Abstract
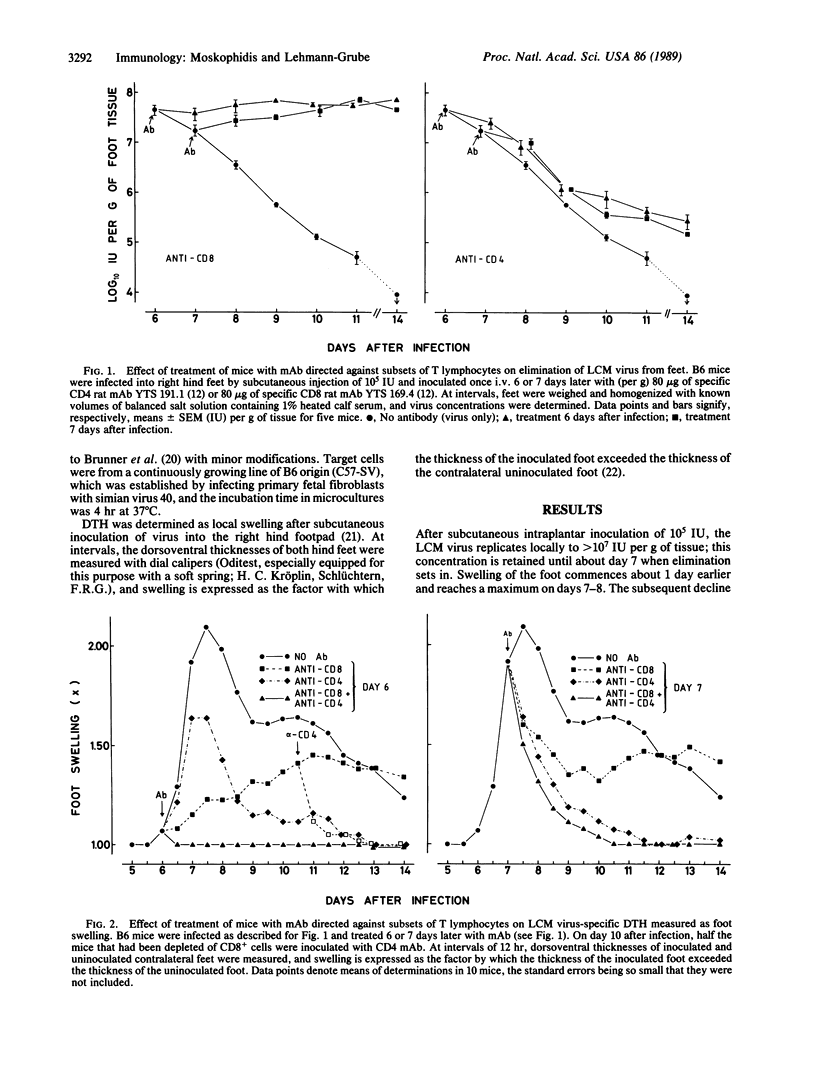
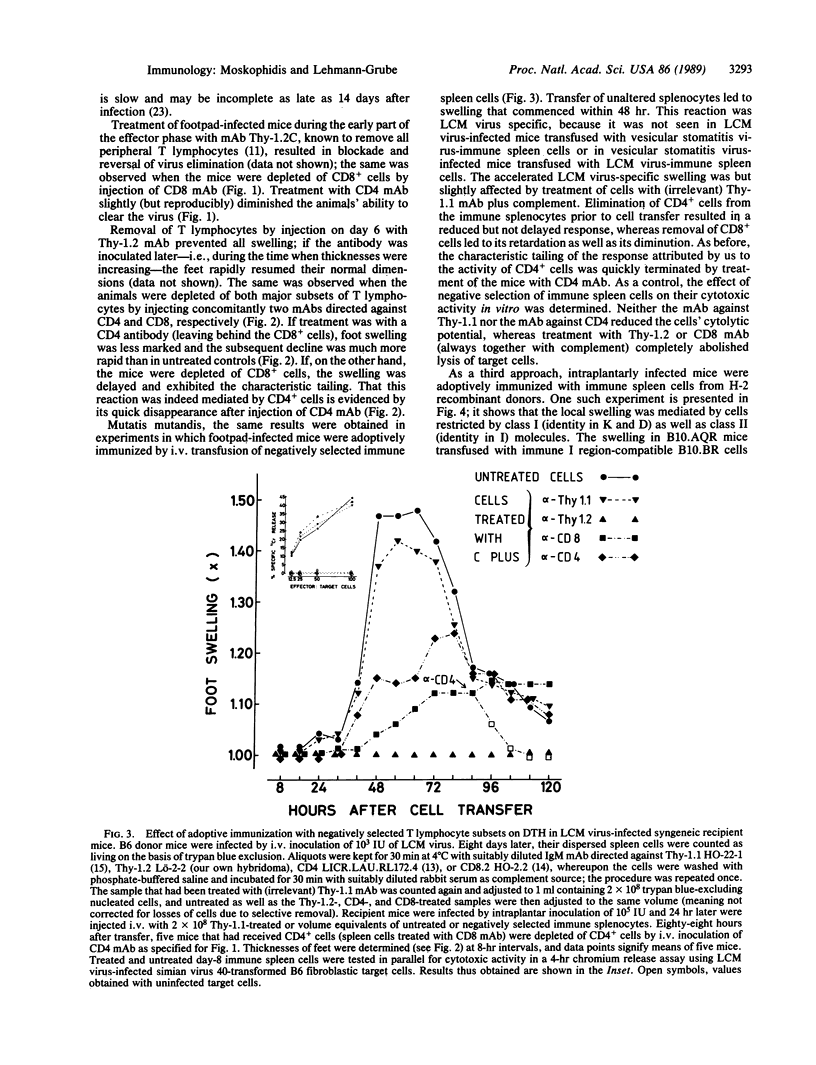
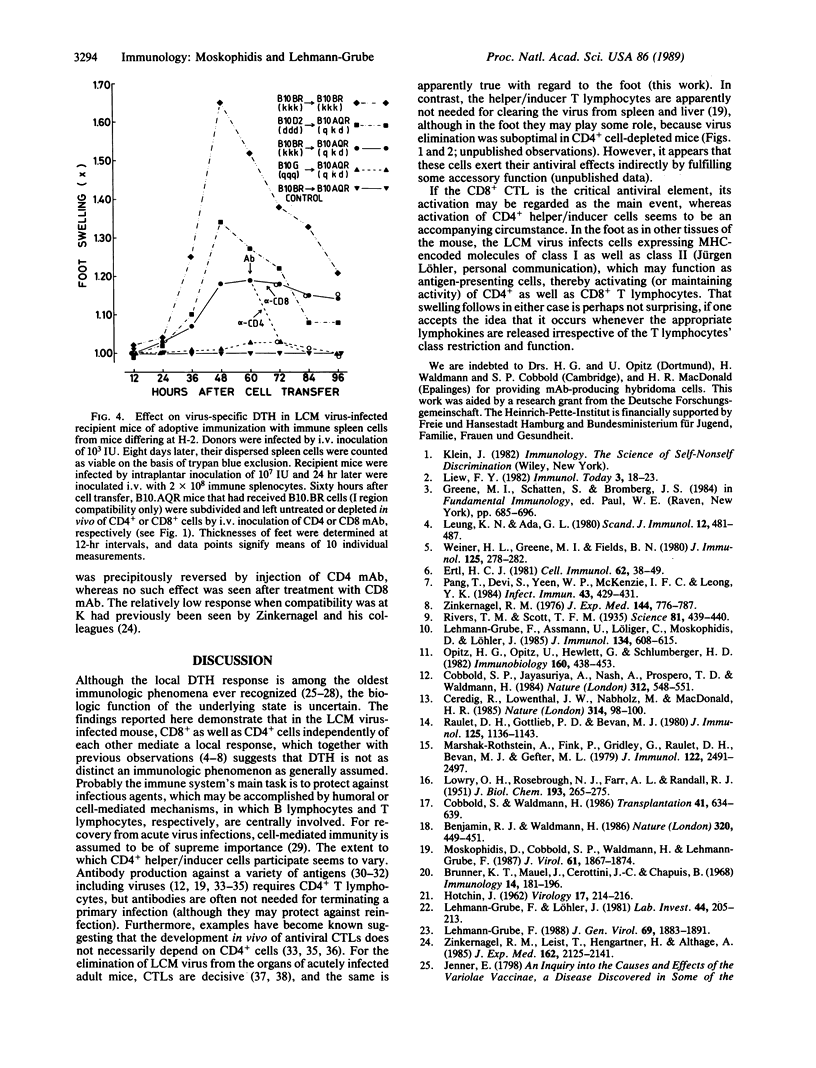
After subcutaneous inoculation into the hind foot of a mouse, the lymphocytic choriomeningitis (LCM) virus multiplies locally, attaining 10(7)-10(8) mouse infectious units per g of tissue; elimination commences around day 7. About 1 day earlier, the foot begins to swell, which is regarded as a delayed-type hypersensitivity (DTH) reaction. To answer the question of whether the local inflammatory response is involved in virus clearance, we needed to known what cells mediate both these phenomena. With three different procedures--namely, depletion in vivo of defined cells by treatment of mice with monoclonal antibodies ("serologic surgery"), adoptive immunization with negatively selected cells, and adoptive immunization with cells from mice differing at the major histocompatibility gene complex--it is shown that the LCM virus-induced local DTH reaction consists of two phases that are sequentially mediated by (first) class I-restricted cytotoxic/suppressive CD8+ and (second) class II-restricted helper/inducer CD4+ T lymphocytes. In contrast, for virus elimination only the former subset of T lymphocytes was found to be needed. Thus, an association may exist between the CD8+ cell-mediated component of the local DTH response and control of the infection, but the CD4+ cell-mediated part appears to be of doubtful antiviral relevance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Butler L. D., Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988 Jun;62(6):2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. J., Waldmann H. Induction of tolerance by monoclonal antibody therapy. Nature. 1986 Apr 3;320(6061):449–451. doi: 10.1038/320449a0. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Waldmann H. Skin allograft rejection by L3/T4+ and Lyt-2+ T cell subsets. Transplantation. 1986 May;41(5):634–639. doi: 10.1097/00007890-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Coutelier J. P., Uyttenhove C., Lambotte P., Van Snick J. In vivo suppression of T-dependent antibody responses by treatment with a monoclonal anti-L3T4 antibody. Eur J Immunol. 1985 Jun;15(6):638–640. doi: 10.1002/eji.1830150620. [DOI] [PubMed] [Google Scholar]
- Ertl H. C. Adoptive transfer of delayed-type hypersensitivity to Sendai virus. I. Induction of two different subsets of T lymphocytes which differ in H-2 restriction as well as in the Lyt phenotype. Cell Immunol. 1981 Jul 15;62(1):38–49. doi: 10.1016/0008-8749(81)90297-5. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. The foot pad reaction of mice to lymphocytic choriomeningitis virus. Virology. 1962 May;17:214–216. doi: 10.1016/0042-6822(62)90106-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann-Grube F., Assmann U., Löliger C., Moskophidis D., Löhler J. Mechanism of recovery from acute virus infection. I. Role of T lymphocytes in the clearance of lymphocytic choriomeningitis virus from spleens of mice. J Immunol. 1985 Jan;134(1):608–615. [PubMed] [Google Scholar]
- Lehmann-Grube F., Löhler J. Immunopathologic alterations of lymphatic tissues of mice infected with lymphocytic choriomeningitis virus. II. Pathogenetic mechanism. Lab Invest. 1981 Mar;44(3):205–213. [PubMed] [Google Scholar]
- Lehmann-Grube F. Mechanism of recovery from acute virus infection. VI. Replication of lymphocytic choriomeningitis virus in and clearance from the foot of the mouse. J Gen Virol. 1988 Aug;69(Pt 8):1883–1891. doi: 10.1099/0022-1317-69-8-1883. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Moskophidis D., Löhler J. Recovery from acute virus infection. Role of cytotoxic T lymphocytes in the elimination of lymphocytic choriomeningitis virus from spleens of mice. Ann N Y Acad Sci. 1988;532:238–256. doi: 10.1111/j.1749-6632.1988.tb36343.x. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Two T-cell populations mediate delayed-type hypersensitivity to murine influenza virus infection. Scand J Immunol. 1980;12(6):481–487. doi: 10.1111/j.1365-3083.1980.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Opitz H. G., Opitz U., Hewlett G., Schlumberger H. D. A new model for investigations of T-cell functions in mice: differential immunosuppressive effects of two monoclonal anti-Thy-1.2 antibodies. Immunobiology. 1982 Feb;160(5):438–453. doi: 10.1016/S0171-2985(82)80007-7. [DOI] [PubMed] [Google Scholar]
- Pang T., Devi S., Yeen W. P., McKenzie I. F., Leong Y. K. Lyt phenotype and H-2 compatibility requirements of effector cells in the delayed-type hypersensitivity response to dengue virus infection. Infect Immun. 1984 Jan;43(1):429–431. doi: 10.1128/iai.43.1.429-431.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Gottlieb P. D., Bevan M. J. Fractionation of lymphocyte populations with monoclonal antibodies specific for LYT-2.2 and LYT-3.1. J Immunol. 1980 Sep;125(3):1136–1143. [PubMed] [Google Scholar]
- Rivers T. M., McNair Scott T. F. MENINGITIS IN MAN CAUSED BY A FILTERABLE VIRUS. Science. 1935 May 3;81(2105):439–440. doi: 10.1126/science.81.2105.439-a. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Greene M. I., Fields B. N. Delayed hypersensitivity in mice infected with reovirus. I. Identification of host and viral gene products responsible for the immune response. J Immunol. 1980 Jul;125(1):278–282. [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Zinkernagel R. M. H-2 restriction of virus-specific T-cell-mediated effector functions in vivo. II. Adoptive transfer of delayed-type hypersensitivity to murine lymphocytic choriomeningits virus is restriced by the K and D region of H-2. J Exp Med. 1976 Sep 1;144(3):776–787. doi: 10.1084/jem.144.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Leist T., Hengartner H., Althage A. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J Exp Med. 1985 Dec 1;162(6):2125–2141. doi: 10.1084/jem.162.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]


