Abstract
Neutrophil (PMN) infiltration into tissues is a hallmark of acute inflammation and is crucial for the rapid removal of microbial pathogens. Previous studies have shown that PMN transmigration is regulated by the cell surface protein CD47. However this phenomenon in the context of microbial invasion and subsequent TLR signaling is poorly understood. In this study, we assessed the role of TLR2 and CD47 costimulation in regulating PMN transmigration. Human PMN transmigration across acellular collagen-coated filters toward the bacterial chemoattractant fMLP was more significantly inhibited by MALP-2 (TLR2/6 agonist) than Pam3CSK4 (TLR2/1 agonist). Subsequent experiments demonstrated that treatment with MALP-2 or anti-human CD47 mAbs delayed human PMN transfilter migration, while combined treatment led to further delayed inhibition. Interestingly, stimulation of PMNs with MALP-2 resulted in an increase in surface expression of CD11b, but not CD47. In experiments addressing the role of TLR agonists in regulating CD47-mediated PMN transmigration, incubation with MALP-2 or with anti-mouse CD47 mAbs did not inhibit transfilter migration of TLR2−/− or MyD88−/−-deficient murine bone marrow-derived PMNs. Similarly, inhibition of MyD88 homodimerization reversed the attenuation of human PMN transmigration induced by MALP-2 or anti-human CD47 mAbs. Separate experiments demonstrated that CD47−/− murine bone marrow-derived PMNs exhibited 4-fold decreased sensitivity toward MALP-2. Collectively, these findings suggest that activation of CD47 signaling enhances PMN sensitivity toward TLR2 activation which, in turn, signals their arrival at a site of invasion and may facilitate antimicrobial function.
Neutrophils (PMNs)3 play a central role in host defense by migrating to the site of infection and eliminating pathogenic microorganisms. However, the release of proteolytic enzymes, reactive oxygen species, and inflammatory mediators associated with PMN antimicrobial function results in extensive tissue injury. Thus, the ensuing massive PMN infiltration is a prevalent feature in a number of inflammatory conditions and correlates with disease severity (1, 2).
During bacterial invasion, n-formylated chemotactic peptides such as fMLP are released from bacterial cell walls and diffuse from the site of injury to the microcirculation forming a potent chemoattractant gradient that stimulates the recruitment of PMNs (3). In addition, bacteria and other microbes release a variety of products, which contain specific pathogen-associated molecular patterns (PAMPs) that are able to trigger a family of innate immune receptors also known as TLRs (3, 4). TLRs are expressed on all immune cells and are involved in the cellular recognition of PAMPs such as mycoplasmal (TLR2/6) or bacterial (TLR2/1) lipoproteins, LPS or LPS (TLR4), flagellin (TLR5), imidazoquinoline and viral compounds (TLR7), and bacterial CpG DNA motifs (TLR9). Stimulation of TLRs subsequently leads to the activation of an adaptor molecule termed MyD88, which triggers the activation of a downstream cascade that culminates in the activation of NF-κB. NF-κB induces the production of inflammatory cytokines and chemoattractants necessary to recruit other cells (5). Although it has been shown that PMNs express all TLRs except TLR3 (6), very few studies have yet to detail the role of TLRs in these cells. Direct stimulation with LPS has been shown to enhance PMN migration (7), and up-regulate cell surface CD11b/CD18, which can facilitate subsequent transmigration (8, 9). Conversely, others have reported that exposure of PMNs to TLR agonists resulted in reduced chemotaxis and increased rate of phagocytosis and production of superoxide (6). Despite these studies, many have not focused on how cell surface signaling events that regulate PMN transmigration are influenced in the context of TLR activation, a condition that is likely to occur during microbial invasion. Furthermore, studies have not focused on how TLR activation regulates the rate or speed by which PMNs reach their target. Indeed, it is ultimately the kinetics of PMN migration that will determine the success or failure of the inflammatory response.
The sequence of molecular events that occur during PMN migration remain incompletely understood. Studies focusing on how receptor-ligand signaling interactions at the PMN surface can fine-tune PMN transmigration and ultimately regulate inflammatory diseases remains poorly understood. Previously, we and others have demonstrated that the ubiquitously expressed Ig superfamily transmembrane glycoprotein termed CD47 (also known as integrin-associated protein) has been shown to regulate the rate of PMN transmigration in mice infected with bacteria as well as across cell monolayers and matrix (10–13). In addition, CD47 has been implicated in multiple cellular functions such as a marker of self (14), platelet activation (15, 16), macrophage multinucleation (17), immune cell apoptosis (18), and dendritic cell maturation (19). CD47 has been shown to associate with various molecules (thrombospondins, integrins, and SIRPα) (20–22), however the CD47 signaling pathways have not been clearly elucidated. Furthermore, the signaling mechanisms that regulate PMN transmigration remain unclear, however past in vivo and in vitro studies suggest that CD47 acts as a fine-tuning mechanism that regulates the rate of PMN transmigration and may facilitate recognition of microbial pathogens (13, 23, 24).
In this study, we determined that activation of TLR2/6 by mycoplasmal lipopeptide potently inhibits PMN transmigration. Furthermore, we describe a cross-talk mechanism by which CD47-mediated inhibition of PMN transmigration is dependent on TLR2 and MyD88, and that CD47 may facilitate PMN responses to mycoplasmal lipopeptide by acting as a microbial sensor.
Materials and Methods
Reagents
Macrophage-activating lipopeptide-2 (MALP-2; S-(2,3-bis(Palmityloxy)-(2R)-propyl-cysteinyl-GNNDESNISFKEK)) and Pam3CSK4 ((S)-(2,3-Bis(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH-3HCl) were obtained from Alexis Biochemicals. The leukocyte chemoattractant n-formyl-met-leu-phe and n-formyl-norleu-leu-phe were obtained from Sigma-Aldrich. C5D5 and PF3.1 functionally inhibitory anti-human CD47 murine mAbs (IgG1) were produced in our laboratory as previously reported (13, 24). B6H12.2, anti-human (mouse IgG1) and miap301 anti-mouse (rat IgG2a) CD47 functionally inhibitory mAbs as well as respective isotype controls MOPC-21 (mouse IgG1) and R35–95 (rat IgG2a) were obtained from BD Biosciences. M1/70 functionally inhibitory anti-mouse (rat IgG2b) CD11b and respective isotype control A95-1 (rat IgG2b) were obtained from BD Biosciences. The MyD88 homodimerization inhibitor was obtained from Imgenex.
PMN isolation
Normal human PMNs were isolated as described previously (25) with approval from the Emory University Institutional Review Board on human subjects. In brief, citrated peripheral blood from healthy human volunteers was subjected to RBC dextran 500 (Amersham Biosciences) sedimentation followed by density sedimentation using Ficoll-Paque (Amersham Biosciences) as per the manufacturer’s instructions. The purified PMNs were resuspended in calcium and magnesium-free HBSS containing 0.4g/l KCl, 0.06g/l KH2PO4, 0.35g/l NaHCO3, 8.0g/l NaCl, 0.048g/l Na2HPO4, 1.0g/l glucose, and 10 mM HEPES at 4°C. For all experiments, PMNs were suspended in HBSS containing 0.185g/l CaCl2•2H2O and 0.098g/l MgSO4. PMN purity was >99% as assessed by differential staining with Diff-Quik (Dade-Behring).
Animals
C57BL/6 mice were obtained from The Jackson Laboratory. TLR2−/− and MyD88−/− mice were generously provided by Drs. Andrew Gewirtz and Melanie Sherman (Emory University, Atlanta, GA). TLR2−/− and MyD88−/− mice were produced on a C57BL/6 background as previously described (26, 27). All experimental procedures using mice were reviewed and approved by the Emory University Institutional Animal Care and Use Committee and were performed according to the National Institutes of Health criteria.
Murine bone marrow PMN isolation
The femurs and tibia were removed from mice that were euthanized with isoflurane (Novaplus). Bone marrow cells were flushed from the bones using ice cold 0.38% sodium citrate in HBSS (0.4g/l KCl, 0.06g/l KH2PO4, 0.35g/l NaHCO3, 8.0g/l NaCl, 0.048g/l Na2HPO4, 1.0g/l glucose, and 10 mM HEPES (pH 7.4)) without Ca2+ and Mg2+. Cells were pelleted with centrifugation at 200 × g for 10 min at 4°C. Erythrocytes were lysed with 5 ml hypotonic buffer (8.2g/l NH4Cl, 1g/l NaHCO3, 0.038g/l EDTA) for 2 min on ice followed by addition of 45 ml of ice cold HBSS without Ca2+ and Mg2+. Cells were pelleted with centrifugation at 200 × g for 10 min at 4°C, were washed and resuspended in ice-cold HBSS without Ca2+ and Mg2+. Given that isolation of PMNs from murine bone marrow is both costly and difficult, especially from the transgenic animals, semipure bone marrow-derived PMNs were used in the transmigration experiments. The semipure bone marrow cell preparations were stained specifically for Ly6G (clone 1A8; BD Biosciences), a PMN surface marker (28, 29), followed by flow cytometric analysis to assess purity which was determined to be 43.1 ± 2.0% PMNs.
PMN transfilter migration
PMN transmigration experiments were performed with Transwells (Corning) consisting of 5-µm pore-sized filters coated with collagen I as described previously (25). In brief, PMNs (1 × 106 purified human or 5 × 106 semipure murine) were added to the upper reservoir and were induced to migrate toward the lower reservoir containing 10−7M fMLP (human) or 10−6M fNLP (murine) at 37°C. At the end of the indicated time, migrated PMNs in the lower reservoirs and unmigrated PMNs in the upper reservoirs were quantified by assaying for myeloperoxidase (30). PMN migration into the lower reservoirs is expressed as a percentage of total applied PMNs per Transwell. For the animal experiments, 84 ± 2.4% PMNs migrated through the Transwells while 22.7 ± 0.8% PMNs remained in the upper chamber.
Flow cytometry
PMNs (106) were labeled with 5 µg/ml PE-conjugated B6H12.2 (anti-CD47), M1/70 (anti-CD11b), fluorescein-conjugated anti-Ly6G, or respective isotype controls for 45 min at 4°C in a sterile 96-well round-bottom plate. Cells were washed three times with ice cold HBSS and fixed in 2% paraformaldehyde for 1 h at 4°C. Surface expression of human CD47 and CD11b was assessed using CellQuest analysis software on a FACScalibur flow cytometer.
Statistical analysis
Results were expressed as means ± SEM and compared by one-way ANOVA, followed by Tukey’s test for multiple comparison analysis where applicable. Statistical significance was established at p < 0.05.
Results
Activation of TLR2/6 inhibits PMN transmigration
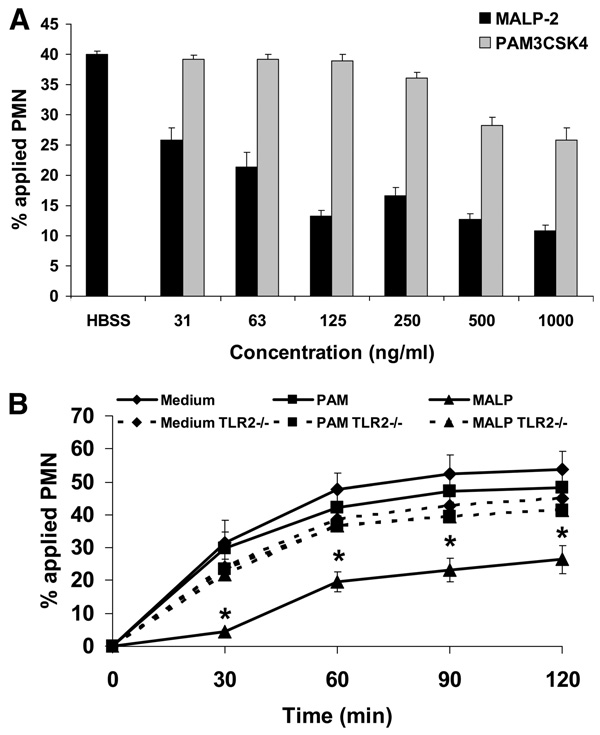
Given that PMNs play an important role in host defense, we sought to determine how microbial products may regulate PMN transmigration. For these studies, we initially examined the effects of bacterial or mycoplasmal lipoproteins that activate TLR2/1 or TLR2/6, respectively. Purified human PMNs were pretreated with either the specific TLR2/1 agonist Pam3CSK4 or the specific TLR2/6 agonist MALP-2, and then they were assayed for transmigration across collagen-coated filters. To simulate an inflammatory environment, either Pam3CSK4 or MALP-2 were added to both the upper and lower compartments of the Transwells, and then PMN transmigration was elicited upon addition of fMLP to the lower compartment. As shown in Fig. 1A, MALP-2 significantly inhibited migration at concentrations as low as 31 ng/ml (14.5 nM) as compared with Pam3CSK4 which exhibits inhibitory activity at 500 ng/ml. Furthermore, MALP-2 inhibited PMN transmigration >50% at 125 ng/ml (Fig. 1A).
FIGURE 1.
TLR2/6 (MALP-2) exhibits more potent inhibition of PMN transmigration than TLR2/1 (Pam3CSK4). A, Purified human PMNs were incubated with MALP-2 and Pam3CSK4 at various concentrations for 15 min at 37°C and then induced to migrate across acelllular collagen-coated filters in the presence of an fMLP gradient. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs incubated in HBSS). B, Purified murine bone marrow-derived PMNs isolated from C57BL/6 wild-type, TLR2−/−, and MyD88−/− mice were incubated with 500 ng/ml MALP-2 or Pam3CSK4 for 15 min at 37°C, and then induced to migrate across acellular collagen-coated filters in the presence of an fNLP gradient. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs incubated in HBSS medium).
Because we measured PMN transmigration by assessing myeloperoxidase activity, we considered the possibility that TLR2 activation may enhance degranulation, which could lead to underestimation of transmigrated PMNs after MALP-2 treatment. To address this concern, we assayed for myeloperoxidase activity (percentage of applied PMNs) in supernatants from isolated human peripheral blood PMNs which were treated with HBSS vehicle (1.2 ± 0.2%), 10−6M fMLP (1.3 ± 0.4%), 125 ng/ml MALP-2 (1.3 ± 0.3%), 10−6M fMLP plus 125 ng/ml MALP-2 (1.5 ± 0.3%), 5 µg/ml cytochalasin B plus 10−6M fMLP (16.3 ± 3.0%), or 1% Triton X-100 (100%). From this separate experiment, it is apparent that TLR2 stimulation does not appreciably induce PMN azurophilic degranulation as compared with baseline stimulation with HBSS vehicle control, mobilization of the actin cytoskeleton for granule release by cytochalasin B or to nonspecific cell lysis with the nonionic detergent Triton X-100. Therefore, we used PMN myeloperoxidase contents as the method of quantifying PMN transmigration for the remainder of our study.
Next, we performed experiments to confirm that the potent inhibitory effects of MALP-2 are attributable to TLR2 activation. For these experiments, we obtained semipure preparations of bone marrow-derived PMNs from C57BL/6 wild-type and TLR2−/− mice and pretreated them with MALP-2 at a concentration which exhibits maximal inhibitory effects (125 ng/ml). As shown in Fig. 1B, pretreatment with 125 ng/ml MALP-2 inhibited C57BL/6 wild-type PMN transmigration by ~50% as compared with TLR2−/− PMNs pretreated in the same manner. In a similar fashion observed with human PMNs, pretreatment with 125 ng/ml Pam3CSK4 failed to significantly inhibit murine PMN transmigration (Fig. 1B). These results suggest that activation of TLR2 by mycoplasmal lipoproteins potently inhibit PMN transmigration.
Activation of TLR2/6 or ligation of CD47 similarly inhibit PMN transmigration
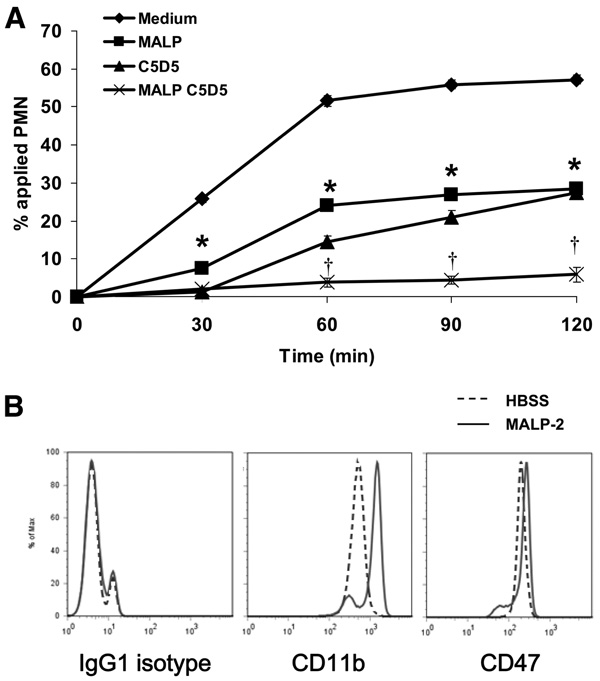
Although previous studies have determined that CD47 on PMNs significantly regulate PMN transmigration (12, 13, 24), the relevance of this phenomenon in the context of microbial invasion has not been explored. Given that the above data suggest that MALP-2 potently inhibits PMN transmigration, we further examined whether this effect may influence the inhibitory effect of CD47 ligation. As shown in Fig. 2A, incubation with MALP-2 and anti-CD47 Abs, similarly inhibited human PMN transmigration across collagen-coated filters by ~50%. Furthermore, combined treatment with MALP-2 and anti-CD47 Abs blocked human PMN transmigration by ~90% (Fig. 2A).
FIGURE 2.
TLR2/6 activation and CD47 ligation block PMN transmigration without affecting CD47 surface expression. A, Purified human PMNs were incubated with 125 ng/ml MALP-2 and/or 20 µg/ml anti-human CD47 mAbs (C5D5) for 15 min at 37°C, and then induced to migrate in the presence of a fMLP gradient across acellular collagen-coated filters. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs incubated in HBSS medium). B, Purified human PMNs were incubated with either HBSS medium (dashed line) or 125 ng/ml MALP-2 (solid line) for 15 min at 37°C, stained for CD11b or CD47, and then analyzed for surface expression via flow cytometry.
To determine whether the combined treatment with MALP-2 and anti-CD47 Abs is inducing further inhibitory effects by enhancing CD47 surface expression and producing more epitopes available for Ab binding, human PMNs were pretreated with MALP-2, immunostained for CD47, and then subjected to flow cytometric analysis. As shown in Fig. 2B, pretreatment with MALP-2 failed to increase CD47 surface expression. However, pretreatment with MALP-2 enhanced the surface expression of CD11b, a crucial β2 integrin, which regulates leukocyte adhesion and migration (Fig. 2B). These results suggest that the inhibition of PMN transmigration induced by combined treatment with MALP-2 and anti-CD47 Abs is not due to the increase in surface CD47 expression. Instead, these results suggest that activation of TLR2 with MALP-2 and ligation of CD47 induces signaling events that may be independent or that may converge and combine to inhibit PMN transmigration.
CD47 ligation is dependent on TLR2 and MyD88 signaling
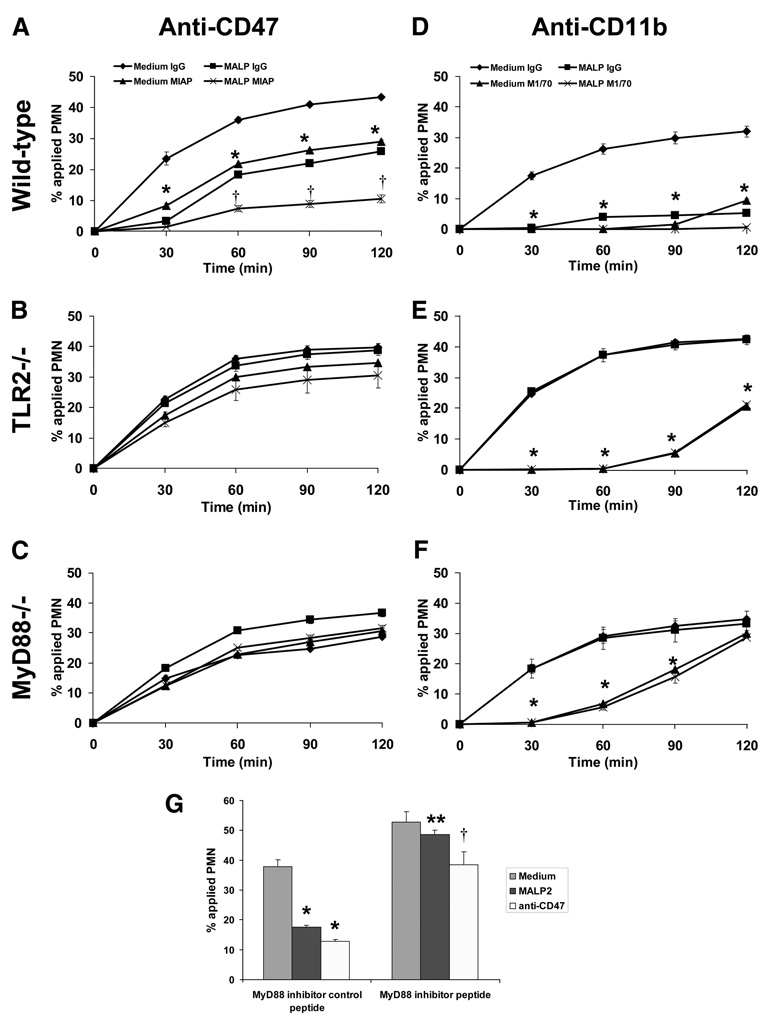
To explore the possibility that TLR2 activation and CD47 ligation may share similar signaling pathways, PMN transmigration assays were performed on semipure murine bone marrow-derived PMNs that were isolated from C57BL/6 wild-type, TLR2−/−, and MyD88−/− mice. In a similar manner exhibited by human PMNs, C57BL/6 wild-type PMN transmigration across collagen-coated filters were similarly inhibited after individual or combined treatment with MALP-2 and anti-CD47 Ab (Fig. 3A). As expected, the inhibitory effect of MALP-2 on wild-type PMNs was reversed when the same assay was performed on TLR2−/− and MyD88−/− mice (Fig. 3, B and C). However, both TLR2−/− and MyD88−/− PMNs did not respond to CD47 ligation and migrated across collagen-coated filters in a similar manner to their respective vehicle-treated control PMNs (Fig. 3, B and C). To demonstrate whether this phenomenon is restricted to CD47, the experiments were internally controlled by assaying for PMN transmigration in the presence or absence of anti-CD11b Abs. As shown in Fig. 3D, individual or combined treatment with MALP-2 or anti-CD11b Abs completely blocked wild-type PMN transmigration. Furthermore, anti-CD11b Abs still retained their ability to inhibit TLR2−/− and MyD88−/− PMN transmigration regardless of the presence of MALP-2 (Fig. 3, E and F).
FIGURE 3.
CD47-mediated inhibition of PMN transmigration is dependent on TLR2 and MyD88. A–C, Purified murine bone marrow-derived PMNs isolated from C57BL/6 wild-type, TLR2−/−, and MyD88−/− mice were incubated with 20 µg/ml anti-murine CD47 mAbs (miap301) for 15 min at 37°C, and then induced to migrate across acellular collagen-coated filters in the presence of an fNLP gradient. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs incubated with isotype control). D–F, Purified murine bone marrow-derived PMNs isolated from C57BL/6 wild-type, TLR2−/−, and MyD88−/− mice were incubated with 20 µg/ml anti-murine CD11b mAbs (M1/70) for 15 min at 37°C, and then induced to migrate across acellular collagen-coated filters in the presence of an fNLP gradient. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs incubated with isotype control). G, Purified human PMNs were pretreated with an MyD88 homodimerization inhibitor or control peptide for 30 min at 37°C followed by incubation with 125 ng/ml MALP-2 or 20 µg/ml anti-human CD47 mAbs (C5D5) for 15 min at 37°C. PMNs were induced to migrate across acellular collagen-coated filters in the presence of an fMLP gradient for 1 h. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs pretreated with control peptide followed by HBSS medium); **, p< 0.05 vs PMNs pretreated with control peptide followed by MALP-2; †p< 0.05 vs PMNs pretreated with control peptide followed by anti-human CD47 mAbs (C5D5).
To further confirm that the inhibition of PMN transmigration by anti-CD47 Abs is dependent on MyD88, human PMNs were pretreated with an MyD88 homodimerization inhibitor (31). As shown in Fig. 3G, inhibition of MyD88 reversed the decrease in human PMN transmigration induced by MALP-2 and by anti-CD47 Abs. These results strongly suggest that the inhibition of PMN transmigration induced by CD47 ligation is dependent on TLR2 and MyD88 signaling.
CD47 enhances TLR2 activation
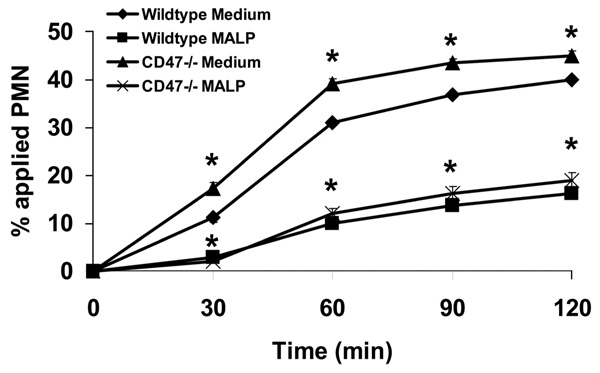
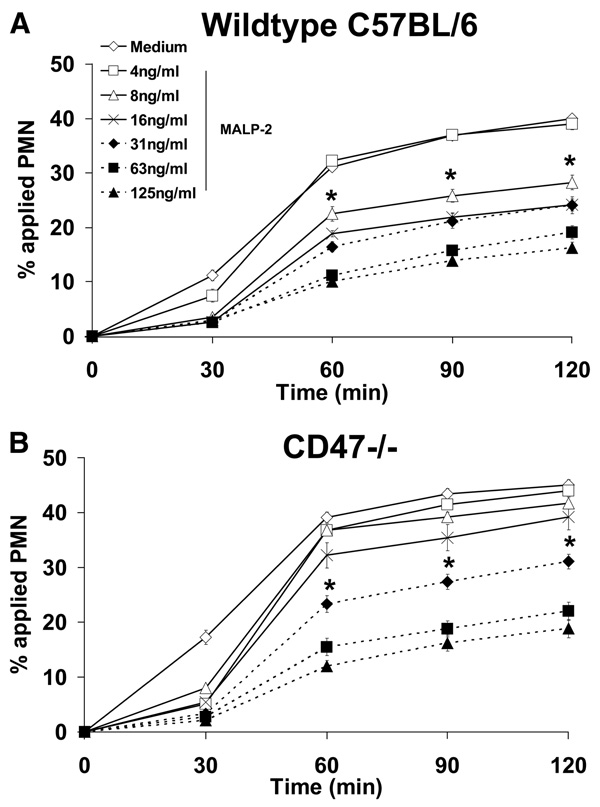
Because our results strongly suggest that inhibition of PMN transmigration by anti-CD47 Abs is dependent on TLR2 and MyD88, we further explored the possibility whether a cross-talk mechanism exists between CD47 and TLR2 signaling. For these experiments, semipure bone marrow-derived PMNs were isolated from CD47−/− mice and were pretreated with 125 ng/ml MALP-2. As shown in Fig. 4, CD47−/− PMNs still retained their ability to respond to MALP-2 and exhibited similar inhibition as wild-type PMNs treated in the same manner. However, untreated CD47−/− PMNs consistently migrated at a significantly higher rate as compared with wild-type PMNs (Fig. 4). Given this data, we rationalized that the MALP-2 concentration (125 ng/ml) was too high and may be saturating the transmigration response of CD47−/− PMNs as compared with wild-type controls. To further investigate this phenomenon, we performed dose response experiments to determine whether CD47−/− PMNs may exhibit different sensitivities to MALP-2. As shown in Fig. 5A, wild-type PMNs retained sensitivity to MALP-2 at 8 ng/ml. However, CD47−/− PMN transmigration was inhibited at a 4-fold higher dose of 31 ng/ml MALP-2 (Fig. 5B). These results suggest that CD47 is necessary for the action of MALP-2 on PMN migration and that the inhibitory effect of anti-CD47 Abs on PMN transmigration is dependent on surface CD47 cross-linking but not on blocking potential adhesion interactions with the substratum. Furthermore, given that we used semipure bone marrow-derived murine PMN preparations as discussed in the Materials and Methods, it was determined that the purity of transmigrated cells was 84 ± 2.4% PMNs in the bottom chamber of our Transwell assays. These findings indicate for the most part that the murine transmigration results obtained are mostly PMN specific and parallel the results of our human PMN experiments.
FIGURE 4.
CD47−/− murine PMNs exhibit increased PMN transmigration. Purified murine bone marrow-derived PMNs isolated from C57BL/6 wild-type, or CD47−/− mice were incubated with 125 ng/ml MALP-2 for 15 min at 37°C, and then induced to migrate across acellular collagen-coated filters in the presence of an fNLP gradient. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (wild-type PMNs incubated in HBSS medium).
FIGURE 5.
CD47−/− murine PMNs exhibit decreased sensitivity to MALP-2. Purified murine bone marrow-derived PMNs isolated from wild-type C57BL/6 (A) or CD47−/− (B) mice were incubated with various concentrations of MALP-2 for 15 min at 37°C, and then induced to migrate across acellular collagen-coated filters in the presence of an fNLP gradient. Values are means ± SEM, n = 3 per group. *, p< 0.05 vs control (PMNs incubated in HBSS medium).
Discussion
In this study, we report a novel mechanism by which CD47 and TLR2 signaling undergo cross-talk to regulate PMN transmigration. Our data suggests that activation of TLR2/6 by mycoplasmally derived MALP-2 potently inhibits human and murine PMN transmigration. Further investigation into the role of microbial activation on influencing other signaling pathways that mediate PMN transmigration revealed that combined pretreatment with MALP-2 and anti-CD47 mAbs almost completely blocked human and murine PMN transmigration. Pretreatment with MALP-2 increased cell surface expression of CD11b, but did not affect CD47 levels in human PMNs as determined by flow cytometry. Although MALP-2 and/or anti-CD47 mAbs blocked wild-type PMN transmigration, specificity of response was confirmed as they failed to inhibit TLR2−/− and MyD88−/− PMN transmigration. Furthermore, the inhibitory effects of anti-CD11b mAbs were unaffected in transmigration assays of wild-type, TLR2−/−, and MyD88−/− PMN. Verification in human PMNs revealed that pretreatment with a MyD88 homodimerization peptide inhibitor reversed the inhibition of transmigration by MALP-2 or anti-CD47 mAbs. We further report that although CD47−/− PMN transmigration was significantly higher than that of wild-type PMNs, no difference in the degree of inhibition was observed upon MALP-2 treatment. Further investigation using different MALP-2 concentrations revealed that CD47−/− PMN transmigration is inhibited at 4-fold higher levels than that of wild-type PMNs. To our knowledge, this is the first report demonstrating cross-talk between CD47 and TLR2 signaling.
Invasion by microbial pathogens at mucosal surfaces elicits the infiltration of PMNs, which subsequently use potent effector mechanisms such as phagocytosis, production of reactive oxygen species, and release of inflammatory mediators and antimicrobial agents to eliminate pathogens. Within this inflammatory milieu, PMNs detect the presence of microbial pathogens and their products via TLRs. Although it is clear that PMN migration is essential to host defense, it is unclear how microbial-derived TLR activation affects this process. Thus, we characterized the effects of TLR activation on PMN transmigration. In the present study, we found that the TLR2-specific agonist MALP-2 possesses potent inhibitory activity as low as 31 ng/ml on PMN migration. In contrast, the bacterially derived lipoprotein Pam3CSK4 inhibited PMN transmigration at a much higher concentration of 500 ng/ml. Thus, MALP-2 appears to display a higher inhibitory effect than Pam3CSK4. Furthermore, we also found that lipoprotein-free LPS recognized by TLR4 as high as 1000 ng/ml does not significantly inhibit PMN migration (data not shown), suggesting that this process may depend on lipopeptide and/or TLR2.
TLR2 is a pleiotropic receptor able to bind PAMPs as various as lipoteichoic acid of Gram-positive bacteria, yeast zymosan, or bacterial lipopeptides (4, 5). TLR2 needs to be associated in heterodimers to recognize some of its ligands such as lipopeptide whereby its specificity is controlled by TLR1 and TLR6 (32–34). Although it has been previously described that diacylated lipopeptides such as MALP-2 are recognized by the pair TLR2/TLR6, whereas triacylated lipopeptides such as Pam3CSK4 binds to TLR2/TLR1, the model appears to be much more complex than previously envisioned. Indeed, some lipopeptides are able to be recognized by TLR2 without TLR1 or TLR6 (35). Regardless of the PMN sensitivity differences between MALP-2 and Pam3CSK4, these results suggest that PMNs are able to sense ng/ml quantities of microbial products and further studies will determine the signaling pathways that drive PMN responses to MALP-2 and Pam3CSK4.
Previously, we and others have demonstrated that PMN transmigration across cell monolayers and matrix is inhibited by CD47 ligation by mAbs (12, 13, 24). However, the mechanisms of this inhibition have not been elucidated. In the present study, we explored the possible link between PMN recruitment and CD47 in the context of microbial invasion or TLR activation. Given that MALP-2 potently inhibited PMN transmigration, we specifically focused on TLR2 activation in our experiments. The results indicate that TLR2−/− and MyD88−/− PMN transmigration is not affected by CD47 ligation. Similarly, inhibition of MyD88 homodimerization reversed the inhibition of human PMN transmigration induced by CD47 ligation. In vitro activation of TLR2 did not enhance the PMN surface expression of CD47, in contrast to CD11b (36). Interestingly, CD11b ligation resulted in a delayed migratory response in TLR2−/− and MyD88−/− PMNs. Perhaps the sustained inhibition of PMN migration by CD11b requires costimulation of an intact TLR2 signaling pathway. Nevertheless, the inhibitory or delayed effect induced by anti-CD11b Abs was not different in TLR2−/− and MyD88−/− PMNs. Taken together, our results indicate that different mechanisms govern TLR2-CD47 and TLR2-CD11b signaling, and while further studies will need to elucidate the divergent role of TLR2 activation in regulating these two signaling pathways, our present results suggest that CD47 signaling may be triggered by TLR agonists. Previously, it was shown that heterodimerization of TLR2 with TLR1 or TLR6 induces MyD88 homodimerization (37). In our study we found that MyD88 was necessary for the inhibition of PMN transmigration by either MALP-2, or anti-CD47. Furthermore, anti-CD47 did not have any effect on MyD88−/− or TLR2−/− PMNs. Taken together, these results suggest that MyD88 is necessary for the crosstalk between TLR2 and CD47. MyD88 is a key adaptor used by all TLRs except TLR3. This adaptor activates NF-κB, JNK, and p38 through TRAF6 (38). If MyD88 is necessary for the cross-talk between TLR2 and CD47, this suggests that these molecules may take part in a signaling complex to enhance antimicrobial function. Indeed, cross-talk mechanisms between TLR2 and other sensor proteins have been described extensively (39). TLR2 may cooperate with other innate receptors such as NOD2 via protein kinase RICK (40) and dectin-1 through Syk kinase (41), display cross-talk with tyrosine kinases or phosphatases (39), interact with inhibitory TAM receptors (Tyro3, Axl, and Mer) or SHP1 (42, 43), or be modified by ubiquitination (39) such as A20, which is necessary to down-regulate the homeostatic signals initiated by commensal flora (44). Further investigations will focus on the molecular interactions between CD47, TLR2, and MyD88.
Currently, little is known about the signaling pathway(s) used by CD47. Past studies attempted to link CD47 signaling with pertussis toxin-sensitive Gi protein coupling (45). However, in our laboratory we were unable to link CD47-mediated inhibition of PMN transmigration with G proteins or with pertussis-sensitive signaling (data not shown). Furthermore, it was unclear whether our earlier observations were due to anti-CD47 Abs inhibiting PMN migration by blocking interactions with the surface or whether they were inducing cross-linking and activating CD47 signaling pathways. In the present report, we demonstrate the latter and that CD47-deficient PMNs exhibited enhanced transmigration as compared with wild-type PMNs. Therefore, we hypothesized that CD47 may be important in fine-tuning TLR2 signaling. To address this phenomenon, we determined whether CD47-deficient PMNs may exhibit decreased sensitivity to MALP-2. Indeed, the results shown herein demonstrate that CD47-deficient PMNs exhibit decreased sensitivity to MALP-2 and require 4-fold higher concentrations of MALP-2 to achieve inhibition of PMN transmigration. The impaired response exhibited by CD47−/− PMNs to MALP-2 shown in our study may provide a possible explanation of why CD47-deficient mice exhibit delayed PMN recruitment and succumb to bacterial peritonitis (23). In a similar manner, a recent study has reported decreased numbers of PMNs in the air spaces of CD47-deficient mice subjected to LPS-induced acute lung injury and Escherichia coli pneumonia (11). Furthermore, in a model of Staphylococcus aureus-induced arthritis, CD47−/− mice are also more resistant to arthritis probably because of the delayed migration of PMNs to the site of infection (10). Another previous study found that during infection of human brain microvascular endothelial cells by Cryptococcus neoformans, CD47 expression was increased between 4 and 12 h postinfection, suggesting that CD47 expression is indeed regulated in response to pathogens (46). Although a growing number of studies indicate that CD47 plays a key role in PMN infiltration, the mechanisms driving this response remain poorly understood. Based on our observations described herein, it is postulated that CD47 may somehow stabilize TLR2 expression at the cell surface. Additional experiments will need to determine whether CD47, TLR2, and MyD88 interact through an intermolecular manner or through intermediate signaling proteins.
In summary, this study demonstrates that TLR2 and CD47 exhibit cross-talk mechanisms through an MyD88-dependent manner to regulate PMN transmigration. We postulate that CD47 may act as a fine-tuning mechanism by regulating TLR2 function and ultimately PMN transmigration and antimicrobial function. Further characterization of CD47-mediated PMN transmigration may prove to be useful in generating therapeutic strategies aimed at controlling acute inflammatory conditions.
Acknowledgments
We thank Dr. Susan Voss for technical assistance, and Drs. Andrew Gewirtz and Melanie Sherman for providing the TLR2−/− and MyD88−/− mice.
Footnotes
Abbreviations used in this paper: PMN, neutrophil; PAMP, pathogen-associated molecular pattern
Disclosures
The authors have no financial conflict of interest.
References
- 1.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu. Rev. Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 2.Kumar NB, Nostrant TT, Appelman HD. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis) Am. J. Surg. Pathol. 1982;6:523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Parker LC, Whyte MK, Dower SK, Sabroe I. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J. Leukocyte Biol. 2005;77:886–892. doi: 10.1189/jlb.1104636. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill LA. Primer: Toll-like receptor signaling pathways: what do rheumatologists need to know? Nat. Clin. Pract. Rheumatol. 2008;4:319–327. doi: 10.1038/ncprheum0802. [DOI] [PubMed] [Google Scholar]
- 5.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat. Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 8.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Gao XP, Fan J, Liu Q, Anwar KN, Frey RS, Malik AB. LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am. J. Physiol. 2005;288:L655–L662. doi: 10.1152/ajplung.00327.2004. [DOI] [PubMed] [Google Scholar]
- 10.Verdrengh M, Lindberg FP, Ryden C, Tarkowski A. Integrin-associated protein (IAP)-deficient mice are less susceptible to developing Staphylococcus aureus-induced arthritis. Microbes Infect. 1999;1:745–751. doi: 10.1016/s1286-4579(99)80076-8. [DOI] [PubMed] [Google Scholar]
- 11.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J. Immunol. 2008;180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc. Natl. Acad. Sci. USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J. Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 15.Chung J, Gao AG, Frazier WA. Thrombspondin acts via integrin-associated protein to activate the platelet integrin αIIbβ3. J. Biol. Chem. 1997;272:14740–14746. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- 16.Dorahy DJ, Thorne RF, Fecondo JV, Burns GF. Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor. J. Biol. Chem. 1997;272:1323–1330. doi: 10.1074/jbc.272.2.1323. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, Lindberg FP, Vignery A. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J. Biol. Chem. 2000;275:37984–37992. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 18.Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP. CD47 signals T cell death. J. Immunol. 1999;162:7031–7040. [PubMed] [Google Scholar]
- 19.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J. Immunol. 2000;164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 20.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 21.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J. Biol. Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Wang XQ, Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with α2 β1 integrin in vascular smooth muscle cells. Mol. Biol. Cell. 1998;9:865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration: increased rate of migration correlates with increased cell surface expression of CD47. J. Biol. Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 25.Lee WY, Chin AC, Voss S, Parkos CA. In vitro neutrophil transepithelial migration. Methods Mol. Biol. 2006;341:205–215. doi: 10.1385/1-59745-113-4:205. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gramnegative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 27.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 28.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukocyte Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 29.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 30.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J. Clin. Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loiarro M, Sette C, Gallo G, Ciacci A, Fanto N, Mastroianni D, Carminati P, Ruggiero V. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J. Biol. Chem. 2005;280:15809–15814. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- 32.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Tolllike receptor 6. Int. Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 35.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J. Biol. Chem. 2006;281:9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 36.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MK. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 37.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukocyte Biol. 2008;83:692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 41.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An H, Hou J, Zhou J, Zhao W, Xu H, Zheng Y, Yu Y, Liu S, Cao X. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat. Immunol. 2008;9:542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 43.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 44.Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J. Exp. Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frazier WA, Gao AG, Dimitry J, Chung J, Brown EJ, Lindberg FP, Linder ME. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J. Biol. Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- 46.Jong A, Wu CH, Zhou W, Chen HM, Huang SH. Infectomic analysis of gene expression profiles of human brain microvascular endothelial cells infected with Cryptococcus neoformans. J. Biomed. Biotechnol. 2008 doi: 10.1155/2008/375620. 375620. [DOI] [PMC free article] [PubMed] [Google Scholar]