Abstract
Research on eukaryotic cytokinesis using advantageous model systems is rapidly advancing our understanding of most aspects of the process. Cytokinesis is very complicated with more than 100 proteins participating. Both fungi and animal cells use proteins to mark the cleavage site for the assembly of a contractile ring of actin filaments and myosin-II. Formins nucleate and elongate the actin filaments and myosin-II helps to organize the filaments into a contractile ring. Much is still to be learned about the organization of the contractile ring and the mechanisms that disassemble the ring as it constricts. Although fungi and animals share many proteins that contribute to cytokinesis, the extent to which they share mechanisms for the location, assembly, constriction, and disassembly of their contractile rings is still in question.
Introduction
Cytokinesis remains a challenging frontier in cell biology as investigators aim to understand how cells assemble the machinery to cleave themselves in two at the end of mitosis. Superficially the process appears simple enough, at least since work in the 1970s established that animal cells use a contractile ring of actin filaments and myosin-II to constrict a cleavage furrow to separate two daughter cells, each with its nucleus and complement of organelles. However, when it came to understanding molecular mechanisms, investigators quickly learned that cytokinesis is remarkably complicated. In the best-documented case, an incomplete inventory includes more than 130 cytokinesis genes [1]. Furthermore, cytokinesis has resisted biochemical reconstitution, so the field has nothing comparable to the elegant in vitro assays used to study mitosis, motility, and membrane traffic. Fortunately, ancient origins of many cytokinesis genes and powerful tools for genetics, molecular biology, microscopy, and biochemistry in model organisms have come to the rescue.
This review highlights examples of recent work on the mechanisms that specify the cleavage site, assemble the contractile ring, and control the constriction of the cleavage furrow in fungi and animals. Readers can consult recent reviews for comprehensive summaries of work on animals, fungi, plants, and bacteria through 2008 [1–4]. Another review covers abscission [5].
Origins of cytokinesis genes
Genome sequences indicate that genes for some key contractile ring proteins, including myosin-II, appeared in the common ancestor of amebas, fungi, and animals about 1 billion years ago [6,7]. The four large groups of eukaryotes that diverged earlier (‘bikonts’) lack myosin-II. Plants, algae, and ciliates have other types of myosins, but none have been implicated conclusively in cytokinesis. Other bikonts such as Giardia and trypanosomes lack myosin genes altogether. Plants have genes required to separate the plasma membranes at the end of cytokinesis (abscission) [8]. Accordingly, plants depend largely on membrane fusion for cytokinesis. Cells with contractile rings also use the membrane fusion machinery to separate the daughter cells after ring constriction forms a cleavage furrow [5]. Ameboid cells such as Dictyostelium can also divide by migration of the daughter cells apart from each other [9].
Among the eukaryotes with contractile rings, the inventory of cytokinesis genes is most complete for fission yeast (reviewed by Pollard and Wu [1]). Fission yeast uses about half of the 130 documented cytokinesis genes to position, assemble, constrict, and disassemble the contractile ring and the other half to participate in cell wall formation and membrane abscission. Less complete cytokinesis gene inventories in other organisms [2] overlap heavily with the fission yeast list, thus rendering evidence that the common ancestor of amebas, fungi, and animals used these proteins for cytokinesis. A complete cross correlation of these cytokinesis gene lists has yet to be done, so we do not know how many of these ancient genes may have been lost or new cytokinesis genes acquired during the past billion years of eukaryotic evolution.
Given the ancient origins of many cytokinesis genes, work on model systems should reveal widely shared mechanisms. Some will be uncomfortable with this view, since students of cytokinesis like to focus on differences. For example, it is difficult to imagine how the microtubules of the intranuclear mitotic spindle of fission yeast help to position the contractile ring like the mitotic spindle microtubules [10] and chromosomal passenger proteins [11] of animal cells. In spite of obvious anatomical differences, chromosomal passenger proteins are also important for cytokinesis of fission yeast [12•]. Thus, I expect basic strategies to be conserved but with lots of variations in emphasis that have arisen since the divergence of amebas, fungi, and animals from their common ancestor.
Mechanisms specifying the position of the division plane
Fission yeast
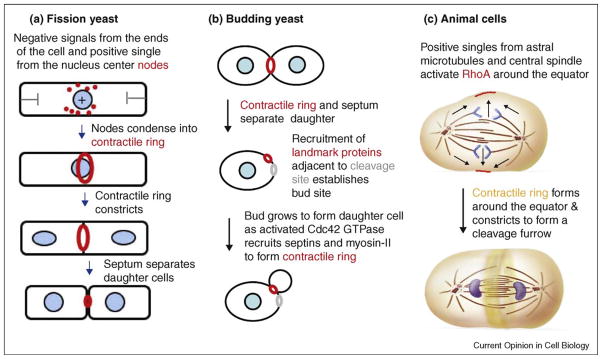
Positional information from the ends of the cylindrical cell and the location of the nucleus combine to determine the plane of cleavage by positioning contractile ring precursors called nodes, which are associated with the inside of the plasma membrane (Figure 1a). During interphase negative signals from the Pom1p kinase and another unknown source at ends of the cell concentrate the nodes around the equator [13••,14••,15•]. These interphase nodes contain the anillin-like protein Mid1p along with three kinases (Cdr1p, Cdr2p, and Wee1p), a kinesin (Klp8) and a RhoGEF (Gef2p). Late in G2 the location of the nucleus provides a positive local influence on the location of the nodes. The action of Polo kinase releases Mid1p from the nucleus, which concentrates in the nodes near the nucleus. Dynamic microtubules keep the nucleus centered in the cell [16], but if the nucleus moves off center, the broad band of cortical nodes follows [17,18,15]. The mechanism coupling the location of the nucleus and to the position of the nodes is still being investigated.
Figure 1.
Drawing of contractile ring assembly and constriction in three model organisms. (a) Fission yeast. (b) Budding yeast. (c) Animal cells.
Budding yeast
Cytokinesis by budding yeast is unusual, since the division site is selected early in interphase (Figure 1b) rather than just before or during mitosis (reviewed by Oliferenko et al. [4]) and because the motor activity of myosin-II is not required for cytokinesis [19,20]. Nevertheless, like other cells, localization of active Rho GTPase (RhoA in animals, Rho1 in budding yeast) at the cleavage site is important for cytokinesis (Figure 1b). Two mechanisms target Rho1 to bud neck, the site of division for budding yeast [21•]. Polo kinase stimulates accumulation of RhoGEFs (nucleotide exchange factors) at the bud neck. The GEFs attract inactive Rho1-GDP and dissociate GDP in exchange for GTP to activate Rho1. During constriction of the cleavage furrow a backup mechanism involving interaction with PIP2 concentrates Rho1 on the membrane between two rings of septins on either side of the contractile ring.
Animal cells
Assembly of the contractile ring depends on concentration of RhoA around the equator at the future site of the cleavage furrow (reviewed by Glotzer [10]) (Figure 1c). Polo kinase promotes the accumulation of active RhoA-GTP in the cleavage furrow [22]. The mechanism depends on the central spindlin complex consisting of a Rho family GAP (GTPase activating protein called MgcRacGAP or CYK-4), a RhoGEF (Ect2), and kinesin-6. This complex moves from the microtubules of the central spindle to the equatorial cortex where RhoGEF activates RhoA (reviewed by Glotzer [10]). Polo kinase phosphorylates RhoGAP, creating a binding site for RhoGEF. Polo kinase also promotes the concentration of a second RhoGEF (MyoGEF) in the central spindle [23•].
The role of the Rho family GAP in the central spindlin complex is still unsettled [24]. If Rho-GAP is depleted from Xenopus eggs and replaced with Rho-GAP lacking the GAP domain or with a point mutation that inhibits GAP activity, the equatorial zone of active Rho spreads out, so GAP activity may help to concentrate active Rho-GTP around the equator [25••]. On the other hand, genetic evidence in nematodes suggests that the target of Rho-GAP is actually the Rac GTPase and that inactivation of Rac may promote cytokinesis by reducing actin filament nucleation by Arp2/3 complex [26••].
Mechanism of contractile ring assembly
Fission yeast
After the spindle pole body divides, formin Cdc12p nucleates and directs the elongation of actin filaments around the equator of the cell, and this is followed by condensation of nodes containing myosin-II into a contractile ring over 10 min (Figure 1a). The most detailed proposal for this mechanism is that myosin-II associated with nodes captures actin filaments growing from adjacent nodes and generates force that pulls two nodes together [27•]. Detecting formin Cdc12p in nodes has been challenging owing to the small numbers of molecules [28], but the latest work detected Cdc12p in >60% of nodes [29]. Computer simulations showed that the ability of this search and capture mechanism to form a ring in 10 min depends on frequent breaks in the connections between nodes, as reflected in the intermittent movements of the nodes during condensation [27•]. Some investigators emphasize the formation of actin filament bundles, called leading cables, during ring formation [28,30]. These bundles form during the condensation of nodes and are probably part of the search and capture mechanism.
Animal cells
Less is known about assembly of contractile rings in animal cells, but the overall impression is that the strategy is similar to fission yeast. Myosin-II concentrates in cortical spots (that may correspond to filaments) independent of actin, followed by formin assembling actin filaments around the equator independent of myosin. Myosin-II applies force to these filaments to assemble the ring. Anillin is not essential for furrow formation, but interacts with RacGAP [31] and appears to contribute to myosin-II activation through interactions with RhoA [32]. It is generally assumed that active RhoA around the equator of the cell promotes contractile ring assembly by activating a formin (presumably directly) and myosin-II (indirectly) via Rho-kinase and phosphorylation of the regulatory light chain [33]. On the contrary, myosin-II lacking a regulatory light chain can assemble and constrict a contractile ring [34•].
Evanescent wave fluorescence microscopy of NRK cells transiently expressing GFP-myosin-II heavy chains showed that the protein appeared around the equator of metaphase cells in small, stationary spots containing multiple myosins [35•]. These myosin-II spots assembled in the presence of inhibitors of myosin-II ATPase (bleb-bistatin), Rho-kinase (Y-27632), or myosin light chain kinase (ML-7), but not C3 transferase, which inhibits Rho family GTPases.
Although formin CYK-1 was firmly established as the source of actin filaments in nematode contractile rings [36], the mammalian cytokinesis formin was in question until it was discovered that more than 40% of 3T3 cells failed to divide if 95% of formin mDia2 was depleted [37•]. Depletion of 70–75% of mDia1 or mDia3 had no effect on cytokinesis. Depletion of mDia2 compromised actin filament nucleation and elongation, since wild type mDia2 restored actin assembly and cytokinesis in the depleted cells, but an mDia2 mutant defective in actin filament nucleation did not. Formins filter out all of the GFP-actin when fission yeast [38] and nematodes [39••] assemble contractile rings, but some GFP-actin appeared around the equator of anaphase NRK cells [35•]. It is not known if the relevant formin accepts GFP-actin or if some filaments assemble independent of formins. Part of the cleavage furrow actin filaments form in the cortex and depend on myosin-II activity for movement toward the equator along irregular paths [35•]. Genetic evidence suggests that actin polymerization by Arp2/3 complex may inhibit cytokinesis in nematodes [26••].
Architecture of the ring
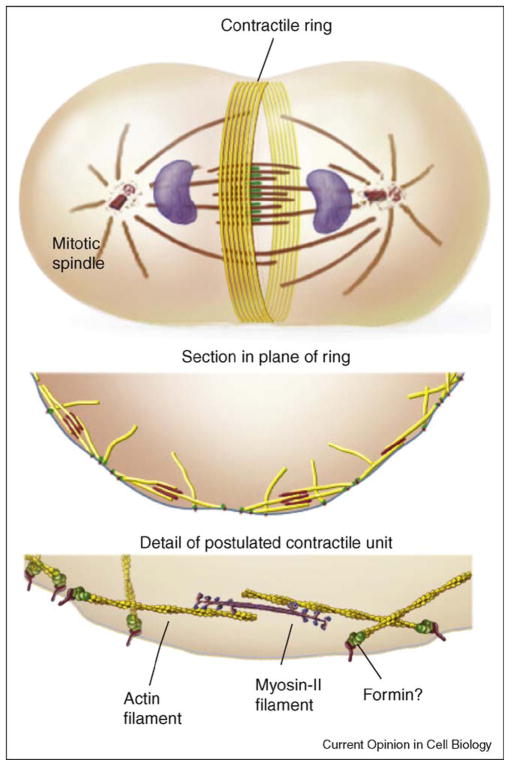
If we knew how contractile rings form, we would probably understand their anatomy. In both animal cells [40,41] and fission yeast [30] contractile ring actin filaments have mixed polarities, so they are oriented in both directions around the ring (Figure 2). In animal cells the filaments are clustered in small bundles [42]. The concentration of polymerized actin in contractile rings is estimated by electron microscopy to be ~1 mM in sea urchin eggs [43] and fission yeast [38]. The lengths of contractile ring actin filaments are not known precisely, because they cannot be followed with certainty for more than 0.25 μm in thin sections [43]. If each formin Cdc12p in the fission yeast contractile ring nucleated an actin filament, they would be about 1 μm long [38], similar to estimates from electron microscopy [30]. In the cleavage furrow of Dictyostelium actin filaments ~100 nm long form a network rather than a tight bundle [9]. Proteins including α-actinin and myosin-II crosslink actin filaments in contractile rings [9,44•]. Formins are attractive but unproven candidates to anchor actin filaments to the plasma membrane, since their lifetimes on the barbed ends of filaments are very long and barbed end attachments might transmit force produced by myosin-II to the plasma membrane.
Figure 2.
Simple drawings of the organization of contractile rings. The example shown is from animal cells but the arrangement of antiparallel actin filaments and interactions with myosin-II are thought to apply to fungi and amebas as well. Attachment of the barbed ends of the actin filaments to the plasma membrane via a formin is hypothetical. As myosin-II walks toward the barbed end of an associated actin filament it will apply tension to the plasma membrane. Illustrations by Graham Johnson.
Local concentrations of myosin-II in contractile rings are 8 μM in Dictyostelium [45], 20 μM rising to 50 μM in fission yeast [38], and unknown in other cells. Myosin-II typically concentrates in small spots that may correspond to filaments [9,35•]. The structures of these myosin aggregates and their arrangements relative to the actin filaments need to be established. Myosin-II interacting with antiparallel actin filaments could constrict a contractile ring by a sliding filament mechanism similar to muscle (Figure 2).
Mechanism of constriction and disassembly of the contractile ring
Actin filaments
Any account of the mechanism of constriction must explain the behavior of the actin filaments. Classic observations by electron microscopy [43] showed that the cross sectional area of the sea urchin embryo contractile ring is constant as it constricts, so the actin filaments must depolymerize in proportion to the circumference. Ideally one would like to measure the local concentration and turnover of actin in contractile rings of live cells, but this has not been possible with GFP-actin in fission yeast [38] or nematode embryos [39••] because the formins filter out all of the GFP-actin. When expressed from plasmids in vertebrate cell lines some GFP-actin accumulates in the cleavage furrow and turns over with a half time of about 30 s in photobleaching experiments [46,47]. It is not known if formins generated these filaments.
As an alternative strategy, one can make functional actin binding proteins with fluorescent protein-tags. In fission yeast the local concentrations of YFP-tagged IQGAP Rng2p, formin Cdc12p and α-actinin Ain1p are constant as the contractile ring constricted, so all of these proteins are lost in direct proportion the circumference [38]. Similarly, the fluorescence of GFP-anillin and GFP-septin are also constant as nematode embryo contractile rings constrict [39••].
Little is known about the mechanisms that trigger constriction and coordinate the removal of actin and associated proteins from contractile rings as they constrict. One source of regulation of formin mDia2 relevant to cytokinesis is degradation by proteasomes after mitosis [48].
Myosin-II
The behavior of contractile ring myosin-II is more variable than actin. In fission yeast two myosin-II isoforms and their light chains are concentrated in the ring as it constricts [38] in spite of the fact that the essential light chain (Cdc4p) exchanges rapidly after photobleaching [49]. Inconstricting contractile rings of C. elegans, embryos the fluorescence intensity of GFP-myosin-II heavy chain is constant per unit length and fills in from the sides of photobleached areas [39••]. In Dictyostelium the myosin-II heavy chain exchanges with a half time of 8 s in the cleavage furrow [50].
Mechanism of constriction
Remarkably, contractile rings constrict at rates proportional to the initial circumference of the rings in nematode embryos [39••] and other organisms. Circumferential constriction relative to the initial circumference is very slow, ranging from 0.5 nm s−1 μm−1 in fission yeast (10 μm circumference) [49] to 3.5 nm s−1 μm−1 in nematode embryos (circumference 50–100 μm) [39••]. In nematodes the shortening rate slows when the ring encounters the bundle of microtubules in the midzone of the mitotic apparatus [39••].
Given the presence of antiparallel actin filaments and myosin-II, most theories for constriction assume a mechanism related to muscle sarcomeres (Figure 2). To account for the dependence of the constriction rate on initial circumference Carvalho et al. [39••] proposed that contractile modules of a certain size are arrayed in series around the ring. As in striated muscles the rate of shortening would depend on the number of modules/sarcomeres in series (at the onset of constriction) and the maximum force would depend on the number of modules/sarcomeres in parallel. This model would work for contractile rings, if the actin filaments were longer than the myosin-II filaments. On the contrary, contractile rings are more complicated than striated muscles, since neither the actin filaments nor the myosin-II are organized so precisely and since the whole apparatus disassembles as it constricts. More information regarding the structure of contractile rings is needed to make further progress.
Sources of drag
Contractile rings constrict very slowly, only 3 nm s−1 μm−1, about 1000 times slower than striated muscle with sarcomeres (2.5 μm long) contracting with no resistance. Cytoplasmic myosin-II moves unloaded actin filaments slower than skeletal muscle myosin, but something else must slow ring constriction. Note that very little myosin activity is required to constrict contractile rings. Severely disabled myosin-II can constrict fission yeast contractile rings [51] and myosin-II having only 10% of normal activity supports cytokinesis of Dictyostelium on a surface but not cells in suspension [9].
The factors contributing to slow contractile ring constriction are not well characterized, but candidate mechanisms are the geometry (few contractile units in series), the load resisting tension produced by myosin-II or a biochemical governor on myosin. Protein crosslinks between the actin filaments may produce an internal load [9]. Over expression of the actin filament crosslinking protein α-actinin in NRK cells increases cortical actin filaments, slows their turnover and can slow or stop constriction on the cleavage furrow, while depletion of α-actinin reduces cortical actin and speeds up constriction of the furrow [44•]. Since the plasma membrane is attached to the cell wall in fungi, the synthesis of the septum may limit the ingression of the furrow.
Modeling
Understanding how force produced by a contractile ring actually produces a cleavage furrow is not trivial and several interesting models have been proposed to address the questions [52,53]. These models raise important questions, but modeling is still limited by the lack of information about the architecture of the ring, questions about the mechanisms that produce force and many missing parameters, as documented in this review. As knowledge regarding molecular mechanisms expands, modeling will be a vital component of the ongoing effort to understand contractile ring constriction and disassembly.
Conclusions
This brief summary of a small sample of recent papers on cytokinesis illustrates that the field is enjoying a phase of rapid growth with new insights coming from a variety of model systems. Novel discoveries continue, such as the role very long chain fatty acids in furrowing in fly spermatocytes [54•]. As knowledge expands, it will be interesting to learn if our understanding of the mechanisms in fungi and animals converge toward a common underlying strategy or if cells on some branches of the eukaryotic phylogenetic tree depend on novel mechanisms.
Acknowledgments
NIH research grant GM-026232 supports work on cytokinesis in the author’s laboratory. The author regrets that space limitations precluded the inclusion of more papers in this review.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Pollard TD, Wu J-Q. Insights regarding cytokinesis from studies of fission yeast. Nat Rev Mol Cell Biol. 2010 doi: 10.1038/nrm2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–566. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 3.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Oliferenko S, Chew TG, Balasubramanian MK. Positioning cytokinesis. Genes Dev. 2009;23:660–674. doi: 10.1101/gad.1772009. [DOI] [PubMed] [Google Scholar]
- 5.Montagnac G, Echard A, Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr Opin Cell Biol. 2008;20:454–461. doi: 10.1016/j.ceb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. doi: 10.1038/nature03949. [DOI] [PubMed] [Google Scholar]
- 7.Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc Natl Acad Sci U S A. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balus ka F, Menzel D, Barlow PW. Cytokinesis in plant and animal cells: endosomes ‘shut the door’. Dev Biol. 2006;294:1–10. doi: 10.1016/j.ydbio.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, Divi S, Iglesias PA, Kuo SC, Robinson DN. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr Biol. 2008;18:471–480. doi: 10.1016/j.cub.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 12•.Bohnert KA, Chen JS, Clifford DM, Vander Kooi CW, Gould KL. A link between aurora kinase and Clp1/Cdc14 regulation uncovered by the identification of a fission yeast borealin-like protein. Mol Biol Cell. 2009;20:3646–3659. doi: 10.1091/mbc.E09-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]; A role for chromosomal passenger proteins in cytokinesis in fission yeast with closed mitosis
- 13••.Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–856. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]; This and the following two papers analyze the mechanism that positions precursors of the contractile ring in the middle of fission yeast cells
- 14••.Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature. 2009;459:857–860. doi: 10.1038/nature08074. [DOI] [PubMed] [Google Scholar]; This and the following two papers analyze the mechanism that positions precursors of the contractile ring in the middle of fission yeast cells
- 15•.Almonacid M, Moseley JB, Janvore J, Mayeux A, Fraisier V, Nurse P, Paoletti A. Spatial control of cytokinesis by Cdr2 kinase and Mid1/anillin nuclear export. Curr Biol. 2009;19:961–966. doi: 10.1016/j.cub.2009.04.024. [DOI] [PubMed] [Google Scholar]; This and the following two papers analyze the mechanism that positions precursors of the contractile ring in the middle of fission yeast cells
- 16.Tran PT, Doye V, Inoué S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daga RR, Chang F. Dynamic positioning of the fission yeast cell division plane. Proc Natl Acad Sci U S A. 2005;102:8228–8232. doi: 10.1073/pnas.0409021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolic-Nørrelykke IM, Sacconi L, Stringari C, Raabe I, Pavone FS. Nuclear and division-plane positioning revealed by optical micromanipulation. Curr Biol. 2005;15:1212–1216. doi: 10.1016/j.cub.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 19.Lord M, Laves E, Pollard TD. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol Biol Cell. 2005;16:5346–5355. doi: 10.1091/mbc.E05-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lister IM, Tolliday NJ, Li R. Characterization of the minimum domain required for targeting budding yeast myosin II to the site of cell division. BMC Biol. 2006;4:19. doi: 10.1186/1741-7007-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Yoshida S, Bartolini S, Pellman D. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 2009:810–823. doi: 10.1101/gad.1785209. [DOI] [PMC free article] [PubMed] [Google Scholar]; The advantages of yeast molecular biology allowed rigorous investigation of two mechanisms that target Rho1 to the cleavage furrow
- 22.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Asiedu M, Wu D, Matsumura F, Wei Q. Phosphorylation of MyoGEF on Thr-574 by Plk1 promotes MyoGEF localization to the central spindle. J Biol Chem. 2008;283:28392–28400. doi: 10.1074/jbc.M801801200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Characterization of a new factor in positioning the contractile ring in animal cells
- 24.Glotzer M. Cytokinesis: GAP Gap. Curr Biol. 2009;19:R162–R165. doi: 10.1016/j.cub.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that both Rho activation and inactivation participate in positioning the cleavage furrow in Xenopus embryos
- 26••.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, Oegema K. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–1546. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that Rac is the target of the centralspindlin GTPase activating protein in C. elegans embryos
- 27•.Vavylonis D, Wu JQ, Hao S, O’Shaughnessy B, Pollard TD. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science. 2008;319:97–100. doi: 10.1126/science.1151086. [DOI] [PubMed] [Google Scholar]; A combination of experimental measurements and simulations of a mathematical model was used to test a hypothesis for the assembly of the contractile ring in fission yeast
- 28.Yonetani A, Lustig RJ, Moseley JB, Takeda T, Goode BL, Chang F. Regulation and targeting of the fission yeast formin cdc12p in cytokinesis. Mol Biol Cell. 2008;19:2208–2219. doi: 10.1091/mbc.E07-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffman VC, Nile AH, Lee I-J, Wu J-Q. Formin nodes and myosin motor activity are important for anillin mid1p-dependent contractile ring assembly in fission yeast cytokinesis. Mol Biol Cell. 2009 Oct 28; doi: 10.1091/mbc.E09-05-0428. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamasaki T, Osumi M, Mabuchi I. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J Cell Biol. 2007;178:765–771. doi: 10.1083/jcb.200612018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol. 2008;18:25–29. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 32.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18:30–36. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 33.Dean SO, Spudich JA. Rho kinase’s role in myosin recruitment to the equatorial cortex of mitotic Drosophila S2 cells is for myosin regulatory light chain phosphorylation. PLoS ONE. 2006;1:e131. doi: 10.1371/journal.pone.0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Beach JR, Egelhoff TT. Myosin II recruitment during cytokinesis independent of centralspindlin-mediated phosphorylation. J Biol Chem. 2009;284:27377–27383. doi: 10.1074/jbc.M109.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]; A surprising observation that cells depending on myosin-II lacking its regulatory light chain complete cytokinesis
- 35•.Zhou M, Wang YL. Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol Biol Cell. 2008;19:318–326. doi: 10.1091/mbc.E07-08-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]; Valuable new information regarding the timing of events during contractile ring formation in an animal cell
- 36.Severson AF, Baillie DL, Bowerman B. A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol. 2002;12:2066–2075. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- 37•.Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, Ishizaki T, Narumiya S. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell. 2008;19:2328–2338. doi: 10.1091/mbc.E07-10-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that formin mDia2 produces the actin filaments for cytokinesis in mammals
- 38.Wu JQ, Pollard TD. Counting cytokinesis proteins globally and locally in fission yeast. Science. 2005;310:310–314. doi: 10.1126/science.1113230. [DOI] [PubMed] [Google Scholar]
- 39••.Carvalho A, Desai A, Oegema K. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell. 2009;137:926–937. doi: 10.1016/j.cell.2009.03.021. [DOI] [PubMed] [Google Scholar]; Careful quantitative analysis revealed that the contractile ring in C. elegans embryos constricts at a constant rate that is proportional to the original circumference of the ring
- 40.Schroeder TE. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci U S A. 1973;70:1688–1692. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanger JM, Sanger JW. Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol. 1980;86:568–575. doi: 10.1083/jcb.86.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maupin P, Pollard TD. Arrangement of actin filaments and myosin-like filaments in the contractile ring and of actin-like filaments in the mitotic spindle of dividing HeLa cells. J Ultrastruct Mol Struct Res. 1986;94:92–103. doi: 10.1016/0889-1605(86)90055-8. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder TE. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972;53:419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Mukhina S, Wang Y-L, Murata-Hori M. α-Actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev Cell. 2007;13:554–565. doi: 10.1016/j.devcel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that the actin filament crosslinking protein α-actinin modulates contractile ring constriction
- 45.Robinson DN, Cavet G, Warrick HM, Spudich JA. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biol. 2002;3:4. doi: 10.1186/1471-2121-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 47.Guha M, Zhou M, Wang Y-L. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2006;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 48.Deward AD, Alberts AS. Ubiquitin-mediated degradation of the formin mDia2 upon completion of cell division. J Biol Chem. 2009;284:20061–20069. doi: 10.1074/jbc.M109.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelham RJ, Chang F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature. 2002;419:82–86. doi: 10.1038/nature00999. [DOI] [PubMed] [Google Scholar]
- 50.Yumura S. Myosin II dynamics and cortical flow during contractile ring formation in Dictyostelium cells. J Cell Biol. 2001;154:137–145. doi: 10.1083/jcb.200011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lord M, Pollard TD. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J Cell Biol. 2004;167:315–325. doi: 10.1083/jcb.200404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zumdieck A, Kruse K, Bringmann H, Hyman AA, Julicher F. Stress generation and filament turnover during actin ring constriction. PLoS ONE. 2007;2:e696. doi: 10.1371/journal.pone.0000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shlomovitz R, Gov NS. Physical model of contractile ring initiation in dividing cells. Biophys J. 2008;94:1155–1168. doi: 10.1529/biophysj.107.111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Szafer-Glusman E, Giansanti MG, Nishihama R, Bolival B, Pringle J, Gatti M, Fuller MT. A role for very-long-chain fatty acids in furrow ingression during cytokinesis in Drosophila spermatocytes. Curr Biol. 2008;18:1426–1431. doi: 10.1016/j.cub.2008.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]; A new factor in cleavage furrow formation