Summary
Antiviral drug resistance is an increasing concern in immunocompromised patient populations, where ongoing viral replication and prolonged drug exposure lead to the selection of resistant strains. Rapid diagnosis of resistance can be made by associating characteristic viral mutations with resistance to various drugs as determined by phenotypic assays. Management of drug resistance includes optimization of host factors and drug delivery, selection of alternative therapies based on knowledge of mechanisms of resistance, and the development of new antivirals. This article discusses drug resistance in herpesviruses and hepatitis B.
MeSH keywords: drug resistant, transplant, cytomegalovirus, herpes simplex virus, varicella zoster virus, hepatitis B virus
In the setting of intensive immunosuppression for the management of rejection in solid organ transplant (SOT) recipients, or graft-versus-host disease (GVHD) in hematopoietic stem cell transplant (HSCT) recipients, antiviral therapy is commonly used and drug resistant viruses are increasingly encountered. Prolonged antiviral drug exposure and ongoing viral replication due to immunosuppression are key factors in the development of antiviral drug resistance, which may manifest as persistent or increasing viremia or disease despite therapy. Consequences of drug resistance range from toxicity inherent in use of second-line antivirals, to severe disease and even death from progressive viral infection when no effective alternative treatments are available. In this article, we review the mechanisms, implications, and management of resistance to antiviral drugs used to treat several viral infections that play a significant role in the clinical course of transplant recipients and oncology patients: cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus (VZV), and hepatitis B virus (HBV).
Herpesviruses
Antiviral agents and mechanism of action
All of the currently licensed drugs for systemic therapy of herpesvirus infections share the same target, viral DNA polymerase. The most commonly used drugs are the nucleoside analogs acyclovir and ganciclovir. Acyclovir, its more bioavailable prodrug valacyclovir, and famciclovir (the prodrug of penciclovir) are used for HSV and VZV infections but have weak anti-CMV activity. Ganciclovir and its valine ester prodrug valganciclovir have in vitro activity against HSV, VZV, and CMV, and are FDA-approved for CMV infection, where antiviral potency outweighs the increased toxicity as compared with acyclovir.
Acyclovir is mono-phosphorylated by thymidine kinase (TK) expressed by HSV (UL23) or VZV (ORF36) and then converted by cellular kinases to the active form, acyclovir triphosphate. Acyclovir triphosphate inhibits HSV and VZV replication by competitive inhibition of viral DNA polymerase and by chain termination of viral DNA strands [1, 2]. Selectivity is related to preferential activation of acyclovir by viral TK and to the greater sensitivity of viral compared with cellular DNA polymerase to acyclovir triphosphate. Penciclovir, the active metabolite of famciclovir, has a similar mechanism of activation and action. Ganciclovir is mono-phosphorylated by the CMV UL97 kinase, or HSV or VZV TK, with subsequent antiviral action analogous to acyclovir. Unlike acyclovir, ganciclovir is not an obligate chain terminator, but rather causes a slowing and subsequent cessation of viral DNA chain elongation [3].
Foscarnet, a pyrophosphate analog, and cidofovir, a nucleotide analog, do not depend on prior activation by viral enzymes. Foscarnet binds selectively to viral DNA polymerase at the pyrophosphate-binding site, blocking cleavage of the pyrophosphate moiety from deoxynucleotide triphosphates, in turn halting DNA chain elongation. Cidofovir is phosphorylated by cellular enzymes, and once activated acts as a potent inhibitor of the viral DNA polymerase. Foscarnet and cidofovir are typically used as second- and third-line herpesvirus drugs respectively, when there is either suspected or documented resistance to initial therapy or dose-limiting toxicities of first-line drugs.
Use of these antiviral drugs may be affected by dose-limiting toxicities. While acyclovir is usually considered relatively nontoxic, high doses are associated with nephrotoxicity [4], and encephalopathy [5, 6]. High-dose valacyclovir has been associated with thrombotic microangiopathy in immunocompromised hosts [7]. Ganciclovir and valganciclovir frequently cause myelosuppression, especially neutropenia [8, 9]. Foscarnet is associated with significant nephrotoxicity and electrolyte abnormalities [10, 11]. Cidofovir is associated with nephrotoxicity and neutropenia when administered intravenously [12], and with application site irritation when administered topically [13].
In vitro evaluation of antiviral susceptibility
In vitro drug susceptibility testing of herpesviruses is by phenotypic and/or genotypic assays. Phenotypic assays measure drug susceptibility by culturing a calibrated viral inoculum under serial drug dilutions, thereby arriving at the drug concentration required to inhibit viral growth by 50 or 90% from the level observed without drug, referred to as the IC50 or IC90 respectively. The IC50 is the value usually reported because it is more reproducible than the IC90 value. The IC50 threshold for susceptible strains is assay-dependent, with the cutoff for sensitivity typically set at three to five times the mean IC50 for susceptible strains. In the classical plaque reduction assay (PRA), viral growth is measured as the number of visible plaques formed in cell culture monolayers after a fixed incubation period. The PRA is poorly standardized as to what constitutes a viral plaque, is labor-intensive and affected by a variety of culture conditions such as the type, density and growth phase of cells, the viral inoculum, and the drug concentration range. Efforts were made to standardize a PRA technique for CMV susceptibility testing [14], though in practice a great deal of variability remains, and the assay is clinically impractical because of the slow growth of CMV and the increasing use of molecular diagnostic assays that do not yield a live isolate for phenotypic testing. On the other hand, phenotypic testing for the more rapidly growing HSV is a preferred approach to resistance testing for this virus. In order to improve assay efficiency and reduce subjectivity, plaque counting can be replaced by viral quantitation methods that depend on assay of viral antigen or nucleic acid, or a reporter gene that is activated by viral infection. A reporter-based yield reduction system has been used for rapid phenotypic testing of HSV clinical isolates and laboratory strains [15].
Genotypic assays depend on knowledge of the viral mutations causing resistance to specific antiviral drugs and the level of resistance and cross-resistance conferred by single and multiple mutations. These assays work best when a limited number of characteristic mutations are regularly encountered in connection with resistance to a specific drug, and are well supported by an accessible information database necessary for accurate interpretation. Genotypic tests have a faster turnaround time than phenotype assays and use a common technology of PCR amplification of viral sequences followed by analysis for diagnostic mutations. A viral culture isolate is not needed and viral DNA can be amplified directly from blood, fluid or tissue specimens. Limitations of genotypic assays include difficulties with interpretation of viral sequence changes not found in the current information database, and the effective levels of resistance that result from combinations of mutations. There are also technical issues relating to DNA amplification and the sensitivity of detection of viral mutations when present as a minor subpopulation mixed with wild type virus.
Genotype-phenotype correlations are confirmed by recombinant phenotyping, also known as marker transfer, where individual mutations suspected of causing drug resistance are transferred to baseline viral strains and their effect on drug susceptibility is established by phenotypic assays. A large volume of this work has been done for CMV because of the dominant role of genotypic resistance testing for this virus. Recombinant phenotyping has also been done to determine the significance of various TK and DNA polymerase gene mutations for HSV and VZV drug resistance [16, 17], but given the number and variety of TK resistance mutations, resistance testing of HSV and VZV isolates is more reliant on phenotypic approaches.
Herpes Simplex Virus
Epidemiology of antiviral resistance
Acyclovir, valacyclovir and famciclovir are drugs of choice for mucocutaneous HSV infections and for preventive treatment, while intravenous acyclovir is used for serious invasive disease such as encephalitis. The first clinical cases of acyclovir-resistant HSV were reported in 1982, shortly after initial use of systemically-administered acyclovir [18, 19]. Despite the subsequent widespread use of acyclovir, clinically evident drug-resistance remains largely confined to the immunocompromised population, and the frequency of isolation of acyclovir-resistant HSV has remained stable over time [20]. Drug-resistant HSV disease is rare in immunocompetent hosts (less than 1% in various reports), and typically is cleared without adverse clinical outcome [20–24]. In immunocompromised hosts the prevalence ranges from 3.5% to 14%, with the most the most immunosuppressed subset having the highest risk for resistance [20–25]. Prolonged use of acyclovir is an important risk factor for resistant HSV, but drug-resistant HSV has been isolated in the absence of a known history of acyclovir exposure [26].
Mechanisms of resistance
Resistance of HSV to acyclovir is related to viral TK or DNA polymerase mutations [27]. As viral TK is not essential for HSV replication, over 90% of acyclovir resistance in clinical isolates is associated with TK mutations [28]. TK mutations may result in either a loss of TK activity (TK deleted or deficient virus) or, less commonly, an alteration in TK substrate specificity (TK altered virus) [28]. Mutations in the TK gene are often due to addition or deletion of nucleotides in homopolymer runs of guanines and cytosines, resulting in frameshifting and loss of TK function [29, 30]. The specific TK mutations resulting from penciclovir exposure differ from those selected by acyclovir, but cross-resistance is expected with TK deficient mutants, though certain acyclovir-resistant TK altered mutants appear to retain in vitro sensitivity to penciclovir [31]. Additionally, resistance to ganciclovir is presumed in the case of TK deficient mutants [32]. Drug-resistant TK mutants retain susceptibility to drugs that are not dependent on virally mediated phosphorylation, including foscarnet and cidofovir, unless a viral DNA polymerase mutation is also present. Given the essential role of viral DNA polymerase in viral replication, mutations in this gene occur less frequently and have been observed to cluster in functional domains II and III. The cross-resistance patterns of these mutations vary and are evaluated by recombinant phenotyping [3, 33].
Clinical implications and management of resistant virus
The clinical implications of antiviral-resistant HSV are related both to the direct effects of viral infection as well as the toxicities of second-line agents. Unchecked viral replication can lead to progressive and sometimes fatal invasive HSV disease [32, 34, 35]. Recurrent, chronic, and extensive mucocutaneous HSV ulcerations have been observed in immunocompromised individuals with drug-resistant virus [36]. Drug-resistant HSV has been associated with decreased neurovirulence in murine models when compared with wild-type virus [37, 38], with TK null mutants having the greatest reduction in virulence [39]. While previously thought to lack the ability to establish and reactivate from latency, it is now appreciated that TK null mutants may be able to do so by way of reversion, due to ribosomal frameshifting or replication errors that create subpopulations of TK altered virus [40, 41]. Human data for decreased pathogenicity of drug-resistant HSV are lacking.
In clinical practice, management of suspected or proven acyclovir-resistant HSV is generally with foscarnet, or less often with cidofovir. This is often done empirically based on the frequency of TK mutations, but cross-resistance may result from DNA polymerase mutations, and emergence of both foscarnet and cidofovir resistance while on therapy has been reported [36, 42]. Vidarabine, a purine analog phosphorylated by cellular kinases with selectivity for HSV DNA polymerase, has in vitro activity against HSV [43], but clinical experience has been disappointing for acyclovir-resistant HSV in the HIV-infected population [44]. Topical imiquimod, an immunomodulatory agent, or topical cidofovir have been used successfully to treat some cases of drug-resistant mucocutaneous HSV infection [45, 46]. Topical treatments avoid the potential nephrotoxicity of systemically administered foscarnet or cidofovir. Management of drug-resistant HSV should include efforts to improve the immune status of the patient, when possible, by decreasing immunosuppressive therapy.
Varicella Zoster Virus
Epidemiology of antiviral resistance
The same antiviral drugs are used for VZV as for HSV. Given that acyclovir has less potent activity against VZV than HSV, intravenous administration, frequent and high oral doses, or the more bioavailable oral prodrugs (valacyclovir or famciclovir) are needed to ensure therapeutic antiviral blood levels [47]. Acyclovir-resistant VZV clinical isolates have been reported uncommonly and mostly in the HIV population [48–51] with a few cases in oncology and transplant recipients [52, 53]. Unlike HSV, there are no large surveillance studies of antiviral drug-resistant VZV, and available information exists as case reports and series. Interestingly, there are two cases in the pediatric oncology literature of chronic disseminated varicella disease attributable to the VZV vaccine strain Oka with in vitro documentation of acyclovir resistance [54, 55].
Mechanisms of resistance
Like HSV, VZV also expresses a TK, and VZV drug resistance is for the most part attributable to TK mutations [3], which often result in a premature stop codon that makes the virus TK deficient. Other resistance mutations appear to cluster at particular VZV TK gene loci [3, 53]. Overall data, which are quite limited, suggest that acyclovir-resistance mutations in this gene are generally distinct from those conferring resistance to foscarnet and cidofovir [3]. Not much is known of penciclovir-resistant clinical isolates. While acyclovir and penciclovir may select in vitro for different patterns of cross-resistance to other antivirals, cross-resistance between the two drugs is expected [56].
Clinical implications and management of resistant virus
Similar to HSV, the clinical implications of drug-resistant VZV relate to the direct effects of viral replication and to the toxicities of alternative antiviral agents. Cases of visceral dissemination and death due to progressive VZV infection unresponsive to antiviral treatment were reported in HIV-infected subjects [51]. A chronic verrucous form of VZV is associated with drug-resistant virus in immunocompromised hosts [52, 55, 57, 58]. Some VZV DNA polymerase mutants selected under foscarnet in cell culture have a slow-growth phenotype [59], perhaps suggesting attenuated virulence, though this has not been clinically validated.
Management of suspected or proven acyclovir-resistant VZV is generally with foscarnet, as described mostly in HIV-infected individuals [51, 60], and some oncology patients [52, 54, 55]. Emergence of foscarnet resistance was detected in a few patients being treated with the drug for acyclovir-resistant VZV [60, 61], and attributed to a viral DNA polymerase mutation [61] While the literature on cidofovir treatment for drug-resistant VZV is very limited [62], cidofovir is expected to retain activity against acyclovir-resistant TK mutants [63]. Vidarabine shows in vitro activity against VZV DNA polymerase mutants [64], though clinical experience is limited [65]. Susceptibility testing of VZV isolates should be performed when drug resistance is suspected on clinical grounds, and any immunosuppressive therapy should be minimized.
Cytomegalovirus
Epidemiology of antiviral resistance
CMV is a well-recognized opportunistic pathogen in those with AIDS, in SOT and HSCT recipients, and occasionally in non-transplant oncology patients, particularly following major T-cell suppressive regimens [66]. Ganciclovir and valganciclovir are currently the principal drugs used for prevention and treatment of CMV infection and are widely used in transplant populations. Shortly following the introduction of ganciclovir in the late 1980s, cases of ganciclovir resistance in immunocompromised hosts began to appear in the literature [67]. Much of our knowledge about CMV drug resistance comes from studies of CMV retinitis in the AIDS population in the 1990s [68, 69]. More recently, studies have highlighted the problem in the SOT population [70–78]. The overall incidence of ganciclovir resistance among SOT recipients is 0% to 13%, and varies according to the type of organ transplant, the immunosuppressive regimen and antiviral prophylaxis used, and the specific criteria for determining resistance [79]. CMV seronegative recipients of organs from seropositive donors (D+/R− subset), those with prolonged ganciclovir exposure and potent immunosuppression, and lung transplant recipients are at higher risk for developing antiviral drug resistance. In the HSCT setting, the development of ganciclovir resistance is reported to be uncommon and generally limited to case reports and small case series [80–85], with the exception of the pediatric population where there have been reports of rapid emergence of resistance [86–88]; this may relate to less ganciclovir exposure in the HSCT population, where a preemptive as opposed to a prophylactic approach to CMV disease prevention is favored. Emergence of resistance to foscarnet and cidofovir has also been reported in the SOT and HSCT population [76, 80, 81, 86, 89–91].
Mechanism of resistance
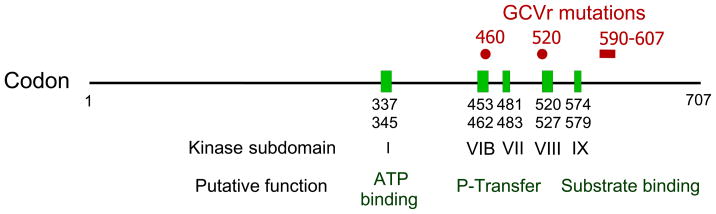
The literature on CMV drug resistance mutations is extensive [92–99], especially for ganciclovir. Over 90% of resistant CMV isolates obtained following ganciclovir exposure contain one or more characteristic mutations in the viral UL97 kinase gene [98], which apparently decrease the phosphorylation of ganciclovir without impairing the important functions of this kinase in viral replication [98, 100, 101]. Unlike the case with HSV TK mutations, CMV UL97 drug-resistance mutations cluster tightly at codons 460, 520, and 590–607 (Figure 1). Mutations M460V/I, H520Q, C592G, A594V, L595S, and C603W are among the most frequently encountered in ganciclovir-resistant isolates [98]. These mutations individually confer moderate ganciclovir resistance, with an IC50 ratio of 5 to 10, except for C592G which confers low-level ganciclovir resistance, with an IC50 ratio of about 2.5 [98]. These IC50 ratios are based on recombinant phenotyping data [99], which are also available for many other less common UL97 mutations. The accumulated genotype-phenotype correlations are the basis for the CMV genotypic resistance testing that is available in various commercial and academic laboratories.
Figure 1.
Map of CMV UL97 gene functional domains and resistance mutations. Ganciclovir resistance (GCVr) mutations are clustered at codons 460, 520 and 590–607. In the latter region mutations A594V, L595S, C592G and C603W are some of the most common, but a variety of point and in-frame deletion mutations are known to confer varying degrees of GCV resistance. Not all sequence changes at codons 590–607 confer ganciclovir resistance.
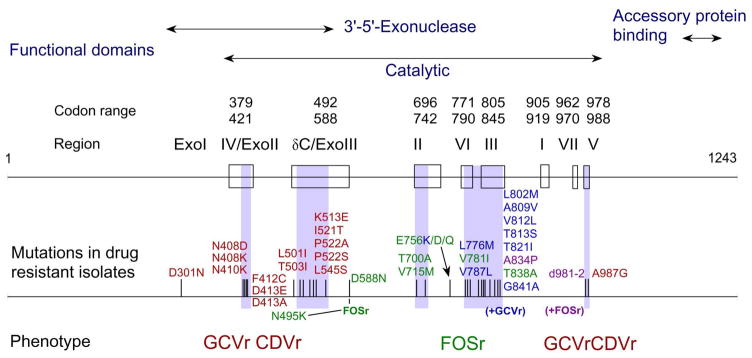
CMV UL54 DNA polymerase mutations can confer resistance to any or all of the current anti-CMV drugs Many ganciclovir resistance mutations are located in the exonuclease domains (Figure 2) and typically confer cross-resistance to cidofovir [92, 94]. Mutations in and between catalytic regions II (e.g., codons 700 and 715), III (e.g., codons 802 and 809), and VI (e.g., codon 781) and at some nonconserved loci (e.g., codon 756) confer foscarnet resistance, as well as low-grade ganciclovir or cidofovir cross-resistance in the case of mutations at region III [3, 92, 95]. Uncommonly, single UL54 mutations can confer simultaneous resistance to ganciclovir, cidofovir, and foscarnet [89, 94, 97]. The serial emergence of multiple mutations in patients on prolonged CMV antiviral therapy is well-documented [93, 102]. Typically, a UL97 mutation conferring ganciclovir appears first, followed by the addition of one or more UL54 polymerase mutations after prolonged therapy. The eventual phenotype of these isolates is often high-level resistance to ganciclovir, with additional resistance to foscarnet and/or cidofovir.
Figure 2.
Map of CMV DNA polymerase functional domains, resistance mutations, and associated phenotypes. All listed mutations have been found in clinical isolates and validated by recombinant phenotyping. Shaded regions indicate where resistance mutations are clustered, with associated phenotypes indicated below. Updated from [92].
GCVr = ganciclovir resistance. CDVr = cidofovir resistance. FOSr = foscarnet resistance.
Clinical implications and management of resistant virus
As with untreated CMV infection, the clinical consequences of infection with drug-resistant CMV range from asymptomatic to severe. While asymptomatic infection with drug- resistant virus has been noted especially in clinical antiviral trials for disease prevention [103], and persistent viremia without overt disease also occurs, severe or fatal disease has been reported more commonly in connection with drug-resistant CMV [75, 77, 80, 83, 84], probably because the host factors that predispose to serious CMV disease are the same as those that favor the emergence of drug resistance. There is insufficient evidence to assess the relative clinical virulence of wild type and drug-resistant CMV strains, even though a number of drug-resistant CMV DNA polymerase mutants have been reported to have a slow-growth phenotype in vitro [3, 80, 86, 96].
CMV drug resistance should be suspected in the setting of high or rising viral load and/or progressive CMV disease despite appropriate induction doses of antiviral therapy for at least 2 weeks, and with a history of cumulative antiviral drug exposure of at least 6 weeks, except in some pediatric settings as noted above. When resistance is suspected, laboratory testing for resistance should be pursued and immunosuppressive therapy should be minimized. It is important to note that there are no controlled studies to guide the treatment of drug-resistant CMV infection. The degree of drug resistance, the antiviral drug(s) and dose used, the competence of host immune response, and the site and extent of CMV disease all play a role in determining outcome.
In the absence of immediate, life- or sight-threatening CMV disease, selection of antiviral therapy should be guided by genotypic analysis of UL97 and UL54 genes. The degree of phenotypic resistance known to be associated with a particular gene mutation(s) has significant implications for choice of therapy. Low-grade ganciclovir resistance in the case of non life- or sight-threatening disease can potentially be addressed with higher dose IV ganciclovir [78, 79, 104, 105]. High-grade ganciclovir resistance with a major UL97 resistance mutation and suspected resistance in the case of life- or sight-threatening disease is best managed with foscarnet. Use of foscarnet is often complicated by nephrotoxicity, and long-term use is rarely tolerated. Cidofovir is another option for ganciclovir-resistant CMV, providing there is not a polymerase mutation conferring cross-resistance to ganciclovir and cidofovir. Significant nephrotoxicity has been associated with cidofovir use in HSCT recipients [106], however, the experience in SOT recipients is limited. Combination therapy with ganciclovir and foscarnet has been recommended for treatment of drug-resistant CMV infection, based on limited in vitro data [107] and a small case series advocating reduced-dose ganciclovir and escalating-dose foscarnet [108]. Despite the lack of controlled studies, combination treatment is a common practice in cases of documented multi-drug resistance or cases of life- or sight-threatening disease unresponsive to monotherapy.
Given the significant limitations of the currently available therapies for drug-resistant CMV infection, alternative agents, both investigational compounds and drugs currently licensed for other indications, have been studied for this indication. Maribavir, a benzimidazole riboside, is a potent inhibitor of the CMV UL97 kinase, an enzyme important in various aspects of CMV replication. Since maribavir inhibits UL97-mediated ganciclovir phosphorylation, it antagonizes the antiviral action of ganciclovir, but may have an additive anti-CMV effect when combined with foscarnet or cidofovir [109]. No cross-resistance has been observed between maribavir and other current anti-CMV drugs [110]. Maribavir-resistant laboratory CMV strains have been isolated in vitro [111, 112], and found to contain mutations in the UL97 and/or UL27 genes, which confer high- and low-grade resistance respectively [111–113]. Maribavir was successfully tested in Phase I and II trials, which suggested low toxicity and in vivo antiviral activity [114]. However, two Phase III trials as a CMV prophylactic agent in HSCT and liver transplant recipients did not meet expectations of antiviral efficacy at the dosing regimens chosen. Higher doses of maribavir could still be useful in treating drug-resistant CMV, though clinical experience to date is limited to several transplant recipients, some of whom may have benefited, but maribavir-resistant virus was isolated in one case [115].
Other experimental anti-CMV therapies are considerably less clinically developed than maribavir. Inhibitors of viral DNA cleavage and processing include tomeglovir (BAY-384766), and a benzimidazole D-riboside, GW-275175X, both of which underwent preliminary clinical studies to demonstrate tolerability, but neither one has proceeded to more advanced clinical trials [116]. In vitro resistance to tomeglovir maps to the CMV UL89, UL56 and UL104 genes [117], supporting the novel mechanism of action and expected lack of cross-resistance to current drugs. A lipid ester oral prodrug of cidofovir (hexadecyloxypropyl-CDV, or CMX001) has been shown to have in vitro and in vivo activity against CMV, with excellent oral bioavailability and minimal nephrotoxicity in preclinical studies [118–120]. This may offer a better alternative to the intravenous cidofovir formulation currently available. Cyclopropavir, a purine nucleoside analog, has been shown to have potent in vitro and in vivo activity against CMV [121, 122] but has not undergone clinical trials. While cyclopropavir appears to have a mechanism of action similar to ganciclovir, one study reported that some ganciclovir-resistant isolates exhibited only slightly reduced susceptibility to cyclopropavir [122]; more data are needed on the extent of cross-resistance between the two drugs.
Several drugs licensed for other indications and with no defined viral target appear to have anti-CMV activity, though clinical experience is limited to case reports, small case series, and retrospective cohort studies, with no controlled treatment data available. Their role in the treatment of drug-resistant CMV is unclear at this time but would likely be adjunctive to other antivirals. A number of retrospective studies in SOT recipients [123–129], as well as a few studies in HSCT recipients [130, 131], have demonstrated a lower incidence of CMV infection in patients who have received immunosuppressive regimens that included a target of rapamycin inhibitor (TOR-I), either sirolimus or everolimus. Leflunomide, an immunosuppressive drug with an indication for the treatment of rheumatoid arthritis, has been demonstrated to inhibit CMV replication in vitro and in a rat model [132]. Clinical data on the use of leflunomide for treatment of CMV infection in transplant recipients is mixed. When used as adjunctive therapy, a few successes have been reported in the treatment of drug-resistant CMV [81, 133]; however, leflunomide is associated with significant hematologic and hepatic toxicity, and treatment failures have been reported as well [134]. Lastly, the antimalarial drug artesunate has been shown to have inhibitory activity against CMV in vitro and in vivo [135, 136]. Artesunate appears to have additive effects with ganciclovir, foscarnet, and cidofovir [135]. There is one report of successful use in a HSCT recipient with foscarnet- and ganciclovir-resistant CMV infection [137].
Hepatitis B virus
Antiviral agents and mechanism of action
There are currently seven FDA-approved agents for the treatment of hepatitis B. Three are nucleoside analogs (lamivudine, entecavir, and telbivudine) and two are nucleotide analogs (adefovir and tenofovir). Alpha interferon, approved in 1992 for this indication, and more recently pegylated interferon, remains an important treatment option. Lastly, passive immunization with hepatitis B immune globulin (HBIG) remains a mainstay of therapy following liver transplantation, when it is used in combination with a nucleoside or nucleotide analog for the prevention of HBV recurrence [138, 139].
All of the nucleoside and nucleotide analogs selectively target HBV DNA polymerase, which includes reverse transcriptase activity. Drugs in this class are phosphorylated by cellular enzymes to active form and then incorporated into growing DNA, resulting in premature chain termination, amongst other inhibitory functions related to viral replication. While drug-related side effects are generally minimal with this class, adefovir is associated with nephrotoxicity in up to 12% of liver transplant recipients [140, 141], and caution is advised in patients receiving concomitant nephrotoxins. While these antiviral compounds are effective to varying degrees in providing long-term suppression, they do not eradicate HBV, which persists in hepatocytes in the form of covalently closed circular DNA (cccDNA) [142]. In vitro studies have demonstrated that antiviral therapy has little or no effect on cccDNA [143]. Therefore, treatment for chronic HBV infection is typically prolonged and issues of antiviral drug resistance become quite important.
Historically, sequential and combination therapy was used to treat chronic HBV infection, with changes made in response to the frequent emergence of antiviral drug resistance. More recently, because of higher potency and lower rates of resistance, entecavir and tenofovir have largely supplanted lamivudine and adefovir as preferred first-line agents for antiviral naïve individuals [144]. For the significant number of patients who have been successfully treated with lamivudine and adefovir, with undetectable serum HBV DNA, there is no recommendation to change therapy
In vitro evaluation of antiviral susceptibility
Genotypic resistance testing involves the detection of characteristic HBV polymerase gene mutations, which can be performed at varying levels of sensitivity using various broadly applicable methods, such as standard sequencing of PCR products, restriction fragment length polymorphism, reverse hybridization, and single genome sequencing [145]. Automated dideoxy sequencing is insensitive at detecting minor subpopulations of mutant virus that comprise <20% of the circulating virus population. The more sensitive assays can detect HBV DNA mutants that represent 5% to 10% of the entire HBV quasispecies, potentially allowing for earlier identification of genotypic resistance. With the advent of newer sequencing technologies, such as “ultra-deep” pyrosequencing, mutants comprising <1% of the viral pool can be identified and characterized [146]. The clinical utility and value of these more sensitive techniques remains to be determined. Genotypic testing is standard clinical practice as it is rapid and practical, but subject to the usual limitation that it cannot interpret novel or previously uncharacterized mutations and cannot directly assess such properties as the replication fitness of drug-resistant mutants.
Standardized phenotypic testing for HBV drug susceptibility has been limited by the absence of a cell culture system that allows fully permissive infection. A human hepatoma cell line maintained with DMSO and hydrocortisone to promote cell differentiation and phenotypic stability [147] has been developed as a means of comparing the relative antiviral susceptibility and growth fitness of HBV mutants [148]. Cell culture systems may involve transient transfection of HBV clones or construction of cell lines that permanently express drug-resistant mutants [149]. Alternatively, biochemical assays of expressed HBV polymerase have been used to assess inhibition by drug, independent of cell culture. Although current HBV recombinant phenotyping approaches may not accurately model viral replication in vivo, they are necessary for validating the interpretation of genotypic resistance testing data.
Epidemiology of antiviral resistance
Given the high viral replication rate and the error-prone nature of HBV reverse transcriptase, emergence of drug resistance is expected [150]. Drug resistance has been associated with a variety of patient and viral factors. Host factors that contribute to an increased risk for drug resistance include older age, high body mass index, medication noncompliance, immunosuppression, high pre-treatment HBV DNA levels, baseline hepatic enzyme elevations, and abundant replication space (large number of uninfected hepatocytes, as in a newly transplanted liver) [151–156]. The viral mutation frequency, the magnitude and rate of virus replication, and the overall replication fitness of the mutant are critical viral determinants in risk for drug resistance [157].
Apart from host and virus factors, the potency and genetic barrier to resistance of the antiviral drug is of critical importance in determining risk for drug resistance [150]. The genetic barrier reflects the number and type of mutations that must be accumulated in order for the virus to develop significant drug resistance while maintaining adequate growth. Lamivudine is an intermediate potency drug with a low genetic barrier to resistance, resulting in high resistance rates. Adefovir is a low potency drug with an intermediate genetic barrier to resistance, and therefore an intermediate rate of resistance. Telbivudine is a high potency drug, though with a low genetic barrier to resistance, and so resistance rates are intermediate. Lastly, entecavir and tenofovir are considered high potency antivirals, with a high genetic barrier to resistance, and therefore low rates of resistance. Among antiviral-naïve patients, drug resistance has been reported in up to 70% of patients treated with 5 years of lamivudine therapy, 29% after 5 years of adefovir, 20% after 2 years of telbivudine, and 1% after 5 years of entecavir [152, 158–162]. Resistance rates are significantly higher in patients with prior exposure to lamivudine, with rates of up to 18% at 1 year following switch to adefovir monotherapy and 51% at 5 years following switch to entecavir [150, 163].
Mechanism of resistance
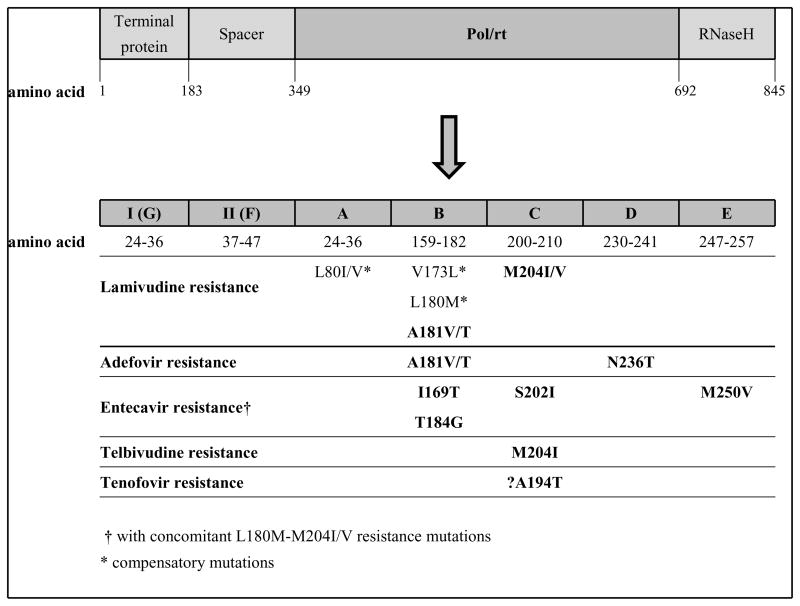
The HBV polymerase gene is the target for nucleoside and nucleotide analogs. The enzyme has four functional domains (terminal protein, spacer, Pol/rt, and RNaseH), with seven catalytic subdomains (A–G) in the Pol/rt region [150] (Figure 3). Antiviral drug-resistant strains have signature mutations in the reverse transcriptase domains of the viral polymerase gene, with most substitutions occurring in domains B, C, and D. Resistance mutations alter the interaction between HBV polymerase and drug [164]. Molecular modeling studies of the interaction of wild-type and mutant HBV polymerase with natural thymidine triphosphate substrate and with anti-HBV agents highlight the important conformational changes in mutants that confer drug-resistance [165]. While the interaction of each nucleoside or nucleotide analog with HBV polymerase appears to be mechanistically unique with regard to binding affinity and shifting after ligand attachment, all drug-resistant mutants seem to exhibit either altered binding of substrate and/or downstream structural changes that interfere with the inhibitory effect of drug on viral polymerase. After emergence of primary resistance mutations, compensatory mutations that restore replication capacity may arise, as well as secondary resistance mutations that increase drug resistance when they accumulate on the same viral genome.
Figure 3.
Map of HBV polymerase gene functional domains (terminal protein, spacer, Pol/rt = polymerase/reverse transcriptase, RNaseH), catalytic subdomains (A–G), and resistance mutations.
High-level lamivudine resistance is most often caused by mutations M204I/V, which are in the YMDD (tyrosine-methionine-aspartate-aspartate) motif in the C domain of the polymerase gene [166], and infrequently by A181V/T mutations [167]. M204V is almost always accompanied by compensatory mutations L180M and/or V173L, resulting in restored fitness of the mutant [166, 168]. The M204I mutation confers high-level cross-resistance to telbivudine, but M204I/V mutations do not appear to reduce susceptibility to adefovir and tenofovir [162, 169]. The signature mutation associated with telbivudine resistance is M204I, either alone or in association with the secondary mutations L80I/V or L180M [162].
N236T and A181V/T are adefovir-resistance mutations [159, 170]. Although the resistance conferred by these mutations is less than that associated with M204I/V and lamivudine resistance, virological breakthrough is seen [169–171]. The N236T mutation reduces viral replicative capacity in vitro and confers cross-resistance to tenofovir but not to lamivudine or telbivudine [172].
Resistance to entecavir appears to occur though a 2-hit mechanism, whereby classic lamivudine-resistant mutants (L180M, M204I/V) are selected in patients on lamivudine, or, less frequently, in patients on primary therapy with entecavir [173]. During continued entecavir treatment, additional mutations at I169T and M250V or T184G and S202I are selected, conferring resistance to entecavir [174–176].
Resistance to tenofovir currently appears to be unusual [177], though more experience with this drug for treatment of chronic HBV is needed. There is a report of virologic breakthrough on tenofovir in two HBV/HIV coinfected patients with prior lamivudine exposure (L180M-M204V mutations) and an A194T mutation [178]. However, A194T was not shown to confer resistance to tenofovir in vitro [179], suggesting that it may instead be a viral sequence polymorphism or a lamivudine compensatory mutation [180].
Clinical implications and management of resistant virus
With the availability of safe and effective oral HBV antiviral agents in the late 1990s and the switch from HBIG monotherapy to combination therapy (HBIG plus antivirals) in HBV-infected liver transplant recipients, HBV recurrence rates have decreased significantly [181–183]. Antiviral drug resistance remains an important factor in HBV reinfection after liver transplantation. Clinical consequences of the emergence of drug-resistant HBV range from asymptomatic viremia to serum transaminase flares, worsening liver histology, hepatic decompensation and occasionally death [152, 171, 184]. Drug resistance is associated with virologic breakthrough [185], defined as an increase in serum HBV DNA by at least 1.0 log10 (10-fold) above nadir or the reappearance of serum HBV DNA with previously undetectable HBV DNA on ≥2 occasions at least 1 month apart while on treatment and after initial response is achieved in a medication-compliant patient [145]. Ultimately, biochemical breakthrough, defined by elevation of hepatic transaminase values (hepatitis flare), occurs.
Genotypic resistance testing is important because not all virologic breakthrough is attributable to drug resistance. As many as 30% to 50% of viral breakthroughs observed in clinical trials are due to medication noncompliance [145], a figure likely to be higher in clinical practice. When virologic breakthrough is associated with the emergence of resistance mutation(s), the inferred cross-resistance phenotype is used to develop a timely plan of action, such as a change to another drug or combination therapy [186].
Management of lamivudine-resistant virus has involved the addition of adefovir, a strategy which has been shown to result in high rates of virologic suppression and a lower rate emergence of adefovir resistance than sequential monotherapy [186, 187]. Tenofovir, a potent antiviral drug with excellent activity against lamivudine-resistant virus, appears to be superior to adefovir monotherapy for treatment of lamivudine-resistant virus [188]; comparison of tenofovir with combination adefovir-lamivudine in this setting, however, has not yet been reported in large-scale clinical studies. Entecavir is not a good option for lamivudine-resistant virus given the observed emergence of resistance [176, 189]. Telbivudine resistance is associated with the M204I mutation, and while there is in vitro data demonstrating telbivudine activity against the M204V lamivudine-resistant mutant [190], clinical data are not available at this time. Telbuvidine should not be relied upon for treatment of lamivudine-resistant virus, and management of telbivudine resistance should be similar to management of lamivudine resistance.
Management of adefovir-resistant virus is dependent upon the type of mutation(s) and the antiviral drug history of the patient. Lamivudine has proven effective in suppressing adefovir-resistant HBV with the N236T mutation [170, 191], and it is presumed that telbivudine would also be effective based on in vitro data [190]. The durability of response in patients with previous lamivudine resistance, however, is unclear, with report of re-emergence of lamivudine resistance after reintroduction of drug [192]. There are in vitro data to suggest that entecavir may be a reasonable choice for N236T mutants [193], with the caveat that the benefit may be short-lived in patients with prior lamivudine resistance. For patients with the N236T mutation, options include switching to or adding entecavir, adding lamivudine (or telbivudine), or switching to tenofovir. The activity of lamivudine (and likely telbivudine) against the A181V adefovir-resistant mutants is decreased compared with wild type HBV [167]. While the A181T mutant has been shown in vitro to have decreased susceptibility to tenofovir [167], in the clinical setting entecavir and tenofovir have been effective in suppressing replication of A181T adefovir-resistant mutants [194, 195]. In the case of an A181T mutation, management options include switching to or adding entecavir, or switching to tenofovir; lamivudine should not be used in this scenario given the risk of cross-resistance [167].
There are no large-scale clinical studies yet available to guide the treatment of entecavir-resistant HBV. From in vitro data and case reports it appears that adefovir and tenofovir are effective for entecavir-resistant HBV [176, 196, 197]. Based for the most part on expert opinion, a recommended approach for entecavir-resistant virus is to add tenofovir or adefovir [145, 150, 194]. Data on management of tenofovir-resistant HBV is not yet available, given the low rate of resistance observed with early use of this drug.
Emtricitabine, a potent nucleoside analog that is currently FDA approved for the treatment of HIV, is currently in late phase clinical trial for management of chronic HBV [198]. At the target treatment dose of 200mg daily, resistance to emtricitabine was observed in 9% of treatment-naïve patients at 1 year and rose to 20% after 2 years [198]. Emtricitabine resistance is conferred by the M204I/V mutation with or without the accompanying L180M and V173L mutations, therefore implying cross-resistance to lamivudine and telbivudine.
The future
As antiviral therapy becomes widely used in immunosuppressed patient populations, concerns about drug resistance will require a better understanding of the relevant virus, host and drug-related factors. Knowledge of genetic mechanisms and associated viral mutations has allowed for development of genotypic techniques for the timely diagnosis of resistance. The accuracy of this testing will be improved by recombinant phenotyping data that validates the drug resistance properties associated with the many viral sequence changes detected in clinical specimens. An accessible and authoritative database of drug resistance mutations needs to be available for each virus in order to guide therapeutic decisions. More comprehensive information on the epidemiologic, host and drug exposure factors that favor the emergence of resistant virus can be used to develop better strategies for prevention, early detection and appropriate treatment change. Ideally, controlled trials are needed to compare sequential and combination use of alternative therapies, optimize dosing schedules, and evaluate adjunctive therapies that seek to improve host conditions for antiviral drug efficacy. There is an ongoing need for less toxic but potent new antiviral drugs that preferably target different aspects of viral replication to reduce the risk of cross-resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynne Strasfeld, Email: strasfel@ohsu.edu, Division of Infectious Diseases, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, mail code L457, Portland, Oregon 97239, phone: 503-418-0136.
Sunwen Chou, Email: chous@ohsu.edu, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, mail code L457, Portland, Oregon 97239, phone: 503-273-5115.
References
- 1.Elion GB, Furman PA, Fyfe JA, et al. The selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Reproduced from Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1002/(sici)1099-1654(199907/09)9:3<147::aid-rmv255>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]; Rev Med Virol. 1999;9(3):147–52. doi: 10.1002/(sici)1099-1654(199907/09)9:3<147::aid-rmv255>3.0.co;2-p. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- 2.Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. J Med Virol. 1993;(Suppl 1):2–6. doi: 10.1002/jmv.1890410503. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C, Bestman-Smith J, Boivin G. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Updat. 2002;5(2):88–114. doi: 10.1016/s1368-7646(02)00021-3. [DOI] [PubMed] [Google Scholar]
- 4.Wagstaff AJ, Faulds D, Goa KL. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47(1):153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Haefeli WE, Schoenenberger RA, Weiss P, et al. Acyclovir-induced neurotoxicity: concentration-side effect relationship in acyclovir overdose. Am J Med. 1993;94(2):212–5. doi: 10.1016/0002-9343(93)90186-s. [DOI] [PubMed] [Google Scholar]
- 6.Lowance D, Neumayer HH, Legendre CM, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N Engl J Med. 1999;340(19):1462–70. doi: 10.1056/NEJM199905133401903. [DOI] [PubMed] [Google Scholar]
- 7.Ormrod D, Scott LJ, Perry CM. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs. 2000;59(4):839–63. doi: 10.2165/00003495-200059040-00013. [DOI] [PubMed] [Google Scholar]
- 8.Kalil AC, Freifeld AG, Lyden ER, et al. Valganciclovir for cytomegalovirus prevention in solid organ transplant patients: an evidence-based reassessment of safety and efficacy. PLoS One. 2009;4(5):e5512. doi: 10.1371/journal.pone.0005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crumpacker CS. Ganciclovir. N Engl J Med. 1996;335(10):721–9. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- 10.Wagstaff AJ, Bryson HM. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48(2):199–226. doi: 10.2165/00003495-199448020-00007. [DOI] [PubMed] [Google Scholar]
- 11.Deray G, Martinez F, Katlama C, et al. Foscarnet nephrotoxicity: mechanism, incidence and prevention. Am J Nephrol. 1989;9(4):316–21. doi: 10.1159/000167987. [DOI] [PubMed] [Google Scholar]
- 12.Safrin S, Cherrington J, Jaffe HS. Clinical uses of cidofovir. Rev Med Virol. 1997;7(3):145–56. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Lalezari J, Schacker T, Feinberg J, et al. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J Infect Dis. 1997;176(4):892–8. doi: 10.1086/516542. [DOI] [PubMed] [Google Scholar]
- 14.Landry ML, Stanat S, Biron K, et al. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob Agents Chemother. 2000;44(3):688–92. doi: 10.1128/aac.44.3.688-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leary JJ, Wittrock R, Sarisky RT, et al. Susceptibilities of herpes simplex viruses to penciclovir and acyclovir in eight cell lines. Antimicrob Agents Chemother. 2002;46(3):762–8. doi: 10.1128/AAC.46.3.762-768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frobert E, Ooka T, Cortay JC, et al. Herpes simplex virus thymidine kinase mutations associated with resistance to acyclovir: a site-directed mutagenesis study. Antimicrob Agents Chemother. 2005;49(3):1055–9. doi: 10.1128/AAC.49.3.1055-1059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzutani T, Saijo M, Nagamine M, et al. Rapid phenotypic characterization method for herpes simplex virus and Varicella-Zoster virus thymidine kinases to screen for acyclovir-resistant viral infection. J Clin Microbiol. 2000;38(5):1839–44. doi: 10.1128/jcm.38.5.1839-1844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns WH, Saral R, Santos GW, et al. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982;1(8269):421–3. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- 19.Sibrack CD, Gutman LT, Wilfert CM, et al. Pathogenicity of acyclovir-resistant herpes simplex virus type 1 from an immunodeficient child. J Infect Dis. 1982;146(5):673–82. doi: 10.1093/infdis/146.5.673. [DOI] [PubMed] [Google Scholar]
- 20.Stranska R, Schuurman R, Nienhuis E, et al. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J Clin Virol. 2005;32(1):7–18. doi: 10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Nugier F, Colin JN, Aymard M, et al. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol. 1992;36(1):1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- 22.Englund JA, Zimmerman ME, Swierkosz EM, et al. Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990;112(6):416–22. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- 23.Christophers J, Clayton J, Craske J, et al. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob Agents Chemother. 1998;42(4):868–72. doi: 10.1128/aac.42.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danve-Szatanek C, Aymard M, Thouvenot D, et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42(1):242–9. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakrabarti S, Pillay D, Ratcliffe D, et al. Resistance to antiviral drugs in herpes simplex virus infections among allogeneic stem cell transplant recipients: risk factors and prognostic significance. J Infect Dis. 2000;181(6):2055–8. doi: 10.1086/315524. [DOI] [PubMed] [Google Scholar]
- 26.Malvy D, Treilhaud M, Bouee S, et al. A retrospective, case-control study of acyclovir resistance in herpes simplex virus. Clin Infect Dis. 2005;41(3):320–6. doi: 10.1086/431585. [DOI] [PubMed] [Google Scholar]
- 27.Coen DM, Schaffer PA. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980;77(4):2265–9. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26(1):29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 29.Morfin F, Souillet G, Bilger K, et al. Genetic characterization of thymidine kinase from acyclovir-resistant and -susceptible herpes simplex virus type 1 isolated from bone marrow transplant recipients. J Infect Dis. 2000;182(1):290–3. doi: 10.1086/315696. [DOI] [PubMed] [Google Scholar]
- 30.Gaudreau A, Hill E, Balfour HH, Jr, et al. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J Infect Dis. 1998;178(2):297–303. doi: 10.1086/515626. [DOI] [PubMed] [Google Scholar]
- 31.Bacon TH, Levin MJ, Leary JJ, et al. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16(1):114–28. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljungman P, Ellis MN, Hackman RC, et al. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J Infect Dis. 1990;162(1):244–8. doi: 10.1093/infdis/162.1.244. [DOI] [PubMed] [Google Scholar]
- 33.Bestman-Smith J, Boivin G. Drug resistance patterns of recombinant herpes simplex virus DNA polymerase mutants generated with a set of overlapping cosmids and plasmids. J Virol. 2003;77(14):7820–9. doi: 10.1128/JVI.77.14.7820-7829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longerich T, Eisenbach C, Penzel R, et al. Recurrent herpes simplex virus hepatitis after liver retransplantation despite acyclovir therapy. Liver Transpl. 2005;11(10):1289–94. doi: 10.1002/lt.20567. [DOI] [PubMed] [Google Scholar]
- 35.Frangoul H, Wills M, Crossno C, et al. Acyclovir-resistant herpes simplex virus pneumonia post-unrelated stem cell transplantation: a word of caution. Pediatr Transplant. 2007;11(8):942–4. doi: 10.1111/j.1399-3046.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Scieux C, Garrait V, et al. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin Infect Dis. 2000;31(4):927–35. doi: 10.1086/314052. [DOI] [PubMed] [Google Scholar]
- 37.Coen DM, Kosz-Vnenchak M, Jacobson JG, et al. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989;86(12):4736–40. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrei G, Fiten P, Froeyen M, et al. DNA polymerase mutations in drug-resistant herpes simplex virus mutants determine in vivo neurovirulence and drug-enzyme interactions. Antivir Ther. 2007;12(5):719–32. [PubMed] [Google Scholar]
- 39.Coen DM. Acyclovir-resistant, pathogenic herpesviruses. Trends Microbiol. 1994;2(12):481–5. doi: 10.1016/0966-842x(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 40.Griffiths A, Chen SH, Horsburgh BC, et al. Translational compensation of a frameshift mutation affecting herpes simplex virus thymidine kinase is sufficient to permit reactivation from latency. J Virol. 2003;77(8):4703–9. doi: 10.1128/JVI.77.8.4703-4709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffiths A, Link MA, Furness CL, et al. Low-level expression and reversion both contribute to reactivation of herpes simplex virus drug-resistant mutants with mutations on homopolymeric sequences in thymidine kinase. J Virol. 2006;80(13):6568–74. doi: 10.1128/JVI.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyles DL, Patel A, Madinger N, et al. Development of herpes simplex virus disease in patients who are receiving cidofovir. Clin Infect Dis. 2005;41(5):676–80. doi: 10.1086/432477. [DOI] [PubMed] [Google Scholar]
- 43.De Clercq E. The antiviral spectrum of (E)-5-(2-bromovinyl)-2′-deoxyuridine. J Antimicrob Chemother. 1984;14(Suppl A):85–95. doi: 10.1093/jac/14.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- 44.Safrin S, Crumpacker C, Chatis P, et al. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1991;325(8):551–5. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- 45.Martinez V, Molina JM, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HSV infection. Am J Med. 2006;119(5):e9–11. doi: 10.1016/j.amjmed.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 46.Sims CR, Thompson K, Chemaly RF, et al. Oral topical cidofovir: novel route of drug delivery in a severely immunosuppressed patient with refractory multidrug-resistant herpes simplex virus infection. Transpl Infect Dis. 2007;9(3):256–9. doi: 10.1111/j.1399-3062.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 47.Biron KK, Elion GB. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980;18(3):443–7. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boivin G, Edelman CK, Pedneault L, et al. Phenotypic and genotypic characterization of acyclovir-resistant varicella-zoster viruses isolated from persons with AIDS. J Infect Dis. 1994;170(1):68–75. doi: 10.1093/infdis/170.1.68. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1990;112(3):187–91. doi: 10.7326/0003-4819-112-3-187. [DOI] [PubMed] [Google Scholar]
- 50.Saint-Leger E, Caumes E, Breton G, et al. Clinical and virologic characterization of acyclovir-resistant varicella-zoster viruses isolated from 11 patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2001;33(12):2061–7. doi: 10.1086/324503. [DOI] [PubMed] [Google Scholar]
- 51.Breton G, Fillet AM, Katlama C, et al. Acyclovir-resistant herpes zoster in human immunodeficiency virus-infected patients: results of foscarnet therapy. Clin Infect Dis. 1998;27(6):1525–7. doi: 10.1086/515045. [DOI] [PubMed] [Google Scholar]
- 52.Crassard N, Souillet AL, Morfin F, et al. Acyclovir-resistant varicella infection with atypical lesions in a non-HIV leukemic infant. Acta Paediatr. 2000;89(12):1497–9. doi: 10.1080/080352500456732. [DOI] [PubMed] [Google Scholar]
- 53.Morfin F, Thouvenot D, De Turenne-Tessier M, et al. Phenotypic and genetic characterization of thymidine kinase from clinical strains of varicella-zoster virus resistant to acyclovir. Antimicrob Agents Chemother. 1999;43(10):2412–6. doi: 10.1128/aac.43.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus, in an immunosuppressed child. J Infect Dis. 2003;188(7):954–9. doi: 10.1086/378502. [DOI] [PubMed] [Google Scholar]
- 55.Bryan CJ, Prichard MN, Daily S, et al. Acyclovir-resistant chronic verrucous vaccine strain varicella in a patient with neuroblastoma. Pediatr Infect Dis J. 2008;27(10):946–8. doi: 10.1097/INF.0b013e318175d85c. [DOI] [PubMed] [Google Scholar]
- 56.Andrei G, De Clercq E, Snoeck R. In vitro selection of drug-resistant varicella-zoster virus (VZV) mutants (OKA strain): differences between acyclovir and penciclovir? Antiviral Res. 2004;61(3):181–7. doi: 10.1016/j.antiviral.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Pahwa S, Biron K, Lim W, et al. Continuous varicella-zoster infection associated with acyclovir resistance in a child with AIDS. JAMA. 1988;260(19):2879–82. [PubMed] [Google Scholar]
- 58.Linnemann CC, Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4(6):577–9. doi: 10.1097/00002030-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 59.Visse B, Huraux JM, Fillet AM. Point mutations in the varicella-zoster virus DNA polymerase gene confers resistance to foscarnet and slow growth phenotype. J Med Virol. 1999;59(1):84–90. [PubMed] [Google Scholar]
- 60.Safrin S, Berger TG, Gilson I, et al. Foscarnet therapy in five patients with AIDS and acyclovir-resistant varicella-zoster virus infection. Ann Intern Med. 1991;115(1):19–21. doi: 10.7326/0003-4819-115-1-19. [DOI] [PubMed] [Google Scholar]
- 61.Visse B, Dumont B, Huraux JM, et al. Single amino acid change in DNA polymerase is associated with foscarnet resistance in a varicella-zoster virus strain recovered from a patient with AIDS. J Infect Dis. 1998;178(Suppl 1):S55–7. doi: 10.1086/514257. [DOI] [PubMed] [Google Scholar]
- 62.Schliefer K, Gumbel HO, Rockstroh JK, et al. Management of progressive outer retinal necrosis with cidofovir in a human immunodeficiency virus-infected patient. Clin Infect Dis. 1999;29(3):684–5. doi: 10.1086/598656. [DOI] [PubMed] [Google Scholar]
- 63.Snoeck R, Andrei G, De Clercq E. Novel agents for the therapy of varicella-zoster virus infections. Expert Opin Investig Drugs. 2000;9(8):1743–51. doi: 10.1517/13543784.9.8.1743. [DOI] [PubMed] [Google Scholar]
- 64.Kamiyama T, Kurokawa M, Shiraki K. Characterization of the DNA polymerase gene of varicella-zoster viruses resistant to acyclovir. J Gen Virol. 2001;82(Pt 11):2761–5. doi: 10.1099/0022-1317-82-11-2761. [DOI] [PubMed] [Google Scholar]
- 65.Reusser P, Cordonnier C, Einsele H, et al. European survey of herpesvirus resistance to antiviral drugs in bone marrow transplant recipients. Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 1996;17(5):813–7. [PubMed] [Google Scholar]
- 66.Laurenti L, Piccioni P, Cattani P, et al. Cytomegalovirus reactivation during alemtuzumab therapy for chronic lymphocytic leukemia: incidence and treatment with oral ganciclovir. Haematologica. 2004;89(10):1248–52. [PubMed] [Google Scholar]
- 67.Erice A, Chou S, Biron KK, et al. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989;320(5):289–93. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- 68.Jabs DA, Enger C, Dunn JP, et al. Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. CMV Retinitis and Viral Resistance Study Group. J Infect Dis. 1998;177(3):770–3. doi: 10.1086/514249. [DOI] [PubMed] [Google Scholar]
- 69.Jabs DA, Enger C, Forman M, et al. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. The Cytomegalovirus Retinitis and Viral Resistance Study Group. Antimicrob Agents Chemother. 1998;42(9):2240–4. doi: 10.1128/aac.42.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy AJ, Zaas AK, Hanson KE, et al. A single-center experience with ganciclovir-resistant cytomegalovirus in lung transplant recipients: treatment and outcome. J Heart Lung Transplant. 2007;26(12):1286–92. doi: 10.1016/j.healun.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Li F, Kenyon KW, Kirby KA, et al. Incidence and clinical features of ganciclovir-resistant cytomegalovirus disease in heart transplant recipients. Clin Infect Dis. 2007;45(4):439–47. doi: 10.1086/519941. [DOI] [PubMed] [Google Scholar]
- 72.Limaye AP, Raghu G, Koelle DM, et al. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis. 2002;185(1):20–7. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- 73.Limaye AP, Corey L, Koelle DM, et al. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000;356(9230):645–9. doi: 10.1016/S0140-6736(00)02607-6. [DOI] [PubMed] [Google Scholar]
- 74.Bhorade SM, Lurain NS, Jordan A, et al. Emergence of ganciclovir-resistant cytomegalovirus in lung transplant recipients. J Heart Lung Transplant. 2002;21(12):1274–82. doi: 10.1016/s1053-2498(02)00463-1. [DOI] [PubMed] [Google Scholar]
- 75.Eid AJ, Arthurs SK, Deziel PJ, et al. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant. 2008;22(2):162–70. doi: 10.1111/j.1399-0012.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 76.Lurain NS, Bhorade SM, Pursell KJ, et al. Analysis and characterization of antiviral drug-resistant cytomegalovirus isolates from solid organ transplant recipients. J Infect Dis. 2002;186(6):760–8. doi: 10.1086/342844. [DOI] [PubMed] [Google Scholar]
- 77.Isada CM, Yen-Lieberman B, Lurain NS, et al. Clinical characteristics of 13 solid organ transplant recipients with ganciclovir-resistant cytomegalovirus infection. Transpl Infect Dis. 2002;4(4):189–94. doi: 10.1034/j.1399-3062.2002.t01-1-02008.x. [DOI] [PubMed] [Google Scholar]
- 78.Kruger RM, Shannon WD, Arens MQ, et al. The impact of ganciclovir-resistant cytomegalovirus infection after lung transplantation. Transplantation. 1999;68(9):1272–9. doi: 10.1097/00007890-199911150-00010. [DOI] [PubMed] [Google Scholar]
- 79.Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis. 2002;35(7):866–72. doi: 10.1086/342385. [DOI] [PubMed] [Google Scholar]
- 80.Marfori JE, Exner MM, Marousek GI, et al. Development of new cytomegalovirus UL97 and DNA polymerase mutations conferring drug resistance after valganciclovir therapy in allogeneic stem cell recipients. J Clin Virol. 2007;38(2):120–5. doi: 10.1016/j.jcv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Avery RK, Bolwell BJ, Yen-Lieberman B, et al. Use of leflunomide in an allogeneic bone marrow transplant recipient with refractory cytomegalovirus infection. Bone Marrow Transplant. 2004;34(12):1071–5. doi: 10.1038/sj.bmt.1704694. [DOI] [PubMed] [Google Scholar]
- 82.Erice A, Borrell N, Li W, et al. Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J Infect Dis. 1998;178(2):531–4. doi: 10.1086/517467. [DOI] [PubMed] [Google Scholar]
- 83.Hamprecht K, Eckle T, Prix L, et al. Ganciclovir-resistant cytomegalovirus disease after allogeneic stem cell transplantation: pitfalls of phenotypic diagnosis by in vitro selection of an UL97 mutant strain. J Infect Dis. 2003;187(1):139–43. doi: 10.1086/346240. [DOI] [PubMed] [Google Scholar]
- 84.Julin JE, van Burik JH, Krivit W, et al. Ganciclovir-resistant cytomegalovirus encephalitis in a bone marrow transplant recipient. Transpl Infect Dis. 2002;4(4):201–6. doi: 10.1034/j.1399-3062.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- 85.Seo SK, Regan A, Cihlar T, et al. Cytomegalovirus ventriculoencephalitis in a bone marrow transplant recipient receiving antiviral maintenance: clinical and molecular evidence of drug resistance. Clin Infect Dis. 2001;33(9):e105–8. doi: 10.1086/323022. [DOI] [PubMed] [Google Scholar]
- 86.Springer KL, Chou S, Li S, et al. How evolution of mutations conferring drug resistance affects viral dynamics and clinical outcomes of cytomegalovirus-infected hematopoietic cell transplant recipients. J Clin Microbiol. 2005;43(1):208–13. doi: 10.1128/JCM.43.1.208-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eckle T, Lang P, Prix L, et al. Rapid development of ganciclovir-resistant cytomegalovirus infection in children after allogeneic stem cell transplantation in the early phase of immune cell recovery. Bone Marrow Transplant. 2002;30(7):433–9. doi: 10.1038/sj.bmt.1703666. [DOI] [PubMed] [Google Scholar]
- 88.Prix L, Hamprecht K, Holzhuter B, et al. Comprehensive restriction analysis of the UL97 region allows early detection of ganciclovir-resistant human cytomegalovirus in an immunocompromised child. J Infect Dis. 1999;180(2):491–5. doi: 10.1086/314877. [DOI] [PubMed] [Google Scholar]
- 89.Scott GM, Weinberg A, Rawlinson WD, et al. Multidrug resistance conferred by novel DNA polymerase mutations in human cytomegalovirus isolates. Antimicrob Agents Chemother. 2007;51(1):89–94. doi: 10.1128/AAC.00633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez J, Casper K, Smallwood G, et al. Resistance to combined ganciclovir and foscarnet therapy in a liver transplant recipient with possible dual-strain cytomegalovirus coinfection. Liver Transpl. 2007;13(10):1396–400. doi: 10.1002/lt.21245. [DOI] [PubMed] [Google Scholar]
- 91.Oshima K, Kanda Y, Kako S, et al. Case report: persistent cytomegalovirus (CMV) infection after haploidentical hematopoietic stem cell transplantation using in vivo alemtuzumab: emergence of resistant CMV due to mutations in the UL97 and UL54 genes. J Med Virol. 2008;80(10):1769–75. doi: 10.1002/jmv.21277. [DOI] [PubMed] [Google Scholar]
- 92.Chou S, Lurain NS, Thompson KD, et al. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J Infect Dis. 2003;188(1):32–9. doi: 10.1086/375743. [DOI] [PubMed] [Google Scholar]
- 93.Chou S, Marousek G, Guentzel S, et al. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J Infect Dis. 1997;176(3):786–9. doi: 10.1086/517302. [DOI] [PubMed] [Google Scholar]
- 94.Chou S, Marousek G, Li S, et al. Contrasting drug resistance phenotypes resulting from cytomegalovirus DNA polymerase mutations at the same exonuclease locus. J Clin Virol. 2008;43(1):107–9. doi: 10.1016/j.jcv.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chou S, Marousek G, Parenti DM, et al. Mutation in region III of the DNA polymerase gene conferring foscarnet resistance in cytomegalovirus isolates from 3 subjects receiving prolonged antiviral therapy. J Infect Dis. 1998;178(2):526–30. doi: 10.1086/515648. [DOI] [PubMed] [Google Scholar]
- 96.Chou S, Marousek GI, Van Wechel LC, et al. Growth and drug resistance phenotypes resulting from cytomegalovirus DNA polymerase region III mutations observed in clinical specimens. Antimicrob Agents Chemother. 2007;51(11):4160–2. doi: 10.1128/AAC.00736-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chou S, Miner RC, Drew WL. A deletion mutation in region V of the cytomegalovirus DNA polymerase sequence confers multidrug resistance. J Infect Dis. 2000;182(6):1765–8. doi: 10.1086/317618. [DOI] [PubMed] [Google Scholar]
- 98.Chou S, Waldemer RH, Senters AE, et al. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J Infect Dis. 2002;185(2):162–9. doi: 10.1086/338362. [DOI] [PubMed] [Google Scholar]
- 99.Chou S, Van Wechel LC, Lichy HM, et al. Phenotyping of cytomegalovirus drug resistance mutations by using recombinant viruses incorporating a reporter gene. Antimicrob Agents Chemother. 2005;49(7):2710–5. doi: 10.1128/AAC.49.7.2710-2715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prichard MN, Gao N, Jairath S, et al. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J Virol. 1999;73(7):5663–70. doi: 10.1128/jvi.73.7.5663-5670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolf DG, Courcelle CT, Prichard MN, et al. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci U S A. 2001;98(4):1895–900. doi: 10.1073/pnas.98.4.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith IL, Cherrington JM, Jiles RE, et al. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176(1):69–77. doi: 10.1086/514041. [DOI] [PubMed] [Google Scholar]
- 103.Boivin G, Goyette N, Gilbert C, et al. Clinical impact of ganciclovir-resistant cytomegalovirus infections in solid organ transplant patients. Transpl Infect Dis. 2005;7(3–4):166–70. doi: 10.1111/j.1399-3062.2005.00112.x. [DOI] [PubMed] [Google Scholar]
- 104.Cytomegalovirus. Am J Transplant. 2004;4(Suppl 10):51–8. doi: 10.1111/j.1600-6135.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 105.West P, Schmiedeskamp M, Neeley H, et al. Use of high-dose ganciclovir for a resistant cytomegalovirus infection due to UL97 mutation. Transpl Infect Dis. 2008;10(2):129–32. doi: 10.1111/j.1399-3062.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- 106.Ljungman P, Deliliers GL, Platzbecker U, et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2001;97(2):388–92. doi: 10.1182/blood.v97.2.388. [DOI] [PubMed] [Google Scholar]
- 107.Drew WL. Is combination antiviral therapy for CMV superior to monotherapy? J Clin Virol. 2006;35(4):485–8. doi: 10.1016/j.jcv.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 108.Mylonakis E, Kallas WM, Fishman JA. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin Infect Dis. 2002;34(10):1337–41. doi: 10.1086/340101. [DOI] [PubMed] [Google Scholar]
- 109.Chou S, Marousek GI. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob Agents Chemother. 2006;50(10):3470–2. doi: 10.1128/AAC.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drew WL, Miner RC, Marousek GI, et al. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J Clin Virol. 2006;37(2):124–7. doi: 10.1016/j.jcv.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Chou S, Wechel LC, Marousek GI. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J Infect Dis. 2007;196(1):91–4. doi: 10.1086/518514. [DOI] [PubMed] [Google Scholar]
- 112.Chou S, Marousek GI, Senters AE, et al. Mutations in the human cytomegalovirus UL27 gene that confer resistance to maribavir. J Virol. 2004;78(13):7124–30. doi: 10.1128/JVI.78.13.7124-7130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chou S, Marousek GI. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J Virol. 2008;82(1):246–53. doi: 10.1128/JVI.01787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403–10. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Avery RK, Marty FM, Strasfeld L, et al. Oral maribavir (MBV) for treatment of resistant or refractory cytomegalovirus (CMV) infection in transplant recipients [abstract 1108]. Programs and abstracts of the 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco. 2009. [Google Scholar]
- 116.Lischka P, Zimmermann H. Antiviral strategies to combat cytomegalovirus infections in transplant recipients. Curr Opin Pharmacol. 2008;8(5):541–8. doi: 10.1016/j.coph.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Buerger I, Reefschlaeger J, Bender W, et al. A novel nonnucleoside inhibitor specifically targets cytomegalovirus DNA maturation via the UL89 and UL56 gene products. J Virol. 2001;75(19):9077–86. doi: 10.1128/JVI.75.19.9077-9086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ciesla SL, Trahan J, Wan WB, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59(3):163–71. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 119.Quenelle DC, Collins DJ, Pettway LR, et al. Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus (MCMV) or human cytomegalovirus (HCMV) in animal models. Antiviral Res. 2008;79(2):133–5. doi: 10.1016/j.antiviral.2008.01.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quenelle DC, Collins DJ, Wan WB, et al. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48(2):404–12. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kern ER, Bidanset DJ, Hartline CB, et al. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob Agents Chemother. 2004;48(12):4745–53. doi: 10.1128/AAC.48.12.4745-4753.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kern ER, Kushner NL, Hartline CB, et al. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob Agents Chemother. 2005;49(3):1039–45. doi: 10.1128/AAC.49.3.1039-1045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Webster AC, Lee VW, Chapman JR, et al. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev. 2006;2:CD004290. doi: 10.1002/14651858.CD004290.pub2. [DOI] [PubMed] [Google Scholar]
- 124.Hill JA, Hummel M, Starling RC, et al. A lower incidence of cytomegalovirus infection in de novo heart transplant recipients randomized to everolimus. Transplantation. 2007;84(11):1436–42. doi: 10.1097/01.tp.0000290686.68910.bd. [DOI] [PubMed] [Google Scholar]
- 125.Kobashigawa JA, Miller LW, Russell SD, et al. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6(6):1377–86. doi: 10.1111/j.1600-6143.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 126.Buchler M, Caillard S, Barbier S, et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant. 2007;7(11):2522–31. doi: 10.1111/j.1600-6143.2007.01976.x. [DOI] [PubMed] [Google Scholar]
- 127.Haririan A, Morawski K, West MS, et al. Sirolimus exposure during the early post-transplant period reduces the risk of CMV infection relative to tacrolimus in renal allograft recipients. Clin Transplant. 2007;21(4):466–71. doi: 10.1111/j.1399-0012.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 128.Demopoulos L, Polinsky M, Steele G, et al. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40(5):1407–10. doi: 10.1016/j.transproceed.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 129.San Juan R, Aguado JM, Lumbreras C, et al. Impact of current transplantation management on the development of cytomegalovirus disease after renal transplantation. Clin Infect Dis. 2008;47(7):875–82. doi: 10.1086/591532. [DOI] [PubMed] [Google Scholar]
- 130.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–36. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 131.Marty FM, Bryar J, Browne SK, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110(2):490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Waldman WJ, Knight DA, Blinder L, et al. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42(5–6):412–8. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- 133.Levi ME, Mandava N, Chan LK, et al. Treatment of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. Transpl Infect Dis. 2006;8(1):38–43. doi: 10.1111/j.1399-3062.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 134.Battiwalla M, Paplham P, Almyroudis NG, et al. Leflunomide failure to control recurrent cytomegalovirus infection in the setting of renal failure after allogeneic stem cell transplantation. Transpl Infect Dis. 2007;9(1):28–32. doi: 10.1111/j.1399-3062.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 135.Kaptein SJ, Efferth T, Leis M, et al. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res. 2006;69(2):60–9. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 136.Efferth T, Romero MR, Wolf DG, et al. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47(6):804–11. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- 137.Shapira MY, Resnick IB, Chou S, et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis. 2008;46(9):1455–7. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 138.Tung BY, Kowdley KV. Hepatitis B and liver transplantation. Clin Infect Dis. 2005;41(10):1461–6. doi: 10.1086/497129. [DOI] [PubMed] [Google Scholar]
- 139.Marzano A, Salizzoni M, Debernardi-Venon W, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001;34(6):903–10. doi: 10.1016/s0168-8278(01)00080-0. [DOI] [PubMed] [Google Scholar]
- 140.Schiff ER, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38(6):1419–27. doi: 10.1016/j.hep.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 141.Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007;13(3):349–60. doi: 10.1002/lt.20981. [DOI] [PubMed] [Google Scholar]
- 142.Wong DK, Yuen MF, Yuan H, et al. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatology. 2004;40(3):727–37. doi: 10.1002/hep.20353. [DOI] [PubMed] [Google Scholar]
- 143.Moraleda G, Saputelli J, Aldrich CE, et al. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71(12):9392–9. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6(12):1315–41. doi: 10.1016/j.cgh.2008.08.021. quiz 286. [DOI] [PubMed] [Google Scholar]
- 145.Lok AS. How to diagnose and treat hepatitis B virus antiviral drug resistance in the liver transplant setting. Liver Transpl. 2008;14(Suppl 2):S8–S14. doi: 10.1002/lt.21616. [DOI] [PubMed] [Google Scholar]
- 146.Margeridon-Thermet S, Shulman NS, Ahmed A, et al. Ultra-deep pyrosequencing of hepatitis B virus quasispecies from nucleoside and nucleotide reverse-transcriptase inhibitor (NRTI)-treated patients and NRTI-naive patients. J Infect Dis. 2009;199(9):1275–85. doi: 10.1086/597808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gripon P, Rumin S, Urban S, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99(24):15655–60. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Villet S, Billioud G, Pichoud C, et al. In vitro characterization of viral fitness of therapy-resistant hepatitis B variants. Gastroenterology. 2009;136(1):168–76. e2. doi: 10.1053/j.gastro.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 149.Zoulim F. In vitro models for studying hepatitis B virus drug resistance. Semin Liver Dis. 2006;26(2):171–80. doi: 10.1055/s-2006-939759. [DOI] [PubMed] [Google Scholar]
- 150.Yuen MF, Fung J, Wong DK, et al. Prevention and management of drug resistance for antihepatitis B treatment. Lancet Infect Dis. 2009;9(4):256–64. doi: 10.1016/S1473-3099(09)70056-8. [DOI] [PubMed] [Google Scholar]
- 151.Fung SK, Chae HB, Fontana RJ, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44(2):283–90. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 152.Lai CL, Dienstag J, Schiff E, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36(6):687–96. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 153.Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30(5):1302–6. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 154.Litwin S, Toll E, Jilbert AR, et al. The competing roles of virus replication and hepatocyte death rates in the emergence of drug-resistant mutants: theoretical considerations. J Clin Virol. 2005;34(Suppl 1):S96–S107. doi: 10.1016/s1386-6532(05)80018-6. [DOI] [PubMed] [Google Scholar]
- 155.Chang ML, Chien RN, Yeh CT, et al. Virus and transaminase levels determine the emergence of drug resistance during long-term lamivudine therapy in chronic hepatitis B. J Hepatol. 2005;43(1):72–7. doi: 10.1016/j.jhep.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 156.Zoulim F, Poynard T, Degos F, et al. A prospective study of the evolution of lamivudine resistance mutations in patients with chronic hepatitis B treated with lamivudine. J Viral Hepat. 2006;13(4):278–88. doi: 10.1111/j.1365-2893.2005.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Locarnini S, Warner N. Major causes of antiviral drug resistance and implications for treatment of hepatitis B virus monoinfection and coinfection with HIV. Antivir Ther. 2007;12(Suppl 3):H15–23. [PubMed] [Google Scholar]
- 158.Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125(6):1714–22. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 159.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131(6):1743–51. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 160.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–14. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 161.Nguyen MH, Keeffe EB. Chronic hepatitis B: early viral suppression and long-term outcomes of therapy with oral nucleos(t)ides. J Viral Hepat. 2009;16(3):149–55. doi: 10.1111/j.1365-2893.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 162.Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136(2):486–95. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 163.Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43(6):1385–91. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 164.Das K, Xiong X, Yang H, et al. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC) J Virol. 2001;75(10):4771–9. doi: 10.1128/JVI.75.10.4771-4779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sharon A, Chu CK. Understanding the molecular basis of HBV drug resistance by molecular modeling. Antiviral Res. 2008;80(3):339–53. doi: 10.1016/j.antiviral.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Allen MI, Deslauriers M, Andrews CW, et al. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27(6):1670–7. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- 167.Villet S, Pichoud C, Billioud G, et al. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol. 2008;48(5):747–55. doi: 10.1016/j.jhep.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 168.Delaney WEt, Yang H, Westland CE, et al. The hepatitis B virus polymerase mutation rtV173L is selected during lamivudine therapy and enhances viral replication in vitro. J Virol. 2003;77(21):11833–41. doi: 10.1128/JVI.77.21.11833-11841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang H, Qi X, Sabogal A, et al. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir Ther. 2005;10(5):625–33. [PubMed] [Google Scholar]
- 170.Angus P, Vaughan R, Xiong S, et al. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology. 2003;125(2):292–7. doi: 10.1016/s0016-5085(03)00939-9. [DOI] [PubMed] [Google Scholar]
- 171.Fung SK, Andreone P, Han SH, et al. Adefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensation. J Hepatol. 2005;43(6):937–43. doi: 10.1016/j.jhep.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 172.Yang H, Westland C, Xiong S, et al. In vitro antiviral susceptibility of full-length clinical hepatitis B virus isolates cloned with a novel expression vector. Antiviral Res. 2004;61(1):27–36. doi: 10.1016/j.antiviral.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 173.Kobashi H, Fujioka S, Kawaguchi M, et al. Two cases of development of entecavir resistance during entecavir treatment for nucleoside-naive chronic hepatitis B. Hepatol Int. 2009;3(2):403–10. doi: 10.1007/s12072-008-9108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tenney DJ, Levine SM, Rose RE, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48(9):3498–507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Langley DR, Walsh AW, Baldick CJ, et al. Inhibition of hepatitis B virus polymerase by entecavir. J Virol. 2007;81(8):3992–4001. doi: 10.1128/JVI.02395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tenney DJ, Rose RE, Baldick CJ, et al. Two-year assessment of entecavir resistance in Lamivudine-refractory hepatitis B virus patients reveals different clinical outcomes depending on the resistance substitutions present. Antimicrob Agents Chemother. 2007;51(3):902–11. doi: 10.1128/AAC.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 178.Sheldon J, Camino N, Rodes B, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10(6):727–34. [PubMed] [Google Scholar]
- 179.Delaney WEt, Ray AS, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50(7):2471–7. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fung S, Mazzulli T, Sherman M, et al. Presence of rtA194T at baseline does not reduce efficacy to tenofovir (TDF) in patients with lamivudine (LAM)-resistant chronic hepatitis B [abstract 880]. Programs and abstracts of the 58th Annual Meeting of the American Association for the Study of Liver Disease; San Francisco. 2008. [Google Scholar]
- 181.Markowitz JS, Martin P, Conrad AJ, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28(2):585–9. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 182.Gane EJ, Angus PW, Strasser S, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132(3):931–7. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 183.Angus PW, Patterson SJ, Strasser SI, et al. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology. 2008;48(5):1460–6. doi: 10.1002/hep.22524. [DOI] [PubMed] [Google Scholar]
- 184.Bock CT, Tillmann HL, Torresi J, et al. Selection of hepatitis B virus polymerase mutants with enhanced replication by lamivudine treatment after liver transplantation. Gastroenterology. 2002;122(2):264–73. doi: 10.1053/gast.2002.31015. [DOI] [PubMed] [Google Scholar]
- 185.Fournier C, Zoulim F. Antiviral therapy of chronic hepatitis B: prevention of drug resistance. Clin Liver Dis. 2007;11(4):869–92. ix. doi: 10.1016/j.cld.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 186.Lampertico P, Vigano M, Manenti E, et al. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42(6):1414–9. doi: 10.1002/hep.20939. [DOI] [PubMed] [Google Scholar]
- 187.Fung J, Lai CL, Yuen JC, et al. Adefovir dipivoxil monotherapy and combination therapy with lamivudine for the treatment of chronic hepatitis B in an Asian population. Antivir Ther. 2007;12(1):41–6. [PubMed] [Google Scholar]
- 188.van Bommel F, Wunsche T, Mauss S, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40(6):1421–5. doi: 10.1002/hep.20464. [DOI] [PubMed] [Google Scholar]
- 189.Sherman M, Yurdaydin C, Sollano J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130(7):2039–49. doi: 10.1053/j.gastro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 190.Seifer M, Patty A, Serra I, et al. Telbivudine, a nucleoside analog inhibitor of HBV polymerase, has a different in vitro cross-resistance profile than the nucleotide analog inhibitors adefovir and tenofovir. Antiviral Res. 2009;81(2):147–55. doi: 10.1016/j.antiviral.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 191.Villeneuve JP, Durantel D, Durantel S, et al. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol. 2003;39(6):1085–9. doi: 10.1016/j.jhep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 192.Yim HJ, Hussain M, Liu Y, et al. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44(3):703–12. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 193.Brunelle MN, Jacquard AC, Pichoud C, et al. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology. 2005;41(6):1391–8. doi: 10.1002/hep.20723. [DOI] [PubMed] [Google Scholar]
- 194.Fung SK, Fontana RJ. Management of drug-resistant chronic hepatitis B. Clin Liver Dis. 2006;10(2):275–302. viii. doi: 10.1016/j.cld.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 195.Trojan J, Stuermer M, Teuber G, et al. Treatment of patients with lamivudine-resistant and adefovir dipivoxil-resistant chronic hepatitis B virus infection: is tenofovir the answer? Gut. 2007;56(3):436–7. doi: 10.1136/gut.2006.108928. author reply 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yatsuji H, Hiraga N, Mori N, et al. Successful treatment of an entecavir-resistant hepatitis B virus variant. J Med Virol. 2007;79(12):1811–7. doi: 10.1002/jmv.20981. [DOI] [PubMed] [Google Scholar]
- 197.Villet S, Ollivet A, Pichoud C, et al. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J Hepatol. 2007;46(3):531–8. doi: 10.1016/j.jhep.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 198.Gish RG, Trinh H, Leung N, et al. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J Hepatol. 2005;43(1):60–6. doi: 10.1016/j.jhep.2005.02.017. [DOI] [PubMed] [Google Scholar]