Abstract
Background
Although combination antiretroviral therapy continues to evolve, with potentially more effective options emerging each year, the ability of therapy to prevent multiple regimen failure and mortality in clinical practice remains poorly defined.
Methods
Sixteen cohorts representing over 60 sites contributed data on all individuals who initiated combination antiretroviral therapy. We identified those individuals who experienced virologic failure (defined as a human immunodeficiency virus [HIV] RNA level >1000 copies/mL), received modified therapy, and subsequently had a second episode of virologic failure. Multivariate Cox regression was used to assess factors associated with time to second regimen failure and the time to death after the onset of second regimen failure.
Results
Of the 42,790 individuals who received therapy, 7159 experienced a second virologic failure. The risk of second virologic failure decreased from 1996 (56 cases per 100 person-years) through 2005 (16 cases per 100 person-years; P < .001). The cumulative mortality after onset of second virologic failure was 26% at 5 years and decreased over time. A history of AIDS, a lower CD4+ T cell count, and a higher plasma HIV RNA level were each independently associated with mortality. Similar trends were observed when analysis was limited to the subset of previously treatment-naive patients
Conclusions
Although the rates of multiple regimen failure have decreased dramatically over the past decade, mortality rates for those who have experienced failure of at least 2 regimens have remained high. Plasma HIV RNA levels, CD4+ T cell counts at time of treatment failure, and a history of AIDS remain independent risk factors for death, which emphasizes that these factors remain important targets for those in need of more-aggressive therapeutic interventions.
Although most patients enrolled in clinical trials are able to achieve durable suppression of human immunodeficiency virus (HIV) replication, a significant proportion of patients in clinical practice did not achieve such a response, at least to their first regimen [1]. The degree to which patients who have experienced failure of a first regimen are at risk for subsequent failure has not been well studied, in part because few cohorts have followed up enough patients to address this question.
The overall clinical impact of virologic failure is poorly defined. A number of studies have shown that patients who are unable to achieve and maintain an undetectable viral load do well clinically and immunologically, compared with those who do not receive therapy [2–4]. In clinical practice, treatment failure appears to be an uncommon cause of mortality [5–8]. Among patients with drug-resistant infection, those with more mutations do not necessarily do worse than those with only a limited number of mutations [9], although other studies have reported that those with multidrug failure or multidrug resistance have an overall poor prognosis, compared with those who experience more limited drug failure [10–14].
Because of the uncertainties regarding the overall incidence and clinical consequences of multiple regimen failure, we analyzed data from an established collaboration of US and Canadian cohorts (North American AIDS Cohort Collaboration on Research and Design; NA-ACCORD). We first investigated the incidence and predictors of progression to virologic failure after receipt of at least 2 combination antiretroviral regimens. We next investigated the rates and predictors of mortality after the onset of a second virologic failure. We focused on failure of 2 regimens, because most HIV-infected individuals in resource-rich and (to a lesser degree) resource-poor regions have access to at least 2 distinct regimens.
METHODS
Study sample
The data for this study were collected as part of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), part of the International Epidemiological Databases to Evaluate AIDS project [15]. The NA-ACCORD was established in 2006 as a regional collaboration of existing single-site and multisite cohorts from the United States and Canada. The participating cohorts have been approved by local institutional review boards and use standardized methods of data collection. Seventeen cohorts contributed data for this study. This included 5 interval cohort studies, in which enrollment is targeted and the timing and content of data collection are part of research studies at prespecified intervals. The other 12 cohorts were based in clinical settings, where the timing and type of data collected is determined by the nature of the health care services provided by the center.
For the current analysis, we identified those individuals who met the following inclusion criteria: (1) age >18 years, (2) initiation of first regimen prior to or while participating in follow-up in the cohort, (3) experience of an initial episode of virologic failure while receiving a standard regimen, (4) modification of therapy after first virologic failure, and (5) provision of a CD4+ T cell count within 1 year before regimen change. Information on pregnancy or use of investigational antiretroviral agents was not available.
Study definitions
A combination antiretroviral treatment regimen was defined as containing at least 3 antiretroviral drugs. One of these drugs had to have been a protease inhibitor (PI) or a nonnucleoside reverse-transcriptase inhibitor (NNRTI), unless the regimen contained 3 nucleoside reverse-transcriptase inhibitors (NRTIs) that included abacavir or tenofovir. We excluded individuals who received the combination of zidovudine and stavudine.
Virologic failure was defined by at least 1 plasma HIV RNA measurement >1000 copies/mL measured >3 months after therapy initiation. A therapy modification after first virologic failure was defined as a switch in the nonnucleoside analogue component of the regimen (eg, a switch from an NNRTI to a PI, a switch from a PI to an NNRTI, a switch from an unboosted PI to ritonavir-boosted PI, or a switch from a triple–nucleoside analogue regimen to a regimen including an NNRTI or a PI).
Data analysis
This study had 2 overall objectives: to define the incidence and predictors of second virologic failure over time and to determine the rates and predictors of mortality after second virologic failure. For the first objective, our study sample included individuals who started an initial regimen, exhibited evidence of virologic failure, and subsequently modified therapy. Follow-up time began when therapy modification was observed, and it extended to the time of either second virologic failure or the last contributed HIV RNA measurement. Subjects were included in this analysis even if they modified therapy after the initial regimen change and before experiencing a second failure. Person-time was allocated to the calendar time of observation in annual intervals (1996 and 1997 were combined because of small numbers of patients, with annual intervals through 2005) for computation of incidence rates. Cohort-stratified Cox regression models were used to identify factors associated with a second treatment failure. Calendar time contributions were time-varying using left-truncation methods. All other predictors occurred at or prior to therapy modification and included demographic factors, clinical factors, initial regimen type, and whether individuals were antiretroviral experienced at the time that the first regimen was initiated. With this model, age was accounted for as a cubic spline term with 5 knots. Assessment of hepatitis C virus infection was available for most (86%) of the patients. A history of injection drug use (IDU), however, was available for only ~40% of patients.
For the mortality objective, we developed a prognostic model using an “intention-to-treat” approach that ignored all treatment changes after the development of second treatment failure. Cox regression models, stratified by cohort with left-truncation methods, were constructed to determine the association of demographic and clinical factors prior to or at the time of second regimen failure. Follow-up ended at the month of death, 1.5 years after the date of the last CD4+ cell count measurement, or on 31 December 2005, whichever occurred first.
For each analysis, we first included all patients, regardless of prior treatment experience, and then we analyzed separately those patients who were antiretroviral-naive before initiation of their first combination regimen. We also investigated the sensitivity of the models to influence from individual cohorts by examining estimates calculated with data from each cohort systematically omitted from the analyses.
RESULTS
Seventeen cohorts that represented >60 sites contributed data on 36,188 HIV-infected individuals who initiated therapy. Of these individuals, 17,820 were identified as having experienced an initial episode of virologic failure, 13,165 had a regimen change after the first virologic failure and provided at least 1 CD4+ T cell count within 1 year prior to the regimen change and at least 1 HIV RNA level after the regimen change (Figure 1). The median duration of time between first virologic failure and the time that therapy was modified was 0.5 years (interquartile range [IQR], 0.2–1.2 years). The median CD4+ cell count at the time of the switch increased from 170 to 293 cells/mm3 with calendar time, whereas the plasma HIV RNA level decreased from 27,470 to 4300 copies/mL (Table 1). The majority of patients who experienced a first regimen failure were receiving a protease inhibitor regimen, although the prevalence of protease inhibitor use decreased from 92% to 48% of patients with calendar time, whereas NNRTI use increased from 6% to 38%. The majority of patients had exposure to mono- or dual-nucleoside reverse-transcriptase analogues before receipt of the first standard combination regimen, although the prevalence of prior antiretroviral exposure decreased from 90% to 37% of patients over calendar time.
Figure 1.
Study population numbers and selected characteristics. Follow-up reflects time from initiation of combination therapy to end of follow-up (death or last contributed viral load measurement [VL]). *Sufficient data was defined as a CD4+ cell count within 1 year before therapy was switched and at least 1 HIV RNA level obtained after the switch. IQR, interquartile range.
Table 1.
Cohort Characteristics Stratified by Year for 8993 Patients who had a Regimen Change after First Virologic Failure and were Evaluated for Second Virologic Failure
| No. of patients with therapy modification |
|||||
|---|---|---|---|---|---|
| Variable | 1996–1997 (n = 1430) | 1998–1999 (n = 4325) | 2000–2001 (n = 3615) | 2002–2003 (n = 2852) | 2004–2005 (n = 943) |
| Second virologic failure event during follow-up | 75 | 69 | 54 | 35 | 20 |
| Second virologic failure event within 1 year after regimen change | 45 | 37 | 29 | 23 | 16 |
| Age at regimen change, median years | 41 | 43 | 44 | 45 | 42 |
| Male sex | 87 | 88 | 88 | 84 | 67 |
| History of injection drug usea | 17 | 22 | 24 | 25 | 25 |
| Hepatitis C virus infectiona | 29 | 34 | 37 | 35 | 27 |
| History of clinical AIDS after first virologic failure | 35 | 28 | 27 | 27 | 33 |
| CD4+ T cell count at time of regimen switch, median cells/mm3 | 170 | 253 | 289 | 313 | 293 |
| HIV RNA level at time of regimen switch, median copies/mL | 27,470 | 12,790 | 7272 | 6764 | 4300 |
| Treatment experienced before first regimen | 90 | 75 | 56 | 42 | 37 |
| CD4+ T cell count before first regimen, median cells/mm3 | 117 | 188 | 205 | 219 | 187 |
| First regimen | |||||
| PI based | 91 | 86 | 73 | 55 | 38 |
| Boosted PI based | 1 | 2 | 3 | 8 | 10 |
| NNRTI based | 6 | 7 | 16 | 23 | 38 |
| PI or NNRTI | 1 | 6 | 7 | 6 | 6 |
| ≥3 NRTIs | <1 | <1 | 1 | 7 | 7 |
NOTE. Data are percentage of patients, unless otherwise indicated. NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Among those patients without missing data.
We also analyzed outcomes for the 19,876 subjects who were treatment naive at the time that their first standard combination regimen was initiated. Of these patients, 5087 exhibited an initial episode of virologic failure after switching to a second regimen, and 2289 exhibited evidence of a second virologic failure. The median duration of time between the first virologic failure and therapy modification was 0.42 years (IQR, 0.17–1.08). At the time of the switch to a second regimen, the median CD4+ T cell count was 175 cells/mm3 and the median plasma HIV RNA level was 9716 copies/mL.
Incidence of multiple regimen failure
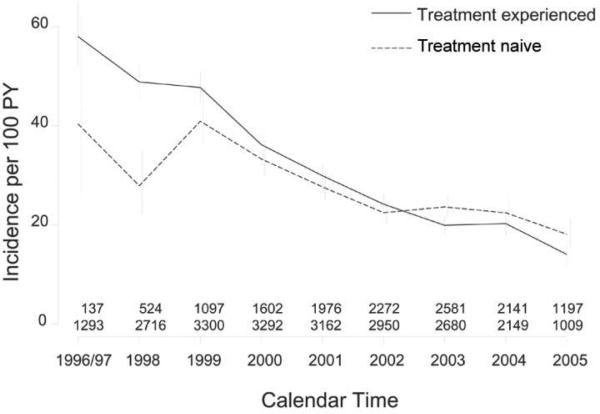
Of the 13,165 patients who switched to a second regimen, 7159 subsequently exhibited evidence of virologic failure. At the time of second virologic failure, the median CD4+ T cell count was 244 cells/mm3 (IQR, 120–405 cells/mm3), and the median plasma HIV RNA level was 13,000 copies/mL (IQR, 3100–60,864 copies/mL). Most patients (66%) had been exposed to all 3 classes at the time of the second treatment failure. The risk of experiencing a second virologic failure decreased dramatically during the first decade that these regimens were available (P < .001; Figure 2). The crude incidence of second treatment failure during 1996–1997 was 56 (95% confidence interval [CI], 50–63) events per 100 person-years. This incidence decreased to 16 (95% CI, 14–18) events per 100 person-years in 2005. In the cohort-stratified multivariate Cox regression analysis, these temporal trends remained strong and statistically significant (Table 2). The trends remained of similar magnitude and significance when the outcomes were censored after the first year of the second regimen.
Figure 2.
Decrease in incidence of second virologic failure over calendar time. The incidence of second virologic failure (defined as a plasma HIV RNA level >1000 copies/mL) is shown over time for those individuals who were treatment-naive before receipt of their initial regimen (dashed line) and those who were treatment-experienced before receipt of their first regimen (solid line). Vertical bars reflect 95% confidence intervals for the incidence estimates. Numbers at the bottom display the number of individuals contributing time at-risk for second virologic failure during each time interval, with the top row indicating the number of treatment-experienced individuals and the bottom row indicating the number of treatment-naive individuals. PY, person-years.
Table 2.
Predictors of Time to Second Virologic Failure from Cohort-Stratified Cox Regression Model
| Full dataset (n = 7159) |
Full dataset censoring at 1 year after regimen switch (n = 6351) |
Analysis of patients who were antiretroviral naive before first combination regimen (n = 2289) |
||||
|---|---|---|---|---|---|---|
| Predictor | Relative hazard (95% CI) | P | Relative hazard (95% CI) | P | Relative hazard (95% CI) | P |
| Calendar time linear trend (per year) | 0.96 (0.94–0.98) | <.01 | 0.94 (0.92–0.97) | <.01 | 0.98 (0.96–1.01) | .29 |
| Female sex | 1.05 (0.96–1.16) | .28 | 0.97 (0.85–1.10) | .59 | 1.08 (0.93–1.25) | .34 |
| History of clinical AIDS | 1.04 (0.99–1.10) | .15 | 1.07 (1.00–1.15) | .05 | 1.09 (0.99–1.20) | .08 |
| Type of therapy at initiation of first regimen | ||||||
| PI based | Reference | Reference | Reference | |||
| NNRTI based | 0.97 (0.91–1.05) | .55 | 0.99 (0.90–1.10) | .90 | 1.11 (0.98–1.24) | .10 |
| PI or NNRTI | 1.00 (0.90–1.10) | .92 | 1.01 (0.88–1.16) | .86 | 1.12 (0.99–1.45) | .06 |
| ≥3 NRTIs | 0.90 (0.73–1.11) | .33 | 1.05 (0.81–1.35) | .73 | 0.81 (0.62–1.07) | .14 |
| Treatment experienced before first regimen | 1.12 (1.05–1.18) | <.01 | 1.09 (1.02–1.18) | .02 | NA | |
| Time from first initiation of therapy to regimen switch | ||||||
| ≤1 Year | Reference | Reference | Reference | |||
| >1 and ≤2 Years | 0.98 (0.93–1.04) | .53 | 0.99 (0.92–1.07) | .79 | 0.95 (0.86–1.06) | .36 |
| >2 and ≤3 Years | 0.93 (0.86–1.00) | .05 | 0.97 (0.88–1.07) | .50 | 0.98 (0.86–1.11) | .70 |
| >3 and ≤4 Years | 0.91 (0.82–1.00) | .05 | 0.88 (0.77–1.00) | .05 | 0.93 (0.79–1.09) | .35 |
| >4 Years | 0.72 (0.64–0.82) | <.01 | 0.75 (0.64–0.87) | <.01 | 0.68 (0.55–0.83) | <.01 |
| CD4+ cell count at regimen switch (per 100 cells/mm3) | 0.94 (0.93–0.95) | <.01 | 0.95 (0.93–0.96) | <.01 | 0.93 (0.91–0.95) | .01 |
| HIV RNA level at regimen switch (per log10 copies/mL) | 1.21 (1.18–1.25) | <.01 | 1.27 (1.22–1.32) | <.01 | 1.30 (1.24–1.37) | <.01 |
| HIV RNA level at first virologic failure (log10 copies/mL) | 1.00 (0.96–1.04) | .97 | 0.97 (0.93–1.02) | .29 | 0.92 (0.87–0.98) | .02 |
NOTE. In addition to the predictors given in the table, the model was adjusted for age using a cubic spline function. CI, confidence interval; NA, not applicable; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Similar trends were observed in the subset of 5087 patients who were treatment naive before receipt of their first regimen and who eventually modified therapy after an initial episode of virologic failure. The crude incidence of second regimen failure among this subset during 1996–1997 was 40 (95% CI, 26–62) events per 100 person-years. This incidence decreased to 18 (95% CI, 15–21) events per 100 person-years in 2005.
Predictors of multiple regimen failure
We identified additional factors associated with experiencing a second treatment failure after regimen switch (Table 2). After adjusting for calendar time, a longer time between the initiation and modification of therapy and a higher CD4+ cell count were associated with a lower risk of second treatment failure. Both a higher viral load at the first virologic failure time point and a higher viral load at the time that the regimen was switched were associated with an increased risk of second treatment failure. As expected, these 2 viral load measurements were highly correlated (ρ = 0.57). When included together in the same model, only the preswitch viral load was independently associated with a second viral failure (Table 2). Neither sex, age, history of AIDS, or initial regimen type was associated with second treatment failure in multivariate analysis. These predictors remained of similar magnitude when the outcomes were censored at 1 year after regimen switch and when the analysis was restricted to those who were antiretroviral naive before receipt of their first regimen. The analyses of data that left out each cohort systematically provided estimates that were generally within the presented confidence limits.
We also investigated the 2 components of the time from therapy initiation to therapy modification; namely, the time from therapy initiation to first virologic failure (or the duration of success) and the time from first virologic failure to therapy modification. After adjusting for the other factors in Table 2, each of these variables was statistically significantly (P < .001) associated with a second virological failure when added to the model separately. When included together in a model, only the time from treatment initiation to first virologic failure was statistically significantly associated with a lower risk of second virologic failure (hazard ratio [HR], 0.90 per year; P < .001).
Among those with data available, both hepatitis C virus infection and IDU were associated with a shorter time to second virological failure (HR, 0.90 and 0.95, respectively), but only hepatitis C virus infection was statistically significant (P < .01). These variables did not materially impact the inferences of the other variables in the model.
Similar trends were observed in the subset of 5087 patients who were treatment naive before receipt of their first regimen (Table 2). In this subset, however, the calendar time trend was attenuated and not statistically significant (P = .29). After adjusting for calendar time, a higher CD4+ T cell count at the time of the first regimen switch was associated with a lower risk of second regimen failure. Both a higher plasma HIV RNA level at the first virologic failure time and a higher plasma HIV RNA level at the time that the regimen was switched were independently associated with an increased risk of second treatment failure.
In our primary analysis, only a singe viral load >1000 copies/mL was necessary to meet our failure definition. Most individuals who had a follow-up viral load determined at 3, 6, or 12 months after their virologic failure time point had a confirmatory viral load (82%, 84%, and 88% of patients, respectively). Censoring individuals who did not have a confirmatory virologic failure did not materially impact the results.
Mortality after second virologic failure
Of the 7159 subjects who experienced a second regimen failure, 6698 had >1 month of follow-up after second treatment failure and had data on all covariates. There were 1532 deaths observed during 25,722 person-years of follow-up after second treatment failure. The cumulative mortality was 5% at 1 year and 26% at 5 years (Figure 3). The crude incidence of mortality among those who experienced a second regimen failure during 1996–1997 was 6.5 (95% CI, 5.4–8.0) per 100 person-years. This incidence decreased to a low of 5 deaths per 100 person-years among those who experienced a second regimen failure beginning in 2003. In multivariate analysis, there was a statistically significant trend towards lower risk of death among those with second treatment failure in later years (P < .01; Table 3).
Figure 3.
Mortality after second virologic failure by calendar time. The time to death after onset of second virologic failure (plasma HIV RNA level >1000 copies/mL) was estimated with use of Kaplan-Meier methods. Individuals were stratified on the basis of the calendar time at which the second virologic failure was identified.
Table 3.
Predictors of Mortality after Second Virologic Failure using Cox Regression Models with Age as the Timescale and Stratified by Cohort
| Full dataseta |
Analysis of patients who were antiretroviral naive before first combination regimenb |
|||
|---|---|---|---|---|
| Predictor | Relative hazard (95% CI) | P | Relative hazard (95% CI) | P |
| Calendar time linear trend (per year) | 0.95 (0.92–0.99) | <.01 | 0.91 (0.85–0.98) | .01 |
| Female sex | 0.81 (0.63–1.04) | .10 | 0.59 (0.33–1.06) | .08 |
| History of clinical AIDS | 1.40 (1.25–1.56) | <.01 | 1.37 (1.07–1.74) | .01 |
| CD4+ T cell count at second virologic failure | ||||
| >200 cells/mm3 | Reference | Reference | ||
| 50–200 cells/mm3 | 1.88 (1.66–2.13) | <.01 | 1.91 (1.45–2.52) | <.01 |
| <50 cells/mm3 | 4.40 (3.79–5.11) | <.01 | 1.62 (1.21–2.15) | <.01 |
| HIV RNA level at second virologic failure | ||||
| <10,000 copies/mL | Reference | Reference | ||
| 10,000 to 100,000 copies/mL | 1.32 (1.16–1.50) | <.01 | 1.62 (1.21–2.15) | <.01 |
| >100,000 copies/mL | 2.05 (1.76–2.37) | <.01 | 2.23 (1.59–3.11) | <.01 |
| Type of therapy at initiation of first regimen | ||||
| PI based | Reference | Reference | ||
| NNRTI based | 0.99 (0.82–1.22) | .99 | 1.02 (0.71–1.46) | .92 |
| PI or NNRTI | 0.99 (0.78–1.25) | .91 | 1.24 (0.79–1.94) | .35 |
| ≥3 NRTIs | 0.78 (0.35–1.72) | .53 | 1.02 (0.35–2.98) | .97 |
| Treatment experienced before initiation of first regimen | 1.11 (0.98–1.27) | .10 | NA | |
NOTE. CI, confidence interval; NA, not applicable; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
There were 1532 deaths among 6698 patients.
There were 355 deaths among 2050 patients.
Of the 2050 patients who had no treatment experience before the initiation of combination therapy and who experienced a second regimen failure, 355 died. The crude incidence of mortality among those who experienced a second regimen failure in 1998 was 7.0 (IQR, 4.8–10.3) deaths per 100 person-years. This incidence decreased to 3.0 (IQR, 1.4–6.3) deaths per 100 person-years among those who experienced a second regimen failure in 2004; this trend remained statistically significant (P = .01; Table 3).
Predictors of mortality after second virologic failure
In addition to the calendar time trends noted above, a lower CD4+ cell count at the time of second virologic failure, a higher viral load at the time of a second virologic failure, and a history of AIDS at the time of second virologic failure were each strongly associated with a greater risk of mortality (Table 3). Sex, pretherapy CD4+ cell count, pretherapy viral load, and regimen type were not associated with mortality in multivariate analysis.
Data on hepatitis C virus infection and a history of IDU were available in a subset of patients. Both variables were associated with a greater risk of mortality (HR, 1.66 and 1.42 for hepatitis C virus infection and IDU, respectively; P < .01for each). However, neither of these variables materially changed the inferences from the analyses displayed in Table 3. Furthermore, the analyses of data that left out each cohort systematically provided estimates that were generally within the presented confidence limits.
These predictors of mortality remained of similar magnitude and significance when the analysis was restricted to those individuals who were antiretroviral treatment naive before receipt of their first regimen (Table 3). Both hepatitis C virus infection and a history of IDU had similar relative hazard magnitudes (1.48 and 1.13) as that observed in the entire cohort; the effect of hepatitis C virus infection remained statistically significant (P < .01), but a history of IDU did not achieve statistical significance (P = .74).
DISCUSSION
The overall objective of this study was to define the incidence and long-term consequences of multiple regimen failure among treated individuals across North America. The overall incidence of second virologic failure decreased dramatically, eventually reaching a stable level in 2005 of ~16 cases per 100 person-years for the entire cohort and 18 cases per 100 persons-years for those patients who were treatment naive at the time that their first combination regimen was initiated. The risk of mortality after a second regimen failure was high (5–6.5 deaths per 100 person-years; as a comparison, among the larger NA-ACCORD population of all treatment-naive patients starting therapy, the crude death rates were 1.3–1.6 deaths per 100 person-years) [16]. Although there was evidence that the mortality rates among those patients who experienced multiple regimen failures decreased over time, the rate of decrease was modest, compared with the far more dramatic improvements in risk of progressing to second regimen failure. Collectively, these data indicate that the effectiveness of combination therapy continues to improve, even among those patients who experienced failure of an initial regimen. However, among those who experience virologic failure of at least 2 distinct regimens, the overall clinical prognosis remains poor.
The ability of an initial antiretroviral regimen to durably suppress virus replication in clinical practice has increased with time [17–20], presumably in part as a result of the greater potency and tolerability of current regimens, compared with those used in the earlier time period. Our data extend these observations to those who had experienced failure of an initial regimen and were switched to a subsequent regimen. The dramatic decreases in the rate of failure of these second line regimens is likely attributable to several factors, including improved therapeutic options (ie, more-potent and better-tolerated regimens), as well as improved therapeutic strategies (ie, initiation of therapy earlier in the disease course), and perhaps better monitoring of patients who are receiving therapy [8, 21–24].
The major strength of our study is the large sample size and the inclusion of cohorts representing a diverse population of patients and a diverse selection of health care delivery systems. Our approach, however, has certain limitations that deserve comment. First, the observation periods ended in 2005, just prior to the introduction of several drugs with proven efficacy in highly treatment-experienced patients. One of the most important questions in the field is whether the efficacy of darunavir, raltegravir, maraviroc, and etravirine, which was observed in clinical trials, will translate into comparable levels of effectiveness when used more broadly [25–27]. A second limitation pertains to the lack of consistent data on important covariates, particularly adherence. Improved adherence in later years likely accounts, in part, for the decrease in the prevalence of a second treatment failure. Although inclusion of adherence measures would have allowed us to comment more precisely on the mechanisms accounting for our observations [28], the lack of this information does not detract from our primary conclusions, which are that multiple regimen failure is now uncommon, whereas the rate of death after multiple regimen failure remains high. Finally, because we defined treatment failure on the basis of a single episode of viremia, it is possible that we included individuals who had an isolated “blip” or whose viral load was still decreasing. However, most individuals with follow-up data had confirmatory viral load measurements, and the inferences remained unchanged when censoring those individuals without confirmed events.
In conclusion, the ability of combination antiretroviral therapy to prevent multiple regimen failure has improved dramatically over the past decade. Among patients who experience multiple regimen failure, mortality rates have also gradually decreased over time. Plasma HIV RNA levels and CD4+ T cell counts, as well as a history of AIDS, remain independent risk factors for mortality after multiple regimen failure, which emphasizes that these factors should be used to target those in need of more-aggressive interventions.
Acknowledgments
Financial support. National Institutes of Health (U01-AI069918, U01-AA013566, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-HD32632, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, P30-AI27757, P30-AI27767, P30-AI50410, P30-AI54999, R01-DA04334, R01-DA12568, R01-MH54907, R24-AI067039, Z01-CP010176, AHQ290–01-0012, N02-CP55504, R01-DA11602, AI-69432, K01-AI071754, R01-AA16893, K24-00432, K23-AI-61-0320).
Footnotes
Potential conflicts of interest. S.G.D. has received consulting fees from GlaxoSmithKline, Roche, Gilead, and Boehringer-Ingelheim and grant support from Merck, Gilead, Bristol-Myers Squibb, and Pfizer. M.S.S. has received consulting fees from Ardea Biosciences, Avexa, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Monogram Biosciences, Pain Therapeutics, Panacos, Pfizer, Progenics, Roche Laboratories, Tibotec, Tobira Therapeutics, and Vicro and research support from Achillion Pharmaceuticals, Boehringer-Ingelheim, Merck, Pfizer, Progenics, and Tibotec. J.J.E. has received consulting fees from Tibotec, Bristol-Myers Squibb, Merck, GlaxoSmithKline, and Pfizer; lecture fees from Roche, Bristol-Myers Squibb, Tibotec, and Merck; and grant support from GlaxoSmithKline, Merck, and Boehringer-Ingelheim. J.G. has received consulting fees from Gilead, Abbott, Merck, Boehringer-Ingelheim, Tibotec, and Pfizer and grant support from GlaxoSmithKline, Abbott, Canadian Institutes of Health Research, Tibotec, and Pfizer. R.S.H. has received payment from a commercial entity that sponsored his study and grant support from Merck. M. B. Klein has received consulting fees from GlaxoSmithKline, Abbott, Pfizer, and Boehringer-Ingelheim; lecture fees from Abbott, Gilead, Tibotec, Bristol-Myers Squibb, and GlaxoSmithKline; and research support from Canadian Institutes of Health Research/Fonds de la recherche en santé du Québec, Canadian HIV Trials Network, Ontario HIV Treatment Network, and Schering Plough Canada. A.R.R. has received consulting fees and lecture fees from GlaxoSmithKline, Abbott, Merck, Pfizer, and Tibotec and grant support from GlaxoSmithKline, Tibotec, Boehringer-Ingelheim, Abbott, Merck, Pfizer, and Roche. M.A.H. has received grant support from Gilead, Abbott, and Bristol-Myers Squibb. M.J.S. has received grant support from Pfizer, Merck, Gilead, Universitywide AIDS Research Program, and Community Benefit/Kaiser Permanente; K.A.G. has received consulting fees from Tibotec and grant support from Johns Hopkins University Richard Ross Award and Agency for Healthcare Research and Quality. R.D.M. has received consulting fees from Bristol-Myers Squibb and Glaxo-SmithKline; lecture fees from Gilead; and grant support from Pfizer, Merck, Gilead, and Agency for Healthcare Research and Quality. A.C.C. has received consulting fees from Merck, Pfizer, and GlaxoSmithKline; has equity ownership/stock options in Bristol-Myers Squibb and Abbott; and has received grant support from Schering-Plough, Tibotec-Virco, Gilead, Koronis, and Merck. C.A.B. has received consulting fees from GlaxoSmithKline, Pfizer, Merck, and Achillion and grant support from Gilead. B.R. has received consulting fees from Gilead and Bristol-Myers Squibb, lecture fees from Bristol-Myers Squibb, and grant support from STERIS. All other authors: no conflicts.
References
- 1.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1–infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Barbour JD, Martin JN, Swanson MS, Grant RM. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J Infect Dis. 2000;181:946–53. doi: 10.1086/315334. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence J, Mayers DL, Hullsiek KH, et al. Structured treatment interruption in patients with multidrug-resistant human immunodeficiency virus. N Engl J Med. 2003;349:837–46. doi: 10.1056/NEJMoa035103. [DOI] [PubMed] [Google Scholar]
- 5.Recsky MA, Brumme ZL, Chan KJ, et al. Antiretroviral resistance among HIV-infected persons who have died in British Columbia, in the era of modern antiretroviral therapy. J Infect Dis. 2004;190:285–92. doi: 10.1086/422007. [DOI] [PubMed] [Google Scholar]
- 6.Sabin CA, Smith CJ, Youle M, et al. Deaths in the era of HAART: contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. AIDS. 2006;20:67–71. doi: 10.1097/01.aids.0000196178.73174.24. [DOI] [PubMed] [Google Scholar]
- 7.Phillips AN, Leen C, Wilson A, et al. Risk of extensive virological failure to the three original antiretroviral drug classes over long-term follow-up from the start of therapy in patients with HIV infection: an observational cohort study. Lancet. 2007;370:1923–8. doi: 10.1016/S0140-6736(07)61815-7. [DOI] [PubMed] [Google Scholar]
- 8.Jones R, Nelson M, Bower M, et al. Triple-class antiretroviral agent resistance in a large cohort: prevalence and clinical outcomes. Arch Intern Med. 2008;168:1926–7. doi: 10.1001/archinte.168.17.1926. [DOI] [PubMed] [Google Scholar]
- 9.Lucas GM, Gallant JE, Moore RD. Relationship between drug resistance and HIV-1 disease progression or death in patients undergoing resistance testing. AIDS. 2004;18:1539–48. doi: 10.1097/01.aids.0000131339.68666.1a. [DOI] [PubMed] [Google Scholar]
- 10.Zaccarelli M, Tozzi V, Lorenzini P, et al. Multiple drug class-wide resistance associated with poorer survival after treatment failure in a cohort of HIV-infected patients. AIDS. 2005;19:1081–9. doi: 10.1097/01.aids.0000174455.01369.ad. [DOI] [PubMed] [Google Scholar]
- 11.Grover D, Copas A, Green H, et al. What is the risk of mortality following diagnosis of multidrug-resistant HIV-1? J Antimicrob Chemother. 2008;61:705–13. doi: 10.1093/jac/dkm522. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Ledergerber B, Viard JP, et al. Time to virological failure of 3 classes of antiretrovirals after initiation of highly active antiretroviral therapy: results from the EuroSIDA study group. J Infect Dis. 2004;190:1947–56. doi: 10.1086/425424. [DOI] [PubMed] [Google Scholar]
- 13.Di Giambenedetto S, Colafigli M, Pinnetti C, et al. Genotypic resistance profile and clinical progression of treatment-experienced HIV type 1–infected patients with virological failure. AIDS Res Hum Retroviruses. 2008;24:149–54. doi: 10.1089/aid.2007.0070. [DOI] [PubMed] [Google Scholar]
- 14.Lohse N, Jorgensen LB, Kronborg G, et al. Genotypic drug resistance and long-term mortality in patients with triple-class antiretroviral drug failure. Antivir Ther. 2007;12:909–17. [PubMed] [Google Scholar]
- 15.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–8. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 18.Moore RD, Keruly JC, Gebo KA, Lucas GM. An improvement in virologic response to highly active antiretroviral therapy in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr. 2005;39:195–8. [PubMed] [Google Scholar]
- 19.Lampe FC, Gatell JM, Staszewski S, et al. Changes over time in risk of initial virological failure of combination antiretroviral therapy: a multicohort analysis, 1996 to 2002. Arch Intern Med. 2006;166:521–8. doi: 10.1001/archinte.166.5.521. [DOI] [PubMed] [Google Scholar]
- 20.Porter K, Walker S, Hill T, et al. Changes in outcome of persons initiating highly active antiretroviral therapy at a CD4 count less than 50 Cells/mm3. J Acquir Immune Defic Syndr. 2008;47:202–5. doi: 10.1097/QAI.0b013e31815b1291. [DOI] [PubMed] [Google Scholar]
- 21.von Wyl V, Yerly S, Burgisser P, et al. Long-term trends of HIV type 1 drug resistance prevalence among antiretroviral treatment-experienced patients in Switzerland. Clin Infect Dis. 2009;48:979–87. doi: 10.1086/597352. [DOI] [PubMed] [Google Scholar]
- 22.Deeks SG. Treatment of antiretroviral-drug-resistant HIV-1 infection. Lancet. 2003;362:2002–11. doi: 10.1016/S0140-6736(03)15022-2. [DOI] [PubMed] [Google Scholar]
- 23.Napravnik S, Keys JR, Quinlivan EB, Wohl DA, Mikeal OV, Eron JJ., Jr Triple-class antiretroviral drug resistance: risk and predictors among HIV-1-infected patients. AIDS. 2007;21:825–34. doi: 10.1097/QAD.0b013e32805e8764. [DOI] [PubMed] [Google Scholar]
- 24.Havlir DV. HIV integrase inhibitors—out of the pipeline and into the clinic. N Engl J Med. 2008;359:416–8. doi: 10.1056/NEJMe0804289. [DOI] [PubMed] [Google Scholar]
- 25.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 26.Madruga JV, Cahn P, Grinsztejn B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1–infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- 27.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–31. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]