Abstract
An estimated one‐third of the general population is affected by insomnia, and this number is increasing due to more stressful working conditions and the progressive aging of society. However, current treatment of insomnia with hypnotics, gamma‐aminobutyric acid A (GABAA) receptor modulators, induces various side effects, including cognitive impairment, motor disturbance, dependence, tolerance, hangover, and rebound insomnia. Ramelteon (Rozerem; Takeda Pharmaceutical Company Limited, Osaka, Japan) is an orally active, highly selective melatonin MT1/MT2 receptor agonist. Unlike the sedative hypnotics that target GABAA receptor complexes, ramelteon is a chronohypnotic that acts on the melatonin MT1 and MT2 receptors, which are primarily located in the suprachiasmatic nucleus, the body's “master clock.” As such, ramelteon possesses the first new therapeutic mechanism of action for a prescription insomnia medication in over three decades. Ramelteon has demonstrated sleep‐promoting effects in clinical trials, and coupled with its favorable safety profile and lack of abuse potential or dependence, this chronohypnotic provides an important treatment option for insomnia.
Keywords: Insomnia, Melatonin, MT1 receptor, MT2 receptor, Ramelteon, Suprachiasmatic nucleus (SCN), TAK‐375
Introduction
Most adults have experienced insomnia or sleeplessness at one time or another in their lives. An estimated one‐third of the general population is affected by insomnia, and 10–15% have chronic insomnia [1]. Although most of us know what insomnia is and how we feel and perform after one or more sleepless nights, few seek medical advice. Many people remain unaware of the behavioral and medical options available to treat insomnia. Insomnia affects all age groups. The incidence increases with age, and among older adults, insomnia affects women more often than men [2].
The first‐generation drugs for insomnia were barbiturates such as pentobarbital and phenobarbital, which were often used as sedative hypnotics/anxiolytics before benzodiazepines largely came to be used for these purposes. However, barbiturates have a high abuse potential, and overdose can cause unconsciousness and even death due to respiratory suppression [3, 4, 5].
Benzodiazepines, the second generation of hypnotics, such as triazolam, lorazepam, and estazolam, have been used for treatment of insomnia, because benzodiazepines have a low potential for abuse and low danger of lethal overdose [3, 4, 6]. However, these hypnotics produce several side effects including cognitive impairment, psychomotor impairment, dependence, tolerance, hangover, rebound insomnia, and so on [7, 8, 9, 10]. Then benzodiazepine receptor agonists, the third generation of hypnotics with non‐benzodiazepine chemical structures, such as zolpidem, zopiclone, and zaleplon, were developed to maintain sleep‐inducing action and reduce side effects such as amnesia and motor dysfunction [11, 12, 13, 14]. It has been reported that there is no tolerance during treatment and no or limited rebound insomnia after therapy discontinuation in the long‐term use of zaleplon, eszopiclone, and modified release formulation of zolpidem [11, 15, 16]. However, at higher doses, non‐benzodiazepine hypnotics also cause similar side effects as those caused by benzodiazepines, although the severity of side effects differs among specific drugs [11, 12, 13, 17, 18, 19, 20]. Benzodiazepine receptor agonists, including non‐benzodiazepines, have abuse and dependence potential. [18, 21, 22, 23]. Furthermore, sleep produced by these agents is electrophysiologically different from that of naturally occurring physiological sleep. Benzodiazepine receptor agonists reduce rapid eye movement (REM) sleep and increase stage 2 sleep [16, 24, 25, 26, 27]. Newer non‐benzodiazepine hypnotics, including zopiclone, zaleplon, and zolpidem, also have been shown to decrease REM sleep in the first half of the night, although the effects were milder than those of benzodiazepines such as triazolam and temazepam [25, 28, 29]. Also, electroencephalographic (EEG) power spectral analyses revealed that there is a decrease in low‐frequency (0.25–10.0 Hz) activity and increase in high‐frequency activity in non‐REM sleep [24, 25, 30].
Since its discovery in 1958 [31], melatonin has been shown to play a vital role in the regulation of circadian rhythms, including the sleep–wake cycle [32, 33, 34]. The cyclic nature of melatonin's production by the pineal gland is controlled by neuronal output from the suprachiasmatic nucleus (SCN) of the hypothalamus. With regard to sleep in humans, melatonin production is concurrent with nocturnal sleep; the increase in endogenous melatonin levels in the evening correlates with the onset of self‐reported evening sleepiness [35, 36] and an increase in sleep propensity [37].
The identification and cloning of melatonin receptors has increased our understanding of melatonin's role in sleep. In 1998, the nomenclature committee of the International Union of Basic and Clinical Pharmacology classified melatonin receptors as MT1, MT2, and MT3. The MT1 receptor (formerly ML1A or Mel1a) and MT2 receptor (formerly ML1B or Mel1b) were initially defined as high‐affinity binding sites (picomolar affinity) in chick and mammalian brains and retina [38, 39, 40, 41, 42]. MT1 receptor mRNA has been detected in the SCN, and studies using an MT1 receptor knockout mouse indicate that this receptor mediates the acute inhibition of SCN firing by melatonin [33]. MT2 receptor mRNA has also been detected in the SCN, and the activity at this receptor has been associated with the phase‐shifting effects of melatonin on circadian rhythms [32, 43, 44, 45]. The MT3 binding site (formerly ML2) was initially defined as a low‐affinity binding site (nanomolar affinity) in mammalian brains and peripheral organs [46], and it has recently been characterized as a melatonin‐sensitive form of quinone reductase 2 [47, 48]. MT3 has a profile that is completely different from that of the MT1 and MT2 receptors, and it is not likely to be involved in the sleep–wake cycle [49].
The notion that direct administration of exogenous melatonin could promote sleep has been dampened by inconsistent efficacy results in clinical trials [50, 51, 52, 53]. According to the 2005 National Institutes of Health (NIH) State‐of‐the‐Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, melatonin's effect on sleep promotion is questionable based on information from meta‐analyses and consensus statements. Because melatonin is not regulated by the FDA, preparations vary in their melatonin content, making comparisons across studies difficult. Although melatonin appears to be effective for the treatment of circadian rhythm disorders [54, 55, 56], little consistent evidence exists for its efficacy in the treatment of insomnia. The limited efficacy of melatonin for insomnia may be partly attributed to its short half‐life [57, 58]. A controlled‐release melatonin has been shown to improve initiation of sleep and increase sleep efficiency and total sleep time in clinical trials in elderly people with insomnia [59, 60]. These finding suggests that a high‐affinity MT1/MT2 receptor agonist with a longer half‐life than that of melatonin might be a useful therapy for sleep disorders in this population. Recently, there has been a focus on the development of hypnotic agents that selectively target melatonin receptors [61, 62, 63].
Ramelteon [(5)‐N‐[2‐(1,6,7,8‐tetrahydro‐2H‐indeno [5,4‐b]turan‐8‐yl)ethyl]propionamide] is a melatonin receptor agonist that shows high selectivity for the MT1/MT2 receptors [64]. Figure 1 compares the chemical structures of melatonin and ramelteon. Ramelteon has a half‐life of 0.83–1.90 h (much longer than that of melatonin [58]) and undergoes a rapid, high first‐pass metabolism [65, 66]. In clinical trials, ramelteon has demonstrated sleep‐promoting effects, with no next‐day residual effects, rebound insomnia, or withdrawal effects, making it the first available nonscheduled prescription insomnia medication [16, 67, 68, 69]. This review summarizes the preclinical data, clinical efficacy, and safety profile of ramelteon.
Figure 1.
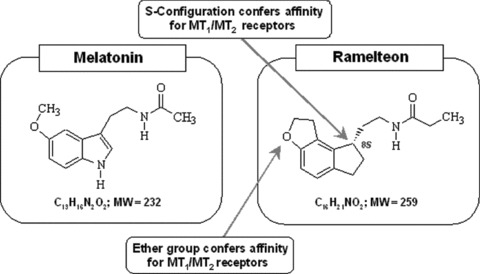
Comparison of chemical structures of melatonin and ramelteon, (S)‐N‐[2‐(1, 6, 7, 8‐tetrahyrdo‐2H‐indeno[4, 5‐b]furan‐8‐yl)ethyl]propionamide.
Preclinical Pharmacology
Neurochemical Effects
High Affinity for MT 1/MT 2 Receptors
In vitro studies have demonstrated that ramelteon is a potent and highly selective MT1/MT2 receptor agonist (Table 1) [70]. In studies of Chinese hamster ovary (CHO) cells expressing human melatonin receptors, ramelteon exhibited a 6‐fold higher affinity for the MT1 receptor than that by melatonin [70]. Similarly, ramelteon had a higher affinity for the MT2 receptor than that of melatonin. The dissociation equilibrium constant (Kd) and maximal number of binding sites (Bmax) values of ramelteon were 15.0 ± 3.0 pM and 555 ± 114 fM/mg protein, respectively, for the MT1 receptor, and 328 ± 12 pM and 133 ± 2 fM/mg protein, respectively, for the MT2 receptor. The affinities of ramelteon for the MT1 and MT2 receptors were comparable to those of 2‐iodomelatonin. N‐acetyl‐5‐HT, a precursor of melatonin with high affinity for the MT3 binding site, showed very low affinity for the MT1 and MT2 receptors. Prazosin, an α1 antagonist with high affinity for the MT3 binding site [71, 72], showed negligible affinity for the MT1 and MT2 receptors [70].
Table 1.
Receptor binding characteristics
| Affinity (Ki) for human MT1 receptor expressed in CHO cells | Affinity (Ki) for human MT2 receptor expressed in CHO cells | Affinity (Ki) for MT3 binding site in hamster brain | |
|---|---|---|---|
| Ramelteon | 14 ± 0.5 pM | 112 ± 5 pM | 2650 ± 180 nM |
| Melatonin | 80.7 ± 2.1 pM | 383 ± 5 pM | 24.1 ± 0.5 nM |
| 2‐iodomelatonin | 13.1 ± 0.3 pM | 188 ± 4 pM | 0.964 ± 0.015 nM |
| N‐acetyl‐5‐HT | 81,300 ± 6900 pM | 3,640,000 ± 30,000 pM | 15.7 ± 2.8 nM |
| Prazosin | >2,730,000 pM | >5,370,000 pM | 6.16 ± 0.46 nM |
Each value represents the mean and standard error [70].
In studies of Syrian hamster brain, the affinity of ramelteon for the MT3 binding site was extremely weak (Ki: 2.65 μM) relative to melatonin and 2‐iodomelatonin; the values being 24.1 nM and 0.924 nM, respectively [70]. The affinity of ramelteon for the MT3 binding site was 1/110 that of melatonin and 1/2750 that of 2‐iodomelatonin. N‐acetyl‐5‐HT and prazosin also showed high affinities for the MT3 binding site [70], as reported previously [38, 71].
Agonistic Effect on MT 1/MT 2 Receptors
Melatonin receptors, when activated, inhibit adenylate cyclase, which leads to a decrease in cAMP [73, 74]. Ramelteon demonstrated this agonist activity at both MT1 and MT2 receptors. In CHO cells expressing the human melatonin receptors, ramelteon inhibited forskolin‐stimulated cAMP production in a concentration‐dependent manner. [70] The half maximal inhibitory concentration (IC50) values of ramelteon and melatonin for the inhibition of cAMP production in cells expressing MT1 receptors were 21.2 and 77.8 pM, respectively, and in cells expressing MT2 receptors, were 53.4 and 904.0 pM, respectively (Table 2) [70]. Taken together, ramelteon's affinity and activity at MT1/MT2 receptors and its low affinity for the MT3 binding site make it a potentially more suitable sleep‐promoting agent than exogenous melatonin.
Table 2.
Effects of ramelteon and other compounds on forskolin‐stimulated cAMP production in CHO cells expressing the human MT1 and MT2 receptors
| Compound | Human MT1 receptor IC50 (pM/L) | Human MT2 receptor IC50 (pM/L) |
|---|---|---|
| Ramelteon | 21.2 ± 0.5 | 53.4 (40.7–70.3) |
| Melatonin | 77.8 ± 14.6 | 904.0 (714.0–1150.0) |
| 2‐iodomelatonin | 26.8 ± 7. 5 | 60.7 (44.0–83.9) |
MT1 receptor: each value represents the mean of 3–4 experiments with the SEM.
MT2 receptor: each IC50 value is calculated from two experiments done in triplicate. Figures in the parentheses indicate 95% confidence intervals of IC50 values.
Effects on Other Receptors and Enzymes
To help differentiate ramelteon from sedative hypnotics that target the GABAA‐receptor complex, investigators assessed ramelteon's affinity to GABA and a wide variety of other CNS binding sites [70]. As expected, ramelteon did not show significant inhibition of binding (>50%) to 131 G‐protein‐coupled receptors, transporters, and ion channels, including monoamine receptors, opioid receptors, central benzodiazepine receptors, and dopamine transporters tested at 10 μM [70]. Moreover, ramelteon showed no effect on enzyme activity of 54 tested enzymes at 10–1000 μM [70]. Melatonin caused no significant inhibition of the binding of almost all receptors, except for 5‐HT1A receptor, for which the Ki value was 5.6 μM. Ramelteon's negligible affinity to GABA, serotonin, acetylcholine, glutamate, noradrenaline, opioid, histamine, and dopamine receptors is noteworthy, as ancillary activity at these receptors may result in unwanted secondary or residual effects.
Sleep‐Promoting Effects
To study the sleep‐promoting effects of ramelteon in cats and monkeys using polysomnography (PSG), a chamber was constructed to allow animals to move freely while being attached to electroencephalogram (EEG) cables [49]. In freely moving cats, the effect of ramelteon on sleep was stronger and lasted longer than the effect of exogenous melatonin (Fig. 2) [49]. Ramelteon (0.001, 0.01, and 0.1 mg/kg, p.o.) was shown to promote sleep, as evidenced by decreases in the percentage of time spent awake and increases in the percentage of time spent in slow wave sleep (SWS) and REM sleep. The median latency to sleep onset (time to first SWS lasting more than 1 min) was 24 min in cats given ramelteon 0.1 mg/kg compared with 60 min in cats given vehicle control. In contrast, melatonin resulted in a mild sleep‐promoting effect; melatonin (0.001–1.000 mg/kg, p.o.) showed no statistically significant effect on sleep latency, and the duration of the sleep‐promoting action of the highest dose of melatonin (2 h) was shorter than that of ramelteon (6 h).
Figure 2.
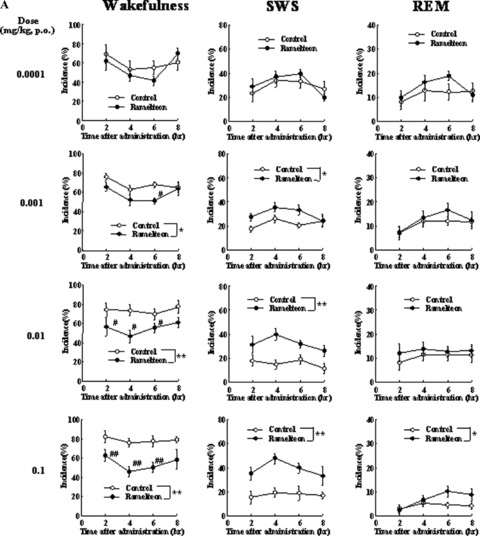
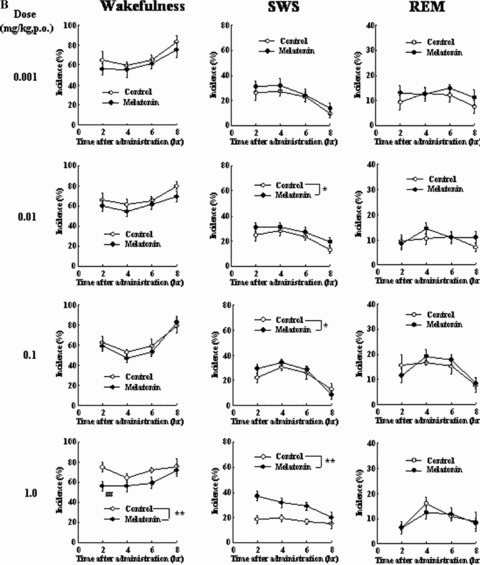
(A) Effects of ramelteon on sleep and wakefulness in freely moving cats. Each value shows the mean (with standard error) percentage of time spent in the stages of wakefulness, SWS, or REM sleep during each block of 2 h after drug administration. Eight of 14 cats were randomly used in each dose group. *P≤ 0.05, **P≤ 0.01, compared with the vehicle‐treated control (ANOVA). #P≤ 0.05, ##P≤ 0.01, compared with the vehicle‐treated control (paired t‐test with Holm's correction) [49]. (B) Effects of melatonin on sleep and wakefulness in freely moving cats. Each value shows the mean (with standard error) percentage of time spent in the stages of wakefulness, SWS, or REM sleep during each block of 2 h after drug administration. Eight of 14 cats were randomly used in each dose group. *P≤ 0.05, **P≤ 0.01, compared with the vehicle‐treated control (ANOVA). #P≤ 0.05, ##P≤ 0.01, compared with the vehicle‐treated control (paired t‐test with Holm's correction) [49].
Ramelteon was shown to cause sleep promotion in freely moving cats. However, it was not enough for starting clinical trial, because the experimental conditions were artificial and the effect of ramelteon was on daytime sleep in cats. It was critical for the project to confirm pharmacological efficacy in primates, focusing on the effect on nighttime sleep, which is much more close to the clinical use of ramelteon. Since there was no evaluation system to assess PSG in freely moving monkeys, the authors developed the PSG assay system through a try and error process [75]. In the study, young adult female crab‐eating macaques (Macaca fascicularis), implanted with electrodes for EEG, electrooculogram (EOG), and electromyogram (EMG) recording, were used, well habituated to the recording chamber located in a soundproof, electrically shielded room that was maintained under conditions similar to those of the home cage under freely moving unrestrained condition. Sleep architecure in the unrestrained monkeys was similar to that of humans, which was also confirmed by recent reports [76].
Ramelteon (0.03 and 0.3 mg/kg, p.o.) showed sleep‐promoting effects, as evidenced by statistically significant increases in total duration of sleep and decreases in latencies to sleep onset (time to the first consecutive 3 min of sleep stage) of light sleep (sleep stages 1 and 2) and SWS (sleep stages 3 and 4) (3, 4, 5) [75]. Melatonin (0.3 mg/kg, p.o.) showed a statistically significant reduction in latency to onset of light sleep but not of SWS and had no statistically significant effect on sleep duration [75]. The higher doses of melatonin (1 and 3 mg/kg) had no statistically significant effect on the latencies to each sleep stage or sleep duration. The benzodiazepine receptor agonist zolpidem (1–30 mg/kg, p.o.) showed no statistically significant effects on sleep latencies to each sleep stage or sleep duration [75]. With regard to observable behavior, monkeys given zolpidem 30 mg/kg displayed sedation and myorelaxation, whereas monkeys given either ramelteon or melatonin at any doses showed no apparent motor dysfunction (Fig. 5) [75]. As zolpidem showed marked sedation including myorelaxation at 100 mg/kg, p.o., the authors did not assess the sleep‐inducing action at this dose. They suggested that, if they conduct the study at 100 mg/kg, it might cause more apparent effect. Therefore, zolpidem might cause sleep‐inducing action at doses exerting marked sedation in monkeys. A number of studies showed that zolpidem causes potent reduction of sleep latency in humans [11, 14, 77], suggesting that there might be a species difference in sleep induction by zolpidem. Ramelteon significantly shortened sleep latency and increased total sleep time in monkeys. Similar clinical effects have been confirmed in clinical studies [69, 78], suggesting that the preclinical study using monkeys was valid for the prediction of clinical efficacy in humans.
Figure 3.
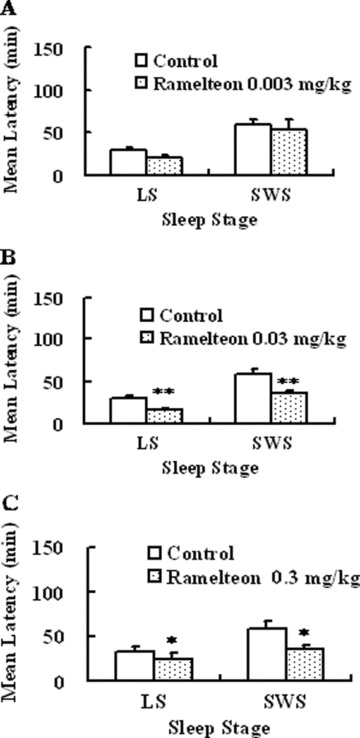
Effects of ramelteon on latency to sleep onset in freely moving monkeys. Each value shows the mean latency ± SE to each light sleep (LS) and SWS at doses of 0.003 (A), 0.03 (B), and 0.3 mg/kg, p.o. (C). Six monkeys were used in each group. *P ≤ 0.05, **P ≤ 0.01, compared with vehicle‐treated controls [paired t test with Holm correction, 103].
Figure 4.
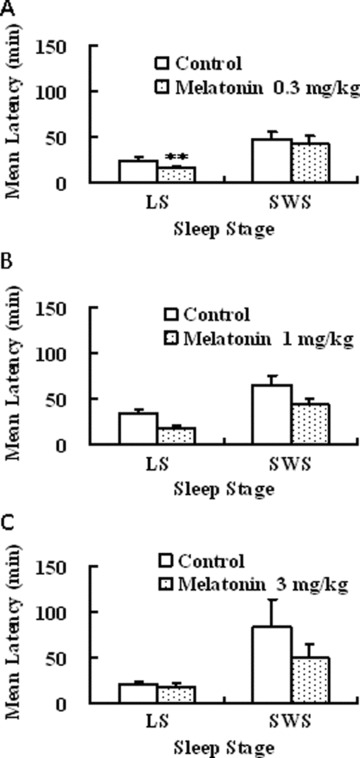
Effects of melatonin on latency to sleep onset in freely moving monkeys. Each value shows the mean latency ± SE to light sleep (LS) and SWS at doses of 0.3 (A), 1 (B), and 3 mg/kg (C). Six or seven monkeys were used in each group. **P ≤ 0.01 compared with the control treated vehicle [paired t test with Holm correction, 103].
Figure 5.
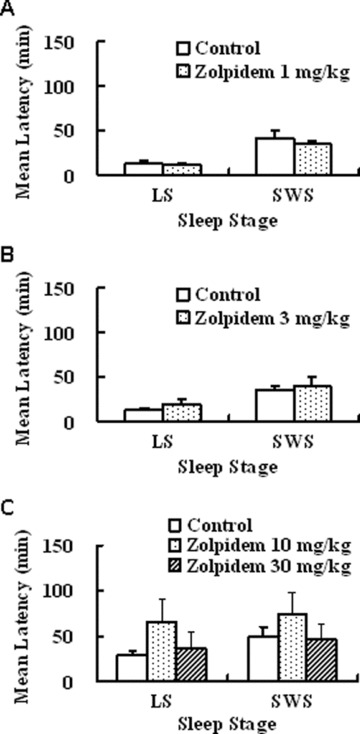
Effects of zolpidem on latency to sleep onset in freely moving monkeys. Each value shows the mean latency ± standard error to light sleep (LS) and SWS at doses of 1 (A), 3 (B), and 10 or 30 mg/kg, p.o. (C). Four monkeys were used in each group.
Unlike the zolpidem, which increased EEG fast waves (>14 Hz) during sleep, EEG spectra and a fast Fourier transform (FFT) after ramelteon and melatonin administration were indistinguishable from those of naturally occurring physiological sleep (Fig. 6) [75]. EEG power spectral analyses in humans have shown that treatment with benzodiazepines or zolpidem caused a significant reduction of low‐frequency activity (0.25–10.0 Hz) and a significant increase in high‐frequency activity in non‐REM sleep observed in healthy young subjects [24, 25, 30]. Therefore, sleep induced by benzodiazepine receptor agonists may be qualitatively different from natural sleep in terms of EEG findings. EEG spectral analyses showed that sleep induced by ramelteon and melatonin was indistinguishable from that of vehicle‐treated control [75], implying that MT1/MT2 receptor agonists may promote physiological sleep in animals.
Figure 6.
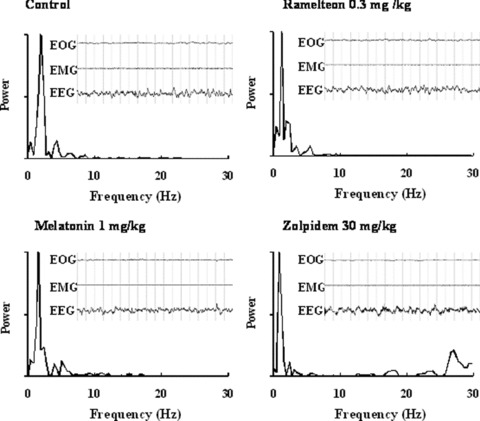
Typical electroencephalographic (EEG) spectra and fast Fourier transform (FFT) analysis in freely moving monkey when treated with ramelteon (0.3 mg/kg, p.o.), melatonin (1 mg/kg, p.o.), and zolpidem (30 mg/kg, p.o.). Typical samples were selected during non‐REM sleep. EEG, electro‐oculogram (EOG) and electromyographic (EMG) activities were recorded [103].
Circadian Reentrainment
To assess the ability of ramelteon to shift the circadian rhythm, a study of running‐wheel activity in rats was conducted [79]. After an abrupt 8‐h advance of the light–dark cycle, the circadian rhythm of running‐wheel activity of rats was gradually resynchronized to the new light–dark cycle. Rats treated with ramelteon (0.1 and 1.0 mg/kg, p.o.) took less time to resynchronize to the new light–dark cycle compared with rats that received vehicle control (Fig. 7). Melatonin 10 mg/kg, p.o., also accelerated reentrainment of running‐wheel activity rhythm following the phase advance [79]. These results indicate that ramelteon was closely mimicking the central actions of melatonin as a circadian rhythm signal, not simply acting as a hypnotic.
Figure 7.
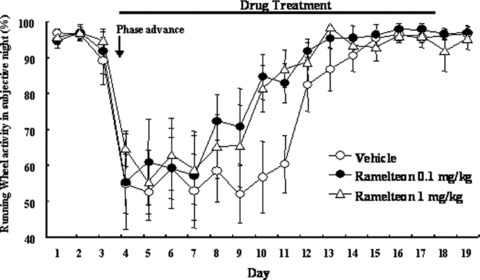
Effects of ramelteon on reentrainment of running‐wheel activity rhythm following an 8‐hour phase advance. Running‐wheel activity in the subjective night as a percentage of total activity in 24 hours is shown with standard error. Ramelteon or vehicle was administered 5–30 min before lights out of the new light‐dark cycle for 14 days starting the day of the phase‐shift. Arrows and black bars show the phase‐shift and the vehicle or ramelteon treatment periods [36].
The MT2 receptor had been shown to be important for the phase‐shifting effects of melatonin in the SCN [32, 43, 44, 45]. Ramelteon induced phase advance by application at zeitgeber time (ZT) 10 but not at ZT 6 in SCN slice in rats. The phase‐advancing effect was inhibited by an MT2 receptor antagonist, 4‐phenyl‐2‐acetamidotetraline [80], suggesting that MT2 receptor might be involved in the phase‐advancing effect of ramelteon.
Learning and Memory and Motor Function
On the Morris water‐maze task for spatial learning and memory, rats treated with ramelteon (3–30 mg/kg, p.o.) and melatonin (10–100 mg/kg, p.o.) showed no statistically significant differences from vehicle control in time to find the submerged platform (Fig. 8) or the number of crossings of the area where the platform had been located during training [79]. In contrast, diazepam (3–30 mg/kg, p.o.) and triazolam (0.1–1 mg/kg p.o.) resulted in statistically significant delays in platform reaching times and reductions in the number of platform crossings, indicating an adverse effect on learning and memory function [79].
Figure 8.
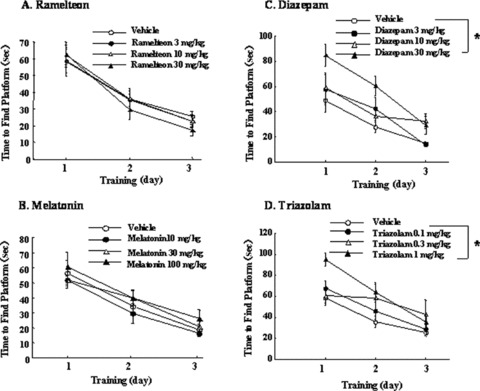
Effects of ramelteon, melatonin, diazepam, and triazolam on the Morris water maze task in rats. Each value shows the mean time to find the platform submerged in the water (A, B, C, D). *P≤ 0.025, compared with the respective vehicle control group [one‐tailed Williams test, 36].
On the delayed matching to position task for memory and attention, rats treated with ramelteon (3–30 mg/kg, p.o.) and melatonin (10–100 mg/kg, p.o.) showed no impairment on task performance [79]. In contrast, high doses of diazepam (30 mg/kg, p.o.) and triazolam (1 and 3 mg/kg, p.o.) resulted in statistically significant reductions in the number of correct responses across delays [79].
On the rota‐rod test for motor coordination, mice treated with ramelteon, melatonin, or N‐acetyl‐5‐HT (3–30 mg/kg p.o.) showed no impairment on rota‐rod performance [81]. In contrast, the benzodiazepine diazepam (3–10 mg/kg, p.o.) induced dose‐dependent impairment of rota‐rod performance; all of the mice in the diazepam group 10 mg/kg failed the test (Fig. 9) [81]. Melatonin and N‐acetyl‐5‐HT exacerbated the diazepam‐induced impairment of performance, whereas ramelteon did not affect the diazepam‐induced motor dysfunction (Fig. 10).
Figure 9.
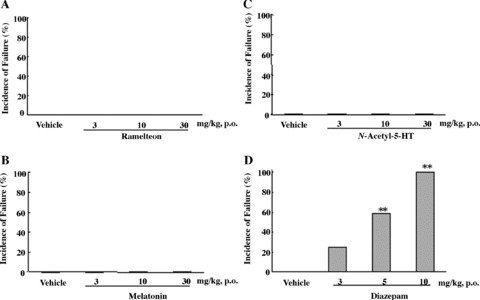
Percentage of mice failing the rota‐rod performance test after administration of ramelteon (A), melatonin (B), N‐acetyl‐5‐HT (C), or diazepam (D) at the indicated doses. Twelve mice were used in each group. **P≤ 0.01, compared with the control group treated with vehicle (chi‐square test with Holm's correction) [81].
Figure 10.
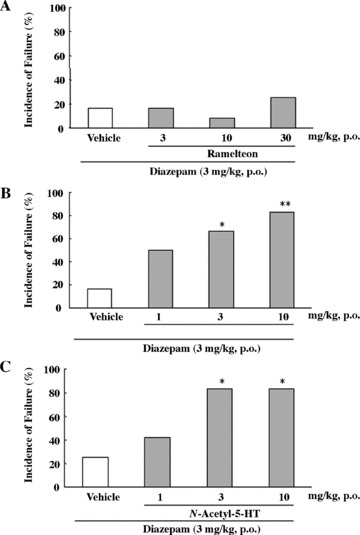
Percentage of mice failing the rota‐rod performance test after administration of diazepam alone or diazepam in combination with ramelteon (A), melatonin (B), N‐acetyl‐5‐HT (C) at the indicated doses. Twelve mice were used in each group. *P≤ 0.05 **P≤ 0.01, compared with the control group treated with vehicle (chi‐square test with Holm's correction) [53].
Rewarding Properties, Abuse Potential, and Dependence
On the conditioned place‐preference test for rewarding property, rats treated with ramelteon (3–30 mg/kg, p.o.) or melatonin (10–100 mg/kg, p.o.) showed no preference in the drug‐associated compartment, indicating a low potential for reinforcing behavior (Fig. 11) [79]. In contrast, morphine (1 mg/kg, s.c.), triazolam (0.5 mg/kg, p.o.), and diazepam (5 mg/kg, p.o.) resulted in statistically significant increases in the time spent in the drug‐associated compartment [79], consistent with reports of the rewarding properties that may occur with the use of benzodiazepines [22, 82].
Figure 11.
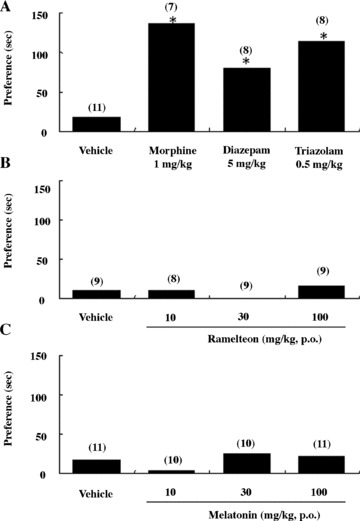
Effect of ramelteon on place preference in rats. Increase in time spent in the compound‐associated compartment in the conditioned place‐preference tests in rats in the experiment 1 (A and C) and experiment 2 (B). The numbers of rats used are shown in parentheses. Vehicle groups in panels A and B are identical. *P≤ 0.05, compared with the value at the preconditioning phase (paired t‐test) [79].
On a task to determine if ramelteon produces benzodiazepine receptor agonist‐like discriminative stimulus effects, the monkeys were trained to reliably discriminate between subcutaneous injections of vehicle and the benzodiazepine midazolam using a standard 2‐lever procedure for shock avoidance [83]. Ramelteon was then administered intravenously in lieu of midazolam at doses of 0.32, 1.0, 3.2, 5.6, and 10.0 mg/kg. After receiving ramelteon, the monkeys responded primarily on the vehicle‐associated lever versus the midazolam‐associated lever, indicating that these animals did not generalize ramelteon to midazolam. In a similar study, monkeys dependent on diazepam were trained to reliably discriminate between subcutaneous injections of vehicle and flumazenil (benzodiazepine antagonist) using a standard 2‐lever procedure for food presentation [83]. Ramelteon was then administered intravenously at doses of 3.2, 5.6, and 10.0 mg/kg 15 min prior to increasing doses of flumazenil. The percentage of responses on the flumazenil‐associated lever was similar between ramelteon 10 mg/kg and vehicle control, indicating that ramelteon did not attenuate the effect of flumazenil.
On an operant task to assess a reinforcing effect, rhesus monkeys were free to self‐administer intravenous ramelteon [84]. In the 2‐h testing periods, there were no significant differences in the number of self‐administrations (fixed ratio of 50) of ramelteon at doses ranging from 0.025–0.400 mg/kg per infusion relative to vehicle control [84]. In contrast, pentobarbital at doses of 0.5 and 1.0 mg/kg per infusion resulted in statistically significant increases in the number of self‐administrations. When the monkeys were free to self‐administer drug (fixed ratio of 5) around the clock, the mean numbers of self‐administrations of ramelteon were comparable to those of vehicle, further indicating that ramelteon showed no positive reinforcing effect. With regard to observable behavior, no gross behavioral changes were noted in monkeys treated with ramelteon or vehicle control. In monkeys treated with pentobarbital, hyporeactivity, slowed motion, and/or ataxia was noted.
In a long‐term study to assess physical dependence liability, monkeys received ramelteon (10 mg/kg, i.g. catheter) daily for 1 year [83]. During weeks 14, 27, and 40, the treatment was temporally discontinued for 5 days to assess the effects of treatment discontinuation on operant behavior (conditioned lever pressing to obtain food and avoid shock) and observable clinical signs (such as yawning and grooming) using a paradigm sensitive to benzodiazepine dependence. Daily treatment with ramelteon had no overall effect on monkey's behavior to obtain food or avoid shock. Operant response rates in individual monkeys did not systematically change over the course of the study. In addition, there were no apparent changes in body weight, motor activity, posture, or behavior observed during the periods of treatment or after discontinuation of treatment.
Clinical Efficacy and Safety
Metabolism
Rameleteon is metabolized primarily via oxidation to hydroxyl and carbonyl derivatives, with secondary metabolism producing glucuronide conjugates [65]. Ring‐opening biotransformation results in the formation of M‐I, and hydroxylation of the carboxy‐metabolite, M‐III, results in the formation of M‐IV (Fig. 12) [65]. The major metabolite of ramelteon in serum is the monohydroxylated metabolite, M‐II. Cytochrome P450 (CYP)1A2 is the major isozyme involved in the hepatic metabolism of ramelteon; the CYP2C subfamily and CYP3A4 isozymes are also involved to a minor degree. The major metabolite in serum was M‐II, having exposure approximately 39‐fold greater than that of ramelteon at the 16‐mg dose. The half‐life of ramelteon ranges from 0.83 to 1.90 h, depending on the dose [65], which is considerably longer than that of melatonin. This might be regarded as a decisive advantage for the use of ramelteon as a sleep promoter, because the relatively poor efficiency of melatonin is presumed to be partly due to its short half‐life [57, 58].
Figure 12.
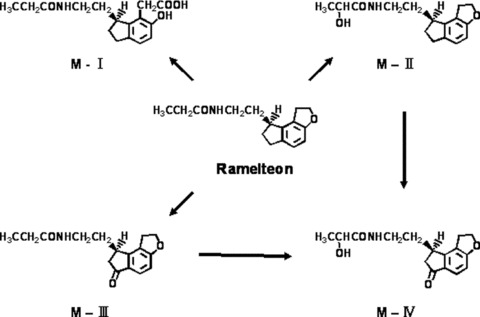
Metabolic pathway of ramelteon in humans. Ramelteon is extensively metabolized, primarily by carboxylation and stereoselective hydroxylation. The major metabolite of ramelteon in serum is the monohydroxylated metabolite, M‐II.
M‐II is a major metabolite and is physiologically active, and its affinity for chick Mel1a/Mel1c receptors is 0.675 nM, one‐fifth to one‐tenth the binding affinity of ramelteon, for the human MT1 and MT2 receptors, and it is 17‐ to 25‐fold less potent than ramelteon in in vitro functional assays [70]. M‐II also showed a potent sleep‐promoting action in freely moving cats [49]. M‐II had no significant affinities for other receptors or various enzyme activities, as those of ramelteon, suggesting that M‐II is also an MT1/MT2 receptor selective agonist [70]. However, the levels of other metabolites are low, and also the affinities for Mel1a/Mel1c receptors are negligible, as demonstrated by Ki values of 36.0 nM for M‐III and >236 nM for both M‐I and M‐IV. Thus, M‐II may contribute to pharmacological effects of ramelteon.
Sleep‐Promoting Effects
In randomized, double‐blind, placebo‐controlled clinical trials, the sleep‐promoting effects of the short‐ and long‐term use of ramelteon have been demonstrated in patients with chronic insomnia as well as in healthy subjects subjected to a first‐night‐effect model of transient insomnia. Overnight monitoring with PSG (EEG, EOG, and EMG) was used to objectively evaluate the ability of ramelteon to reduce latency to persistent sleep, maintain sleep, and increase the duration of sleep. Patients' subjective assessments of sleep were also evaluated with a post‐sleep questionnaire. Latency to persistent sleep (defined as time in minutes to the first of 20 consecutive epochs of sleep on PSG) [78, 85] or patient‐reported sleep latency was the primary measures in these trials.
Transient Insomnia
Ramelteon's ability to improve transient insomnia was assessed in two clinical trials that utilized a first‐night‐effect model of transient insomnia. In Roth et al.'s trial of 375 healthy subjects unfamiliar with a sleep laboratory environment (i.e., experiencing transient insomnia), ramelteon at doses of 16 mg and 64 mg produced statistically significant decreases in latency to persistant sleep and increases in total sleep time, as measured with PSG [85]. The mean latency to persistent sleep was 14.1 and 15.5 min in the ramelteon 16‐mg and 64‐mg groups, respectively, compared with 24.6 min in the placebo group (P≤ 0.001 for both groups vs. placebo). The mean total sleep times were 425.4 and 422.4 min in the 16‐mg and 64‐mg groups, respectively, compared with 411.3 min in the placebo group (P≤ 0.05 for both doses vs. placebo). According to a post‐sleep questionnaire, subjects in the 16‐mg group reported statistically significant improvements in subjective sleep latency (P= 0.013; no P‐value reported for subtotal sleep time). Ramelteon was not associated with next‐day residual psychomotor impairment, as measured by the Digit Symbol Substitution Test (DSST). However, the use of the ramelteon 64‐mg dose was associated with small but statistically significant reductions in subjective next‐day levels of alertness and ability to concentrate relative to placebo. Zammit et al. conducted a similar trial of 289 healthy subjects that showed that latency to persistent sleep, as measured with PSG, was 12.2 min in the ramelteon 8‐mg group and 14.8 min in the ramelteon 16‐mg group compared with 19.7 min in the placebo group; the 8‐mg dose reached statistical significance (P= 0.004) [68]. The mean total sleep time was 436.8 min and 433.1 min in the 8‐mg and 16‐mg groups, respectively, compared with 419.7 min in the placebo group (P≤ 0.05 for both groups vs. placebo). Subjective measures of sleep latency and total sleep time were improved, but the differences from placebo were not statistically significant. In this trial, ramelteon had no significant effects on the DSST and subjective levels of alertness and ability to concentrate as well as on other next‐day residual effect measures including memory recall tests and visual analog scales (VAS) for mood and feeling.
Chronic Insomnia
Patients with a history of chronic insomnia (primary insomnia as defined by Diagnostic and Statistical Manual of Mental Disorders (DSM)‐IV‐TR for at least 3 months) were also evaluated in clinical trials. Figure 13 illustrates the differences from placebo in latency to persistent sleep by dose of ramelteon in these trials. In Erman et al.'s multiple‐dose cross‐over study of 107 adults with chronic insomnia, a 2‐night administration of ramelteon resulted in statistically significant reductions in latency to persistent sleep (P≤ 0.001) and increases in total sleep time (P≤ 0.05), as measured with PSG, at each dose tested (4–32 mg) [78]. Interestingly, all doses of ramelteon produced similar reductions in latency to persistant sleep (13.4–14.8 min more than placebo), with no apparent dose‐dependent effects, and this wide therapeutic window was seen across studies. Patients who received ramelteon 16 mg reported significant reductions in subjective sleep latency (P≤ 0.05); no other statistically significant effects in subjective sleep ratings were observed. Ramelteon had no statistically significant effect on next‐day residual effect measures including the DSST, memory recall tests, and patient‐reported levels of alertness and ability to concentrate. However, ramelteon had no significant effect on the mean wake time after sleep onset (WASO).
Figure 13.
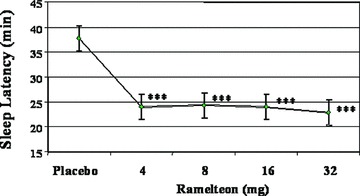
Effects of ramelteon on sleep latency in patients with chronic insomnia under placebo‐controlled, double‐blind, randomized, 5‐period crossover study. All data are shown as the least square means. ***P≤ 0.001, compared with placebo [78].
In Zammit et al.'s extended trial in 405 patients with chronic insomnia, ramelteon 8 mg and 16 mg resulted in improvements in latency to persistent sleep and total sleep time over 5 weeks of nightly treatment, as measured objectively by PSG and subjectively by the post‐sleep questionnaire (Fig. 14) [69]. Ramelteon had no significant effects on WASO. Ramelteon showed no statistically significant effect on the next‐day DSST performance. Patients in the 8‐mg ramelteon group demonstrated a small, but statistically significant, decrease in the mean score compared with placebo on the immediate memory recall test at week 3 and the delayed memory recall test at week 1. At other time points, no significant differences between ramelteon and placebo were found on memory function tests. Subjective levels of alertness and ability to concentrate were similar between the ramelteon groups and placebo with two exceptions: patients in the ramelteon 8‐mg group reported statistically significant improvements in the ability to concentrate at week 1 and the level of alertness at week 5. This trial also included a 2‐day placebo runout period that showed that the discontinuation of ramelteon treatment was not associated with either rebound insomnia or withdrawal effects, as assessed with the Tyer Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ). The BWSQ is a questionnaire that solicits specific information on 20 symptoms commonly experienced during withdrawal from benzodiazepine receptor agonists.
Figure 14.
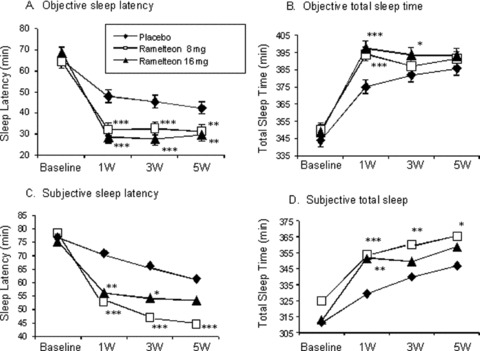
Latency to persistent sleep and total sleep time, as measured by PSG, with ramelteon 8 mg, ramelteon 16 mg, and placebo at weeks 1, 3, and 5 (A and B). Subjective sleep latency and total sleep time, as measured by the post‐sleep questionnaire (C and D). For comparisons between ramelteon dose and placebo, *P≤ 0.05, **P≤ 0.01, and ***P≤ 0.001 [68].
In Roth et al.'s trial of ramelteon in 100 elderly patients (≥65 years) with chronic insomnia, a 2‐night administration of either ramelteon 4 mg or ramelteon 8 mg produced statistically significant reductions in latency to persistent sleep and increases in total sleep time, as measured with PSG [86]. The mean latency to persistent sleep was 28.7 min and 30.8 min in the 4‐mg and 8‐mg groups, respectively, compared with 38.4 min in the placebo group (P≤ 0.01 for both groups vs. placebo). The mean total sleep time was 359.4 min and 362.0 min in the 4‐mg and 8‐mg groups, respectively, compared with 350.4 min in the placebo group (P≤ 0.05 for both groups vs. placebo). According to the post‐sleep questionnaire, patients who received ramelteon 4 mg reported statistically significant reductions in subjective sleep latency (P≤ 0.05). There were no next‐day residual effects with either dose of ramelteon, as measured with the DSST, memory recall tests, and patient‐reported levels of alertness or ability to concentrate.
A large 5‐week outpatient trial in 829 older adult patients (≥65 years) with chronic insomnia was conducted to specifically assess subjective ratings of sleep [87]. In this trial, nightly administration of either ramelteon 4 mg or ramelteon 8 mg produced reductions in patient‐reported sleep latency and increases in patient‐reported total sleep time at week 1, and these improvements were sustained throughout the study. At week 1, subjective sleep latency was 70.2 min in the 4‐mg group and 70.2 min in the 8‐mg group compared with 78.5 min in the placebo group (P≤ 0.01 for both doses vs. placebo). At week 1, subjective total sleep time was 324.6 min in the 4‐mg group and 321.1 min in the 8‐mg group compared with 313.9 min in the placebo group (P≤ 0.01 for 4 mg vs. placebo, P= 0.055 for 8 mg vs. placebo). Ramelteon did not show either rebound insomnia or withdrawal effects, according to subjective sleep latency and BWSQ results during the 7‐day placebo runout period.
In the longest trial of ramelteon to date, 1213 patients with chronic insomnia took open‐label ramelteon (8 mg or 16 mg) nightly for 1 year [88]). Patient‐reported sleep was improved by the first week of treatment and was sustained throughout the remainder of the study. Improvements in sleep latency from baseline at month 1 were 34.0% and 35.1% with the 8‐mg and 16‐mg dose, respectively, and continued to improve through month 6 (44.7% and 49.1%) and month 12 (50.3% and 52.1%). Improvements from baseline in patient‐reported total sleep time at month 1 (15.2% and 16.9%), month 6 (21.6% and 22.7%), and month 12 (25.5% and 23.9%) were also reported with ramelteon 8‐mg and 16‐mg doses, respectively. There was no evidence of rebound insomnia with ramelteon during the 3‐day placebo runout period.
Considering the clinical data thus far, ramelteon has consistently demonstrated significant sleep‐promoting effects, as evidenced by reductions in sleep latency and increases in sleep duration, among patients with chronic insomnia and subjects with transient insomnia, during both short‐term and long‐term treatment, in both the sleep laboratory and outpatient setting by both objective PSG and subjective reports. The efficacy by subjective reports [87] was weaker than that by objective PSG measurement [69, 78]. The efficacy was less than that of sedative hypnotics [11, 15,2008]. However, the subjective efficacy of ramelteon was more prominent in subjective reports to questionnaire done the next morning in the sleep laboratory than that in subjective reports done at home, possibly suggesting that compliance is more important for subjective evaluation of melatonin receptor agonists. In 2005, ramelteon was approved in the United States for the treatment of insomnia characterized by a difficulty in sleep onset. Current data suggest that ramelteon has no significant effects on WASO, and the efficacy for sleep maintenance insomnia has not been confirmed. Further studies are required to clarify the effect on WASO and search for a possible medication for sleep maintenance insomnia, taking into consideration the dosage of ramelteon and appropriate patient population.
Safety
Ramelteon was generally well tolerated across the clinical trials. Most adverse events were considered mild or moderate in severity, and very low incidences of adverse events during ramelteon administration were observed. Only three events were noted to occur at an incidence of ≥2% with ramelteon 8 mg (the recommended therapeutic dose) vs. placebo: somnolence (5% vs. 3%), dizziness (5% vs. 3%), and fatigue (4% vs. 2%).
In the 1‐year trial of ramelteon in 1213 adults with chronic insomnia, the overall incidence of adverse events was similar at 6 months and 1 year [89]. The adverse events were predominantly mild or moderate. At 1 year, the adverse events most frequently reported with ramelteon 8 mg and 16 mg, respectively, were nasopharyngitis (10.5% and 14.9%), somnolence (9.5% and 8.1%), upper respiratory tract infection (7.6% and 11.1%), headache (1.9% and 13.5%), and sinusitis (1.9% and 7.8%). Of 38 subjects (3.1%) reporting a serious adverse event, only three adverse events were possibly treatment related. There were no clinically meaningful changes in vital signs, physical examinations, clinical chemistry, hematology, or urinalysis values and no electrocardiogram trends to suggest adverse effects on cardiac function over the 1 year of ramelteon treatment. No notable changes in endocrine and sexual/reproductive function were observed except for the mean free and total testosterone level, which had a slight decrease in older men that returned to normal by the final visit of the trial.
Abuse Liability
Benzodiazepine receptor agonists, which are the most commonly used prescription medications for treatment of insomnia, have been associated with a potential for abuse [18, 21, 22, 23]. All benzodiazepine receptor agonists (including the newer agents zolpidem, zaleplon, and eszopiclone) are classified as schedule IV controlled substances, meaning they possess some potential for abuse and the adverse consequences of abuse (e.g., withdrawal, psychomotor impairment, and overdose).
In a study of 14 subjects with a history of sedative hypnotic or anxiety drug abuse, ramelteon doses up to 160 mg (20 times the therapeutic dose) had no effect on a wide variety of instruments including subject ratings of mood and behavior, observer rating of mood and behavior, and assessments of behavioral and cognitive performance [67]. Figure 15 shows that ramelteon showed no statistically significant effect at any time point (0.5–24 h post dose) on representative abuse measures including “liking” scales, the DSST, and circular lights task. Ramelteon had no statistically significant effect on other questionnaire items “drug strength,”“good effects,” and “monetary street value.” Similarly, ramelteon had no statistically significant effect on word recall/recognition task, enter and recall task, and balance task performance at any dose compared with placebo. In contrast, triazolam consistently produced a dose‐related effect on all these subjective and behavioral measures, consistent with its profile as a sedative drug with known abuse potential. This trial demonstrates that ramelteon does not share the pharmacologic side effect profile observed among benzodiazepine receptor agonists.
Figure 15.
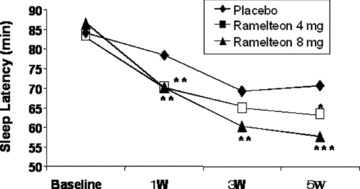
Effects of ramelteon on patient‐reported sleep latency in older adults with chronic insomnia. Patients include older adults (≥65 years; n = 829) with chronic insomnia. Placebo, ramelteon 4 mg, or ramelteon 8 mg was taken nightly for 5 weeks, and patient‐reported sleep data were the collected sleep diaries. *P≤ 0.05, **P≤ 0.01, compared with the placebo control [87].
Future Perspective
Ramelteon has been shown to improve sleep disorders in patients suffering from transient and chronic insomnia without causing significant adverse events. However, it has been suggested that melatonin receptor agonists have opportunities to cure various diseases, including circadian rhythm sleep disorders (CRSD), depression, Alzheimer disease, bipolar disorder, cancer, hypertension, urinary incontinence, and so on.
Patients with CRSD often experience sleep disruption, daytime fatigue, and impaired mental and physical function. The prevalence of CRSD is unknown, but it has been estimated that 5–10% of patients referred to sleep disorder clinics have a CRSD [90]. Among blind individuals, non‐24‐h sleep‐wake syndrome may occur in as many as 50%[91]. Transient CRSD, such as jet lag and shift‐work syndrome, may be quite common in the general population. As a pharmacologic therapy for CRSD, melatonin has shown some effectiveness [92, 93, 94], and a systematic review of 10 trials concluded that melatonin treatment (0.5–5.0 mg per day for 2–5 days) is effective for alleviating the symptoms of jet lag after travel across several time zones [95].
A number of studies have shown altered melatonin levels in depressed patients and suggested that mood disorders may be related to low melatonin levels, although the reports were not consistent [96, 97]. Recently, agomelatin, a melatonin agonist, showed some efficacy in the treatment of major depressive disorder [98, 99]. Agomelatine has been reported to have affinity for 5‐HT2c receptors; however, the affinity was much less than that for melatonin MT1/MT2 receptor, suggesting that antidepressive effect of agomelatine might be related to the effects via activation of MT1/MT2 receptors. Furthermore, reduced melatonin levels and altered timing of melatonin secretion were observed in patients with bipolar affective disorder [100, 101].
The secretion of endogenous melatonin declines with aging [102], and this may be related to the sleep–wake rhythm disturbance in elderly people [103, 104]. Abnormal decreases in nocturnal melatonin have also been observed in patients with Alzheimer disease, another population prone to sleep disturbances [103, 104, 105]. Administration of melatonin to Alzheimer disease patients has been found to improve significantly sleep and circadian abnormality [106, 107, 108]. These findings suggest that melatonin may play a role in significant regulation of various physiological functions. Thus, further studies using ramelteon, a potent and selective MT1/MT2 receptor agonist well tolerated in humans, to examine its clinical efficacy in these disorders would be interesting and may provide an insight into treatment options for these patients.
Conclusion
Ramelteon shows selective affinity for MT1 and MT2 receptors and acts as a full agonist. Currently, MT1 and MT2 receptors have been suggested to be related to sleep promotion and circadian clock such as phase advance. Ramelteon exerts potent sleep promotion in experimental animals including cats and monkeys, without causing any significant adverse effects such as learning and memory impairment, impairment of motor coordination, and drug abuse ability. The sleep induced by ramelteon cannot be distinguished from natural sleep using FFT analysis in monkeys, in contrast to the altered sleep pattern produced by current sedative hypnotics.
In clinical trials, ramelteon decreases sleep latency and increases total sleep time, without causing hangover, addiction, and withdrawal effects. It has no significant effects on psychomotor and cognitive function and does not show drug abuse potential. Taken together, the proven clinical efficacy of ramelteon combined with its safety profile positions it as a fourth‐generation insomnia treatment worth considering for select patients suffering from insomnia (Table 3). Further development of ramelteon for various diseases or disorders is expected.
Table 3.
Classification of therapeutic drugs for insomnia
| Classification | Drug | Adverse events | Sleep pattern |
|---|---|---|---|
| Bariturates | Pentobarbital, phenobarbital | Respiratory suppression, amnesia, loss of motor dysfunction, drug abuse, dependence | Sedative sleep |
| Benzodiazepme receptor agonists (benzodiazepines) | Triazolam, temazepam | Amnesia, loss of motor coordination, drug abuse, dependence | Sedative sleep |
| Benzodiazepme receptor agonists (non‐benzopdiazepines) | Zolpidem, zaleplon, zopiclone | Amnesia, loss of motor coordination, drug abuse, dependence | Sedative sleep |
| MT1/MT2 receptor agonist | Ramelteon | No serious adverse event | Physiological sleep |
Conflict of Interest
The authors have no conflicts of interest.
Re‐use of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.
References
- 1. Silber MH. Chronic insomnia. N Engl J Med 2005;353:803–810. [DOI] [PubMed] [Google Scholar]
- 2. Lamberg L. Several sleep disorders reflect gender differences. Psychiatr News 2007;42:40. [Google Scholar]
- 3. Cohn MA. Hypnotics and the control of breathing: review. Br J Pharmacol 1983;16:245S–250S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitler MM. Nonselective and selective benzodiazepine receptor agonists—where are we today? Sleep 2000;23:S39–S47. [PubMed] [Google Scholar]
- 5. Roache JD, Griffiths RR. Comparison of triazolam and pentobarbital: performance impairment, subjective effects and abuse ability. J Pharmacol Exp Ther 1985;234:120–133. [PubMed] [Google Scholar]
- 6. Barbera J, Shapio C. Benefit‐risk assessment of zaleplon in the treatment of insomnia. Drug Saf 2005;28:301–308. [DOI] [PubMed] [Google Scholar]
- 7. Griffiths RR, Sannerud CA, Ator NA, Brady JV. Zolpidem behavioral pharmacology in baboons: Self‐injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther 1992;260:1199–1208. [PubMed] [Google Scholar]
- 8. Liappas IA, Malitas PN, Dimopoulos NP, Gitsa OE, Liappas AI, Nikolaou CK, Christodoulou GN. Zolpidem dependence case series: Possible neurobiological mechanisms and clinical management. J Psychopharmacol 2003;17:131–135. [DOI] [PubMed] [Google Scholar]
- 9. Preston GC, Ward CE, Broks P, Traub M, Stahl SM. Effects of lorazepam on memory, attention and sedation in man: Antagonism by Ro 15–1788. Psychopharmacology 1989;97:222–227. [DOI] [PubMed] [Google Scholar]
- 10. Sanger DJ, Benavides J, Perrault G, Morel E, Cohen C, Joly D, Zivkovic B. Recent developments in the behavioral pharmacology of benzodiazepine (omega) receptors: Evidence for the functional significance of receptor subtypes. Neurosci Biobehav Rev 1994;18:355–372. [DOI] [PubMed] [Google Scholar]
- 11. Fry J, Scharf M, Mangano R, Fujimori M. Zaleplon improves sleep without producing rebound effects in outpatients with insomnia. Zaleplon Clinical Study Group. Int Clin Psychopharmacol 2000;15:141–152. [DOI] [PubMed] [Google Scholar]
- 12. Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L. New drugs for insomnia: Comparative tolerability of zopiclone, zolpidem and zaleplon. Drug Saf 2003;26:261–282. [DOI] [PubMed] [Google Scholar]
- 13. Troy SM, Lucki I, Unruh MA, Cevallos WH, Leister CA, Martin PT, Furlan PM, Mangano R. Comparison of the effects of zaleplon, zolpidem, and triazolam on memory, learning, and psychomotor performance. J Clin Psychopharmacol 2000;20:328–337. [DOI] [PubMed] [Google Scholar]
- 14. Roth T, Roehrs T, Vogel G. Zolpidem in the treatment of transient insomnia: A double‐blind, randomized comparison with placebo. Sleep 1995;18:246–251. [DOI] [PubMed] [Google Scholar]
- 15. Krystal AD, Wash JK, Laska E, Caron J, Amato DA, Wessel TC, Roth T. Sustained efficacy of eszopiclone over 6 months of nightly treatment: Results of a randomized, double‐blind, placebo‐controlled study in adults with chronic insomnia. Sleep 2003;26:793–799. [DOI] [PubMed] [Google Scholar]
- 16. Roth T, Soubrane C, Titeux L, Wash JK. Efficacy and safety of zolpidem‐MR: a double‐blind, placebo‐controlled study in adults with primary insomnia. Sleep Med 2006;7:397–406. [DOI] [PubMed] [Google Scholar]
- 17. Farber RH, Burke PJ. Post‐bedtime dosing with indiplon in adults and the elderly: Results from two placebo‐controlled, active comparator crossover studies in healthy volunteers. Curr Med Res Opin 2008;24:837–846. [DOI] [PubMed] [Google Scholar]
- 18. Rush CR, Frey JM, Griffith RR. Zaleplon and triazolam in humans: Acute behavioral effects and abuse potential. Psychopharmacology (Berl) 1999;145:39–51. [DOI] [PubMed] [Google Scholar]
- 19. Vermeeren A. Residual effects of hypnotics: Epidemiology and clinical implications. CNS Drugs 2004;18:297–328. [DOI] [PubMed] [Google Scholar]
- 20. Wagner J, Wagner ML. Non‐benzodiazepines for the treatment of insomnia. Sleep Med Rev 2000;4:551–581. [DOI] [PubMed] [Google Scholar]
- 21. Evans SM, Funderburk FR, Griffith RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther 1990;255:1246–1255. [PubMed] [Google Scholar]
- 22. Griffiths RR, Johnson MW. Relative abuse ability of hypnotic drugs: A conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 2005;66:31–41. [PubMed] [Google Scholar]
- 23. Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non‐benzodiazepine hypnotics zolpidem and zopiclone: A review of case reports and epidemiological data. Addiction 2003;98:1371–1378. [DOI] [PubMed] [Google Scholar]
- 24. Borbély AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all‐night sleep EEG spectra. Hum Neurobiol 1985;4:189–194. [PubMed] [Google Scholar]
- 25. Brunner DP, Dijk DJ, Munch M, Borbély AA. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 1991;104:1–5. [DOI] [PubMed] [Google Scholar]
- 26. Feige B, Volderholzer U, Riemann D, Hohagen F, Berger M. Independent sleep EEG slow‐wave sleep and spindle band dynamics associated with 4 weeks of continuous application of short‐half‐life hypnotics in healthy subjects. Clin Neurophysiol 1999;110:1965–1974. [DOI] [PubMed] [Google Scholar]
- 27. Rosenburg R, Caron J, Roth T, Amato D. An assessment of the efficacy and safety of eszopiclone in the treatment of transient insomnia in healthy adults. Seep Med 2005;6:15–22. [DOI] [PubMed] [Google Scholar]
- 28. Drake CL, Roehrs TA, Mangano RM, Roth T. Dose‐response effects of zaleplon as compared with triazolam (0.25 mg) and placebo in chronic primary insomnia. Hum Psychopharmacol 2000;15:595–604. [DOI] [PubMed] [Google Scholar]
- 29. Hemmeter U, Müller M, Bischof R, Annen B, Holsboer‐Trachsler E. Effect of zopiclone and temazepam on sleep EEG parameters, psychomotor and memory functions in healthy elderly volunteers. Psychopharmacology 2000;147:384–396. [DOI] [PubMed] [Google Scholar]
- 30. Feinberg I, Maloney T, Cambell IG. Effects of hypnotics on the sleep EEG of healthy young adults: New data and psychopharmacologic implications. J Psychiatr Res 2000;34:423–438. [DOI] [PubMed] [Google Scholar]
- 31. Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc 1958;80:2587. [Google Scholar]
- 32. Dubocovich ML. Selective MT2 melatonin receptor antagonists block melatonin‐mediated phase advances of circadian rhythms. FASEB J 1998;12:1211–1220. [DOI] [PubMed] [Google Scholar]
- 33. Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 1997;19:91–102. [DOI] [PubMed] [Google Scholar]
- 34. Wehr TA, Aeschbach D Jr., Duncan WC. Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol 2001;535:937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akerstedt T, Froberg JE, Friberg Y, Wetterberg L. Melatonin excretion, body temperature and subjective arousal during 64 hours of sleep deprivation. Psychoneuroendocrinology 1979;4:219–225. [DOI] [PubMed] [Google Scholar]
- 36. Zhdanova IV, Wurtman RJ, Morabito C, Piotrovska VR, Lynch HJ. Effects of low oral doses of melatonin, given 2–4 hours before habitual bedtime, on sleep in normal young humans. Sleep 1996;19:423–431. [DOI] [PubMed] [Google Scholar]
- 37. Tzischinsky O, Shlitner A, Lavie P. The association between the nocturnal sleep gate and nocturnal onset of urinary 6‐sulfatoxymelatonin. J Biol Rhythms 1993;8:199–209. [DOI] [PubMed] [Google Scholar]
- 38. Dubocovich ML. Melatonin receptors: Are there multiple subtypes? Trends Pharmacol Sci 1995;16:50–56. [DOI] [PubMed] [Google Scholar]
- 39. Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994;13:1177–1785. [DOI] [PubMed] [Google Scholar]
- 40. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc Natl Acad Sci U S A 1995;92:8734–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharmacol Sci 1996;17:100–102. [DOI] [PubMed] [Google Scholar]
- 42. Slaugenhaupt SA, Roca AL, Liebert CB, Altherr MR, Gusella JF, Reppert SM. Mapping of the gene for the Mel1a‐melatonin receptor to human chromosome 4 (MTNR1A) and mouse chromosome 8 (Mtnr1a). Genomics 1995;27:355–357. [DOI] [PubMed] [Google Scholar]
- 43. Dubocovich ML. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 2003;8:d1093–d1108. [DOI] [PubMed] [Google Scholar]
- 44. Jin X, Von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol 2003;23:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 2002;309:151–62. [DOI] [PubMed] [Google Scholar]
- 46. Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA. Characterization of 2‐[125I]iodomelatonin binding sites in Syrian hamster peripheral organs. J Pharmacol Exp Ther 1999;290:334–340. [PubMed] [Google Scholar]
- 47. Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F, Fauchere JL, Delagrange P, Canet E, Boutin JA. Identification of the melatonin‐binding site MT3 as the quinone reductase 2. J Biol Chem 2000;275:31311–31317. [DOI] [PubMed] [Google Scholar]
- 48. Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol 2001;61:1369–1379. [DOI] [PubMed] [Google Scholar]
- 49. Miyamoto M, Nishikawa H, Doken Y, Hirai K, Uchikawa O, Ohkawa S. The sleep‐promoting action of ramelteon (TAK‐375) in freely moving cats. Sleep 2004;27:1319–1325. [DOI] [PubMed] [Google Scholar]
- 50. Hughes RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age‐related sleep‐maintenance insomnia: Assessment in a clinical trial of melatonin replacement. Sleep 1998;21:52–68. [PubMed] [Google Scholar]
- 51. Zhdanova IV, Wurtman RJ, Lynch HJ, Ives JR, Dollins AB, Morabito C, Matheson JK, Schomer DL. Sleep‐inducing effects of low doses of melatonin ingested in the evening. Clin Pharmacol Ther 1995;57:552–558. [DOI] [PubMed] [Google Scholar]
- 52. Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age‐related insomnia. J Clin Endocrinol Metab 2001;86:4727–4730. [DOI] [PubMed] [Google Scholar]
- 53. Zhdanova IV, Wurtman RJ. Efficacy of melatonin as a sleep‐promoting agent. J Biol Rhythms 1997;12:644–650. [DOI] [PubMed] [Google Scholar]
- 54. Arendt J. Importance and relevance of melatonin to human biological rhythms. J Neuroendocrinol 2003;15:427–431. [DOI] [PubMed] [Google Scholar]
- 55. Arendt J, Deacon S, English J, Hampton S, Morgan L. Melatonin and adjustment to phase shift. J Sleep Res 1995;4:74–79. [DOI] [PubMed] [Google Scholar]
- 56. Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res 2001;10:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hardeland R, Poeggeler B, Srinivasan V, Trankht I, Pandi‐Perumal SR, Cardinali DP. Melatonergic drugs in clinical practice. Arzneimittelforsung 2008;58:1–10. [DOI] [PubMed] [Google Scholar]
- 58. Yeleswaram K, McLaughlin LG, Knipe JO, Schabdach D. Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications. J Pineal Res 1997;22:45–51. [DOI] [PubMed] [Google Scholar]
- 59. Garfinkel D, Laudon M, Zisapel N. Improvement of sleep quality by controlled‐release melatonin in benzodiazepine‐treated elderly insomniacs. Arch Gerontol Geriatr 1997;24:223–231. [DOI] [PubMed] [Google Scholar]
- 60. Haimov I, Lavie P, Laudon M, Herer P, Vigder C, Zisapel N. Melatonin replacement therapy of elderly insomiacs. Sleep 1995;18:598–603. [DOI] [PubMed] [Google Scholar]
- 61. Faust R, Garratt PJ, Trujillo Perez MA, Piccio VJ, Madsen C, Stenstrom A, Frolund B, Davidson K, Teh MT, Sugden D. 7‐substituted‐melatonin and 7‐substitued‐1‐methylmelatonin analogues: Effect of substituents on potency and binding affinity. Bioorg Med Chem 2007;15:4543–4551. [DOI] [PubMed] [Google Scholar]
- 62. Pandi‐Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, Cardinali DP. Drug insight: The use of melatonergic agonists for the treatment of insomnia‐focus on ramelteon. Nat Clin Pract Neurol 2007;3:221–228. [DOI] [PubMed] [Google Scholar]
- 63. Rivara S, Lodola A, Mor M, Bedini A, Spandoni G, Lucini V, Pannacci M, Fraschini F, Scaglione F, Sanchez RO, et al N‐(substituted‐anilinoethyl)amides: Design, synthesis, and pharmacological characterization of a new class of melatonin receptor ligands. J Med Chem 2007;50:6618–6626. [DOI] [PubMed] [Google Scholar]
- 64. Uchikawa O, Fukatsu K, Tokunoh R, Kawada M, Matsumoto K, Imai Y, Hinuma S, Kato K, Nishikawa H, Hirai K, et al Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem 2002;45:4222–4239. [DOI] [PubMed] [Google Scholar]
- 65. Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high‐affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol 2006;46:140–148. [DOI] [PubMed] [Google Scholar]
- 66. Hibberd M, Stevenson SJ. A phase‐I open‐label study of the absorption, metabolism, and excretion of (14C)‐ramelteon (TAK‐375) following a single oral dose in healthy male subjects. Sleep 2004;27:A54. [Google Scholar]
- 67. Johnson MW, Suess PE, Griffiths RR. Ramelteon: A novel hypnotic lacking abuse liability and sedative side effects. Arch Gen Psychiatry 2006;63:1149–1157. [DOI] [PubMed] [Google Scholar]
- 68. Zammit G, Schwartz H, Roth T, Wright L, Sainati S, Zhang J. Phase III study of ramelteon in a first‐night‐effect model of transient insomnia. Sleep Med 2005;6:S50–S51. [DOI] [PubMed] [Google Scholar]
- 69. Zammit G, Erman M, Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med 2007;3:495–504. [PMC free article] [PubMed] [Google Scholar]
- 70. Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, Miyamoto M. Neurochemical properties of ramelteon (TAK‐375), a selective MT1/MT2 receptor agonist. Neuropharmacology 2005;48:301–310. [DOI] [PubMed] [Google Scholar]
- 71. Niles LP, Pickering DS, Sayer BG. HPLC‐purified 2‐[125I]iodomelatonin labels multiple binding sites in hamster brain. Biochem Biophys Res Commun 1987;147:49–56. [DOI] [PubMed] [Google Scholar]
- 72. Pickering DS, Niles LP. Pharmacological characterization of melatonin binding sites in Syrian hamster hypothalamus. Eur J Pharmacol 1990;175:71–77. [DOI] [PubMed] [Google Scholar]
- 73. Morgan PJ, Lawson W, Davidson G, Howell HE. Melatonin inhibits cyclic AMP in cultured ovine pars tuberalis cells. J Mol Endocrinol 1989;5:R5–R8. [Google Scholar]
- 74. Vanecek J, Vollrath L. Melatonin inhibits cyclic AMP and cyclic GMP accumulation in the rat pituitary. Brain Res 1989;505:157–159. [DOI] [PubMed] [Google Scholar]
- 75. Yukuhiro N, Kimura H, Nishikawa H, Ohkawa S, Yoshikubo S, Miyamoto M. Effects of ramelteon (TAK‐375) on nocturnal sleep in freely moving monkeys. Brain Res 2004;1027:59–66. [DOI] [PubMed] [Google Scholar]
- 76. Daley JT, Turner RS, Freeman A, Bliwise DL, Rye DB. Prolonged assessment of sleep and daytime sleepiness in unrestrained Macaca mulatta . Sleep 2006;29:221–231. [PubMed] [Google Scholar]
- 77. Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, Klassen TP, Witmans M. The efficacy and safety of drug treatments for chronic insomnia in adults: A meta‐analysis of RCTs. J Gen Intern Med 2007;22:1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose‐response study of ramelteon in patients with chronic primary insomnia. Sleep Med 2006;7:17–24. [DOI] [PubMed] [Google Scholar]
- 79. Hirai K, Kita M, Ohta H, Nishikawa H, Fujiwara Y, Ohkawa S, Miyamoto M. Ramelteon (TAK‐375) accelerates reentrainment of circadian rhythm after a phase advance of the light‐dark cycle in rats. J Biol Rhythms 2005;20:27–37. [DOI] [PubMed] [Google Scholar]
- 80. Inoue S, Shimazoe T, Moriya T, Shinohara K, Miyamoto M, Watanabe S. Effects of TAK‐375, a novel ML1‐selective melatonin receptor agonist, on entrainment function in circadian rhythm. J Pharmacol Sci 2003;91:90. [Google Scholar]
- 81. Miyamoto M. Effect of ramelteon (TAK‐375), a selective MT1/MT2 receptor agonist, on motor performance in mice. Neurosci Lett 2006;402:201–204. [DOI] [PubMed] [Google Scholar]
- 82. Spyraki C, Fibiger HC. A role for the mesolimbic dopamine system in the reinforcing properties of diazepam. Psychopharmacology (Berl) 1988;94:133–137. [DOI] [PubMed] [Google Scholar]
- 83. France CP, Weltman RH, Koek H, Cruz CM, McMahon LR. Acute and chronic effects of ramelteon in rhesus monkeys (Macaca mulatta): Dependence liability studies. Behav Neurosci 2006;120:535–541. [DOI] [PubMed] [Google Scholar]
- 84. Nishida N, Sasaki M, Wakasa Y, Awasaki Y, Yamamoto M, Yanagita T. Reinforcing effect of ramelteon assessed by intravenous self‐administration experiments in rhesus monkeys. Sleep 2005;28:A5. [Google Scholar]
- 85. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK‐375), a selective MT1/MT2‐receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303–307. [PubMed] [Google Scholar]
- 86. Roth T, Seiden D, Weigand S, Zhang J. A 2‐night, 3‐period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia. Curr Med Res Opinion 2007;23:1005–1014. [DOI] [PubMed] [Google Scholar]
- 87. Roth T, Seiden D, Sainati S, Wang‐Weigand S, Zhang J, Zee P. Effects of ramelteon on patient‐reported sleep latency in older adults with chronic insomnia. Sleep Medicine 2006;7:312–318. [DOI] [PubMed] [Google Scholar]
- 88. DeMicco M, Wang‐Weigand S, Zhang J. Long‐term therapeutic effects of ramelteon treatment in adults with chronic insomnia: A 1‐year study. Sleep 2006;29:A234. [Google Scholar]
- 89. Richardson G, Wang‐Weigand S, Zhang J, DeMicco M. Long‐term safety of ramelteon treatment in adults with chronic insomnia: A 1‐year study. Sleep 2006;29:A233. [Google Scholar]
- 90. Partinen M. Epidemiology of sleep disorders In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine, 2nd ed Philadelphia , PA : WB Saunders, 1994; 437–452. [Google Scholar]
- 91. Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: Incidence and clinical significance. J Clin Endocrinol Metab 1992;75:127–134. [DOI] [PubMed] [Google Scholar]
- 92. Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep phase response to melatonin. Lancet 1991;337:1121–1124. [DOI] [PubMed] [Google Scholar]
- 93. Oldani A, Ferini‐Strambi L, Zucconi M, Stankov B, Fraschini F, Smirne S. Melatonin and delayed sleep phase syndrome: Ambulatory polygraphic evaluation. Neuroreport 1994;6:132–134. [DOI] [PubMed] [Google Scholar]
- 94. Nagtegaal JE, Kerkhof GA, Smits MG, Swart AC, Van Der Meer YG. Delayed sleep phase syndrome: A placebo‐controlled cross‐over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res 1998;7:135–143. [DOI] [PubMed] [Google Scholar]
- 95. Herxheimer A, Petrie KJ. Melatonin for prevention and treatment of jet lag. Cochrane Database Syst Rev 2001;1:CD001520. [DOI] [PubMed] [Google Scholar]
- 96. Crasson M, Kjiri S, Colin A, Kjiri K, L'Hermite‐Baleriaux M, Ansseau M, Legros JJ. Serum melatonin and urinary 6‐sulfatoxymelatonin in major depression. Psychoneuroendocrinology 2004;29:1–12. [DOI] [PubMed] [Google Scholar]
- 97. Rubin RT, Heist EK, McGeoy SS, Hanada K, Lesser IM. Neuroendocrine aspects of primary endogenous depression. XI. Serum melatonin measures in patients and matched control subjects. Arch Gen Psychiatry 1992;49:558–567. [DOI] [PubMed] [Google Scholar]
- 98. Loo H, Dalery J, Macher JP, Payen A. Pilot study comparing in blind therapeutic effect of two doses of agomelatine, melatonin and 5‐HT2c receptors antagonist, in the treatment of major depressive disorders. Encephale 2002;28:356–362. [PubMed] [Google Scholar]
- 99. Montgomery SA, Kasper S. Severe depression and antidepressants: Focus on a pooled analysis of placebo‐controlled studies on agomelatine. Int Clin Psychopharmacol 2007;22:283–291. [DOI] [PubMed] [Google Scholar]
- 100. Kennedy SH, Kutcher SP, Ralevski E, Brown GM. Nocturnal melatonin and 24‐hour 6‐sulphatoxymelatonin levels in various phases of bipolar affective disorder. Psychiatry Res 1996;63:219–222. [DOI] [PubMed] [Google Scholar]
- 101. Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi‐perumal SR, Parry B, Cardinali DP. Melatonin in mood disorders. World J Biol Psychiatry 2006;7:138–151. [DOI] [PubMed] [Google Scholar]
- 102. Nair NP, Hariharasubramanian N, Pilapil C, Isaac I, Thavundayil JX. Plasma melatonin—an index of brain aging in humans? Biol Psychiatry 1986;21:141–150. [DOI] [PubMed] [Google Scholar]
- 103. Mirmiran M, Swaab DF, Kok JH, Hofman MA, Witting W, Van Gool WA. Circadian rhythms and the suprachiasmatic nucleus in perinatal development, aging and Alzheimer's disease. Prog Brain Res 1992;93:151–162. [DOI] [PubMed] [Google Scholar]
- 104. Skene DJ, Vivien‐Roels B, Sparks DL, Hunsaker JC, Pevet P, Ravid D, Swaab DF. Daily variation in the concentration of melatonin and 5‐methoxytryptophol in the human pineal gland: Effect of age and Alzheimer's disease. Brain Res 1990;528:170–174. [DOI] [PubMed] [Google Scholar]
- 105. Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer's disease. J Pineal Res 2005;38:145–152. [DOI] [PubMed] [Google Scholar]
- 106. Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep‐wake rhythm, cognitive and non‐cognitive functions in Alzheimer type dementia. J Nippon Med Sch 2003;70:334–341. [DOI] [PubMed] [Google Scholar]
- 107. Caldinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer's disease. Neuroendocrinol Lett 2002;23(Suppl):20–23. [PubMed] [Google Scholar]
- 108. Mahlberg R, Kunz D, Sutej I, Kuhl KP, Hellweg R. Melatonin treatment of day‐night rhythm disturbances and sundowning in Alzheimer disease: An open‐label pilot study using actigraphy. J Clin Psychopharmacol 2004;24:456–459. [DOI] [PubMed] [Google Scholar]


