysfunction conveys a greater risk of dementia than either condition alone, even though a prudent interpretation of these findings would raise concern that such could be the case. The future outcome of serial cognitive testing of the present participants will shed light on these questions.
There are distinct advantages to consider when contemplating the role of CAP testing as part of the assessment of older adults. The CAP tests are relatively simple to administer because standardized, prerecorded materials are used. Unlike many cognitive tests, patients find the hearing tests pleasant and non-threatening, and are generally interested in knowing their individual results. The tests are given in training mode to familiarize the patient with the process and to assure sufficient comprehension of the test paradigm. The resources and experience needed to administer and interpret these tests is widely available in audiologic and otologic practices. There are some limitations to the tests, however. Patients must have sufficient vision to read the number of the sentence that was heard and sufficient peripheral auditory function to understand speech at a comfortable loudness level. Because of the need to assure adequate peripheral auditory function, CAP testing would not be suitable for widespread use. Nevertheless, adding these tests to conventional audiometric measures for the elderly is very feasible and we recommend that they be considered and evaluated in the periodic health assessment of people over 65 years, and particularly those in the over 75 and older group and those with a history of memory problems.
Caveats
Central auditory testing in the elderly may be influenced by the effects of age as well as acquired conditions on peripheral (cochlear) function, which may coexist with central auditory dysfunction. Therefore, we limited our testing to people with peripheral function deemed adequate for the central auditory tests presented: i.e. hearing thresholds of 48 dB HL or better and normal word recognition scores at comfortably loud presentation levels. In our population, there was a significant effect of age, cognitive status group, and peripheral hearing status on the SSI but only age and cognitive status group on the DSI and DDT (data not shown). We used other measures to optimize auditory performance of our participants, such as expanding the test time, in order to minimize test methods artifacts contributing to the findings. Thus we believe that our results truly reflect the impact of memory loss on central auditory function rather than peripheral hearing or test-taking ability.
Although the majority of poor CAP test results occurred in the target group, some people in the control group also demonstrated poor CAP test results. We can not explain this finding now. While some of these cognitively-normal, auditory-abnormal cases may represent occult cases of memory impairment or preclinical dementia, only time will prove this assumption. For the present, these outlier cases simply illustrate that the CAD tests have less than perfect specificity for identifying participants with memory impairment.
Summary
People with memory impairment identified by low scores on the CASI perform more poorly on central auditory tests than do control subjects with normal CASI scores. The DSI test demonstrated the greatest sensitivity to memory loss followed by the SSI and DDT. The PPS was the least sensitive to memory loss and yielded in the lowest correlation with age. The ease and availability of central auditory test materials in audiology/otology clinics makes it readily possible to perform such testing as a baseline for people seeking assistance in dealing with their hearing loss.
ACKNOWLEDGMENTS
Aimee Verrall was the research coordinator. Shelby Bellew performed all the audiometric tests. Meredith Pfanschmidt, Sheila O'Connell, Darlene White, and Cathy Hutchison headed up the recruitment efforts as ACT Study staff.
Reference List
- 1.Gates GA, Karzon RK, Garcia P, et al. Auditory dysfunction in aging and senile dementia of the Alzheimer's type. Arch Neurol. 1995;52:626–634. doi: 10.1001/archneur.1995.00540300108020. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JC, Jr., Gates GA. Central auditory processing disorders in the elderly: the effects of pure tone average and maximum word recognition. Ear Hear. 1992;13:278–280. doi: 10.1097/00003446-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bamiou DE, Musiek FE, Luxon LM. Aetiology and clinical presentations of auditory processing disorders--a review. Arch Dis Child. 2001;85:361–365. doi: 10.1136/adc.85.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edland SD, Tobe VO, Rieder MJ, et al. Mitochondrial genetic variants and Alzheimer disease: a case-control study of the T4336C and G5460A variants. Alzheimer Dis Assoc Disord. 2002;16:1–7. doi: 10.1097/00002093-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 9.Dubno JR, Lee FS, Klein AJ, Matthews LJ, Lam CF. Confidence limits for maximum word-recognition scores. J Speech Hear Res. 1995;38:490–502. doi: 10.1044/jshr.3802.490. [DOI] [PubMed] [Google Scholar]
- 10.Feeney MP, Hallowell B. Practice and list effects on the synthetic sentence identification test in young and elderly listeners. J Speech Lang Hear Res. 2000;43:1160–1167. doi: 10.1044/jslhr.4305.1160. [DOI] [PubMed] [Google Scholar]
- 11.Skinner MW. Speech intelligibility in noise-induced hearing loss: effects of high-frequency compensation. J Acoust Soc Am. 1980;67:306–317. doi: 10.1121/1.384463. [DOI] [PubMed] [Google Scholar]
- 12.Chmiel R, Jerger J. Some factors affecting assessment of hearing handicap in the elderly. J Am Acad Audiol. 1993;4:249–257. [PubMed] [Google Scholar]
- 13.Bartolome MV, del CE, Lopez LM, Carricondo F, Poch-Broto J, Gil-Loyzaga P. Effects of aging on C57BL/6J mice: an electrophysiological and morphological study. Adv Otorhinolaryngol. 2002;59:106–111. doi: 10.1159/000059247. [DOI] [PubMed] [Google Scholar]
- 14.Fifer R, Jerger J, Berlin C, Tobey E, Campbell J. Development of a dichotic sentence identification test for hearing impaired adults. Ear Hear. 1983;4:300–305. doi: 10.1097/00003446-198311000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Jerger J. Dichotic listening and attention. J Am Acad Audiol. 2005;16:204. [PubMed] [Google Scholar]
- 16.Musiek FE, Gollegly KM, Kibbe KS, Verkest-Lenz SB. Proposed screening test for central auditory disorders: Follow-up on the dichotic digits test. Am J Otol. 1991;12:109–113. [PubMed] [Google Scholar]
- 17.Strouse AL, Hall JW, 3, Burger MC. Central auditory processing in Alzheimer's disease. Ear Hear. 1995;16:230–238. doi: 10.1097/00003446-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Strouse AL, Hall JW., 3 Test-retest reliability of a dichotic digits test for assessing central auditory function in Alzheimer's disease. Audiology. 1995;34:85–90. doi: 10.3109/00206099509071901. [DOI] [PubMed] [Google Scholar]
- 19.Musiek FE. Assessment of central auditory dysfunction: the dichotic digit test revisited. Ear Hear. 1983;4:79–83. doi: 10.1097/00003446-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Musiek FE, Pinheiro ML, Wilson DH. Auditory pattern perception in ‘split brain’ patients. Arch Otolaryngol. 1980;106:610–612. doi: 10.1001/archotol.1980.00790340018004. [DOI] [PubMed] [Google Scholar]
- 21.Strouse AL, Hall JW, III, Burger MC. Central auditory processing in Alzheimer's disease. Ear Hear. 1995;16:230–238. doi: 10.1097/00003446-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Musiek FE, Pinheiro ML. Frequency patterns in cochlear, brainstem, and cerebral lesions. Audiology. 1987;26:79–88. [PubMed] [Google Scholar]
- 23.Gauthier SG. Alzheimer's disease: the benefits of early treatment. Eur J Neurol. 2005;12(Suppl 3):11–16. doi: 10.1111/j.1468-1331.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- 24.DeCarli C. The role of neuroimaging in dementia. Clin Geriatr Med. 2001;17:255–279. doi: 10.1016/s0749-0690(05)70068-9. [DOI] [PubMed] [Google Scholar]
- 25.Walsh SP, Raman R, Jones KB, Aisen PS. ADCS Prevention Instrument Project: The Mail-In Cognitive Function Screening Instrument (MCFSI) Alzheimer Dis Assoc Disord. 2006;20(Suppl 3):S170–S178. doi: 10.1097/01.wad.0000213879.55547.57. [DOI] [PubMed] [Google Scholar]
- 26.Gates GA, Beiser A, Rees TS, D'Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer's disease. J Am Geriatr Soc. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- 27.Bowen J, Teri L, Kukull W, McCormick W, McCurry SM, Larson EB. Progression to dementia in patients with isolated memory loss. Lancet. 1997;349:763–765. doi: 10.1016/S0140-6736(96)08256-6. [DOI] [PubMed] [Google Scholar]
- 28.Chmiel R, Jerger J. Hearing aid use, central auditory disorder, and hearing handicap in elderly persons. J Am Acad Audiol. 1996;7:190–202. [PubMed] [Google Scholar]


