Abstract
The ability to precisely quantify rare populations of cells has become an essential first step of many cell-based assays in stem cell research. Since current devices for cell quantification require relatively high cell concentrations and/or absolute cell numbers, we have developed a microchannel-based device, allowing precise quantification of limiting cell numbers/concentrations. We anticipate this device will serve as an important tool to overcome a practical obstacle in stem cell research.
Determination of the viable cell number or cell concentration of a given sample is a critical step for virtually all biological experiments. In most laboratories, viable cell numbers are quantified using a device such as a hemacytometer, flow cytometer, capillary-loaded chamber, or Coulter Counter (1–7). Although these devices do provide an accurate and reliable method for counting cells, relatively high cell concentrations are required to make such measurements. For example, a minimum concentration of approximately 10,000 cells/mL in the original sample is required to make an accurate measurement using a hemacytometer. Consequently, samples with small absolute cell numbers are often suspended in very small volumes in order to achieve an effective cell concentration for measurement. However, even then, a significant fraction of the total cells is required to simply perform the measurement, reducing the number of cells left for experimental use. For samples consisting of very small cell numbers, use of currently available devices is therefore impractical, and this restricts the type of analyses that can be done. For example, in most, if not all, assays of somatic stem cell activity, rare cell populations are isolated from their respective tissues, and are then transplanted or cultured in limiting cell dilutions (8). The stem cell frequency of these stem/progenitor cell–enriched populations is then estimated from their ability to produce outgrowths at very low cell numbers. It is therefore critical that the initial cell numbers are estimated precisely prior to these assays to prevent erroneous results and possible misinterpretation.
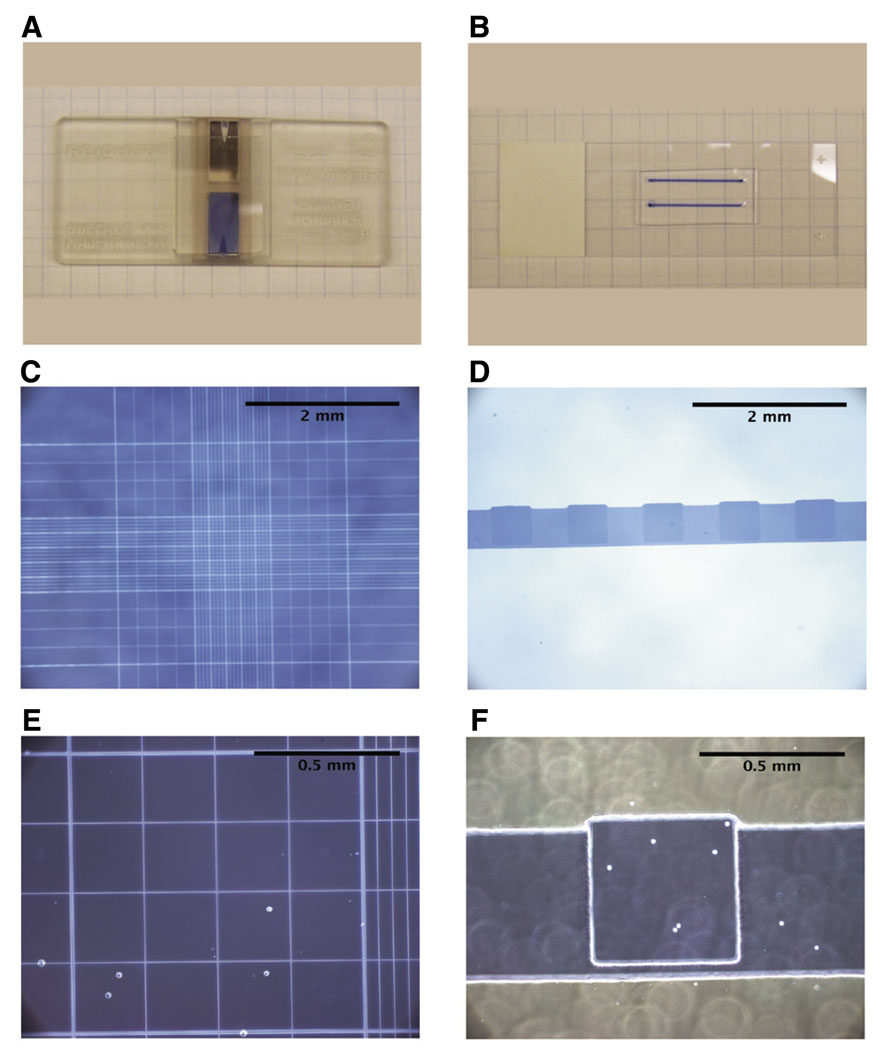
In order to improve quantification of small cell numbers at low concentrations, we have developed a microchannel device (for review of microchannels, see References 9 and 10) in which a minimum concentration of approximately 1000 cells/mL can be measured (Figure 1). The microcounter device was constructed of polydimethylsiloxane (PDMS) using a standard photolithographic and micromolding procedure (11) (see Supplementary Material available online at www.BioTechniques.com for process and channel details). This platform provides an advantage over current conventional devices, since very small volumes, specifically 1 µl, are used for each measurement. Once the cells are placed in the microchannel, the cells are manually counted using phase-contrast microscopy, and the concentration of cells/µl volume is determined. To further aid in visualization of the cells during counting, a grid pattern of 20 0.5 mm squares was molded into the ceiling of the microcounter device (Figure 1, E and F). Since every cell in the 1 µl volume of cells in the microcounter device is counted, no mathematical adjustments are required to estimate the cell concentration. However, as with most cell quantification devices, the concentration of the sample may be adjusted for any dilutions made during counting, including addition of a viability determining dye, such as Trypan blue (Figure 1).
Figure 1. The microcounter device is a versatile tool to enumerate rare populations of cells.
(A) Photographic representation of a typical hemacytometer. (B) Photographic image of two microcounter devices on a standard microscope slide. (C, E) Representative microscopic images of 293 cells loaded on a standard hemacytometer. (D, F) Representative microscopic images of 293 cells loaded in the microcounter device. Images depicted in C and D are 40×, images in E and F are 100× magnification.
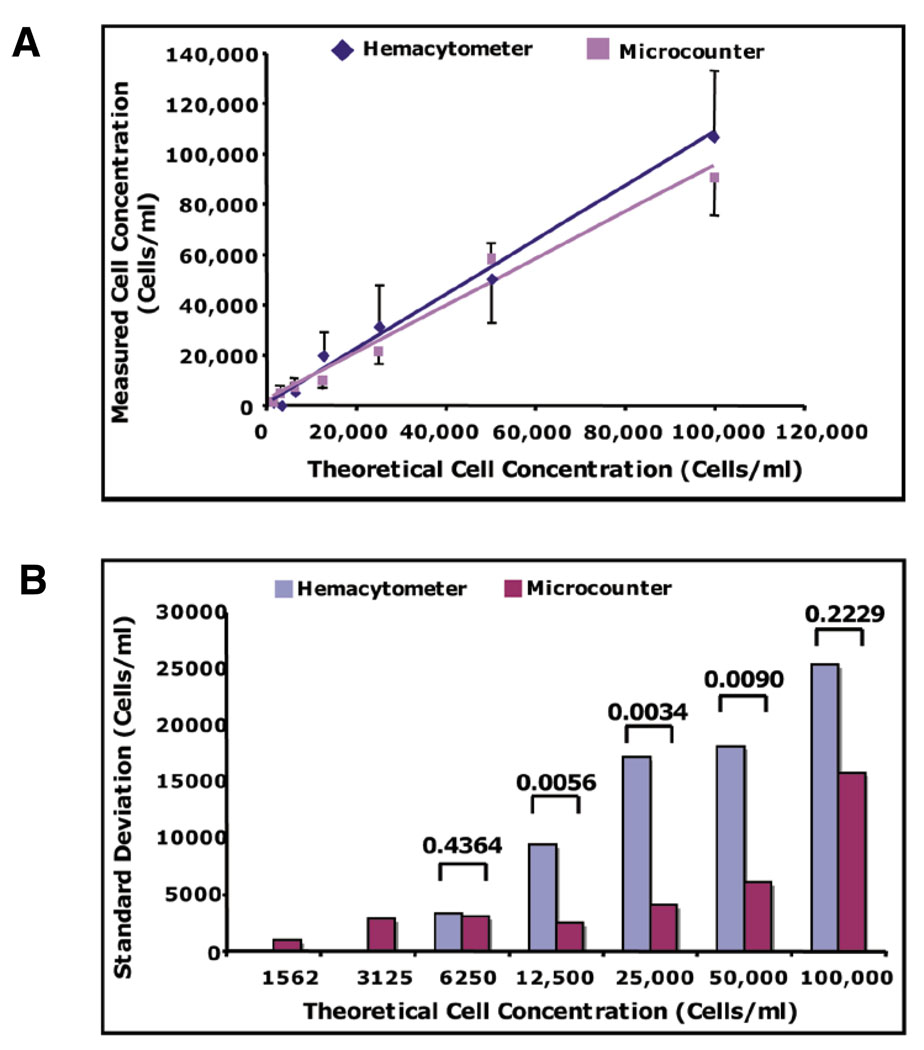
To test the accuracy and precision of our device to count small cell numbers/concentrations, as compared with current counting devices, we measured various cell concentrations of the human 293 epithelial cell line (ATCC, Manassas, VA, USA) in our microcounter device and a standard hemacytometer (Figure 1, C–F). The cells were initially removed from culture by brief trypsin treatment (Invitrogen, Carlsbad, CA, USA), counted with a hemacytometer, and the concentration was adjusted to approximately 100,000 cells/mL by addition of culture media. The cells were then serially diluted 2-fold in culture media and counted using both counting devices, where at least six independent measurements for each device were taken for each cell dilution. As shown in Figure 2, A and B, the effective range for the hemacytometer was limited to 6250 cells/mL, whereas the microcounter was able to accurately measure as low as 1562 cells/mL. In terms of absolute cell number, these results indicate that the lowest possible minimum threshold for the hemacytometer is 62 cells (10 µl sample), whereas the lowest possible minimum threshold for the microcounter is 1–2 cells (1 µl sample).
Figure 2. The microcounter device is able to detect smaller concentrations of cells with significantly less variance than a hemacytometer.
(A) Graphical depiction of the mean measured cell concentrations of 1520–100,000 cells/mL + sd (standard deviation). (B) sd of the means of each measured cell concentration for each device. The sd (cells/mL) were determined from six independent measurements of each theoretical cell concentration. P values were calculated from multiple comparison F-ratios following analysis of variance (ANOVA) for repeated measures.
The variance, depicted as standard deviation, of all measurements by the hemacytometer increased as the theoretical cell concentration increased, as expected (Figure 2B; see also Supplementary Table S1). However, since the variance of the microcounter device remained relatively unchanged with each measurement up to 100,000 cells/mL, this shows that the precision of counting with this device actually increases as the cell concentration increases up to 100,000 cells/mL. The observed increase in precision of the microcounter device is most likely due to the quantification of every cell in each test sample volume. In contrast, only a representative subset of cells, localized to a specific field, are actually enumerated with a hemacytometer, resulting in larger variability for each measurement made. Therefore, at an optimal range of cell concentrations, the variability would, as expected, be significantly less in the microcounter device, since every cell is counted. Indeed, the variance of the microcounter was significantly less than the hemacytometer in a range of 12,500–50,000 cells/mL (Figure 2B, Supplementary Table S1). However, the variability of both devices were not significantly different at low cell concentrations (6250 cells/mL) or at high cell concentrations (100,000 cells/mL), indicating that the greatest precision of the microcounter is achieved at cell concentrations above 6250 cells/mL, but less than 100,000 cells/mL. Nevertheless, the microcounter was able to detect cell concentrations 4-fold smaller than 6250 cells/mL, where the hemacytometer did not, revealing that the microcounter device comprises a larger effective range than a hemacytometer. Therefore, the microcounter device is able to more accurately and precisely evaluate cell concentrations ranging from 1562 to 50,000 cells/mL than a hemacytometer, demonstrating that the microcounter is a superior tool for measuring small cell numbers/concentrations.
Although the microcounter device described here is the first device intended to measure small cell numbers/concentrations, others have used microfluidic-based applications for cell enumeration and sorting (12–15). However, many of these devices still require the use of relatively large cell numbers/concentrations for accurate detection, and thus are not acceptable tools for quantifying rare populations of cells, such as stem cells (12,14,15). Moreover, since many of these devices focus specifically on sorting cells based on size or antibody binding, they are relatively complex devices and may require the use of electrically charged fields, IR lasers, and/or optical tweezers (13,14). Some microfluidic devices that use antibody binding to sort specific and rare cell populations, such as CD4+ T cells from HIV-infected individuals, could potentially be used to analyze stem cell populations (12). However, these devices require initially large numbers of cells, such as the cells present in human blood samples. Moreover, these devices were designed specifically to be used as an experimental end point, which would prevent further use of the sorted rare cell fraction in various stem cell–based assays (12,13).
The use of our microcounter device for cell quantification allows determination of small cell numbers with concentrations as low as 1562 cells/mL. The minimum concentration of cells that can be quantified with currently available cell quantification devices is 6250 cells/mL. Thus, the microcounter device provides a 4-fold increase in sensitivity over existing devices. The use of microchannel devices to measure viable cell concentrations of experimental samples is a simplistic, yet novel application of microfluidic-based culture systems. While the microcounter device described here was initially fabricated with the intention of quantifying fractionated stem cell–enriched subpopulations, the microcounter device could be used to quantify any small or rare cell population. This technology could thus prove to be very useful for experiments involving differentiated blood or immune cell subpopulations, as well as tissue-specific cell populations from small organisms, such as fruit flies or worms. In addition, since the devices are disposable, they could be used in the field as well as any laboratory setting. Therefore, the microcounter device presented here provides a “real world” solution to accurately and quickly quantify biological samples containing small cell numbers and/or low cell concentrations.
Supplementary Material
ACKNOWLEDGEMENTS
Funding for C.A. provided by the United States Department of Defense (no. W81XWH-06-1-0491). Funding for N.M. provided by the National Cancer Institute (no. T32 CA009681). Funding for D.B. and H.Y. provided by the United States Army Breast Cancer Research Program (BCRP) (no. W81XWH-04-1-0572) and National Institutes of Health (no. K25 CA104162). We would also like to acknowledge Jay Warrick for contributing invaluable insight in discussions regarding this manuscript. This paper is subject to the NIH Public Access Policy.
Footnotes
COMPETING INTERESTS STATEMENT
D.J.B. has an ownership interest in Bellbrook Labs LLC, which may license the technology discussed in this article. The other authors declare no competing interests.
To purchase reprints of this article, contact: Reprints@BioTechniques.com
REFERENCES
- 1.Brillard JP, McDaniel GR. The reliability and efficiency of various methods for estimating spermatozoa concentration. Poult. Sci. 1985;64:155–158. doi: 10.3382/ps.0640155. [DOI] [PubMed] [Google Scholar]
- 2.Douglas-Hamilton DH, Smith NG, Kuster CE, Vermeiden JP, Althouse GC. Particle distribution in low-volume capillary-loaded chambers. J. Androl. 2005;26:107–114. [PubMed] [Google Scholar]
- 3.Douglas-Hamilton DH, Smith NG, Kuster CE, Vermeiden JP, Althouse GC. Capillary-loaded particle fluid dynamics: effect on estimation of sperm concentration. J. Androl. 2005;26:115–122. [PubMed] [Google Scholar]
- 4.Maxie MG. Evaluation of techniques for counting bovine platelets. Can. J. Comp. Med. 1977;41:409–415. [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker EV, Thornthwaite JT, Temple WT, Ketcham AS. Flow cytometry: general principles and applications to selected studies in tumor biology. Int. Adv. Surg. Oncol. 1979;2:125–153. [PubMed] [Google Scholar]
- 6.Sundqvist T, Fjallbrant B, Magnusson KE. Computer-aided counting with the Coulter Counter of low numbers of spermatozoa in human semen. Int. J. Androl. 1981;4:18–24. doi: 10.1111/j.1365-2605.1981.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 7.Villas BH. Flow cytometry: an overview. Cell Vis. 1998;5:56–61. [PubMed] [Google Scholar]
- 8.Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 9.Bilitewski U, Genrich M, Kadow S, Mersal G. Biochemical analysis with microfluidic systems. Anal. Bioanal. Chem. 2003;377:556–569. doi: 10.1007/s00216-003-2179-4. [DOI] [PubMed] [Google Scholar]
- 10.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 11.Jo B-H, Van Lerberghe LM, Motsegood KM, Beebe DJ. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000;9:76–81. [Google Scholar]
- 12.Cheng X, Irimia D, Dixon M, Sekine K, Demirci U, Zamir L, Tompkins RG, Rodriguez W, et al. A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects. Lab Chip. 2007;7:170–178. doi: 10.1039/b612966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng X, Liu YS, Irimia D, Demirci U, Yang L, Zamir L, Rodriguez WR, Toner M, et al. Cell detection and counting through cell lysate impedance spectroscopy in microfluidic devices. Lab Chip. 2007;7:746–755. doi: 10.1039/b705082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CC, Chen A, Lin CH. Microfluidic cell counter/sorter utilizing multiple particle tracing technique and optically switching approach. Biomed. Microdevices. 2008;10:55–63. doi: 10.1007/s10544-007-9109-8. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M, Kano K, Tsuda Y, Kobayashi J, Yamato M, Seki M, Okano T. Microfluidic devices for size-dependent separation of liver cells. Biomed. Microdevices. 2007;9:637–645. doi: 10.1007/s10544-007-9055-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.