Abstract
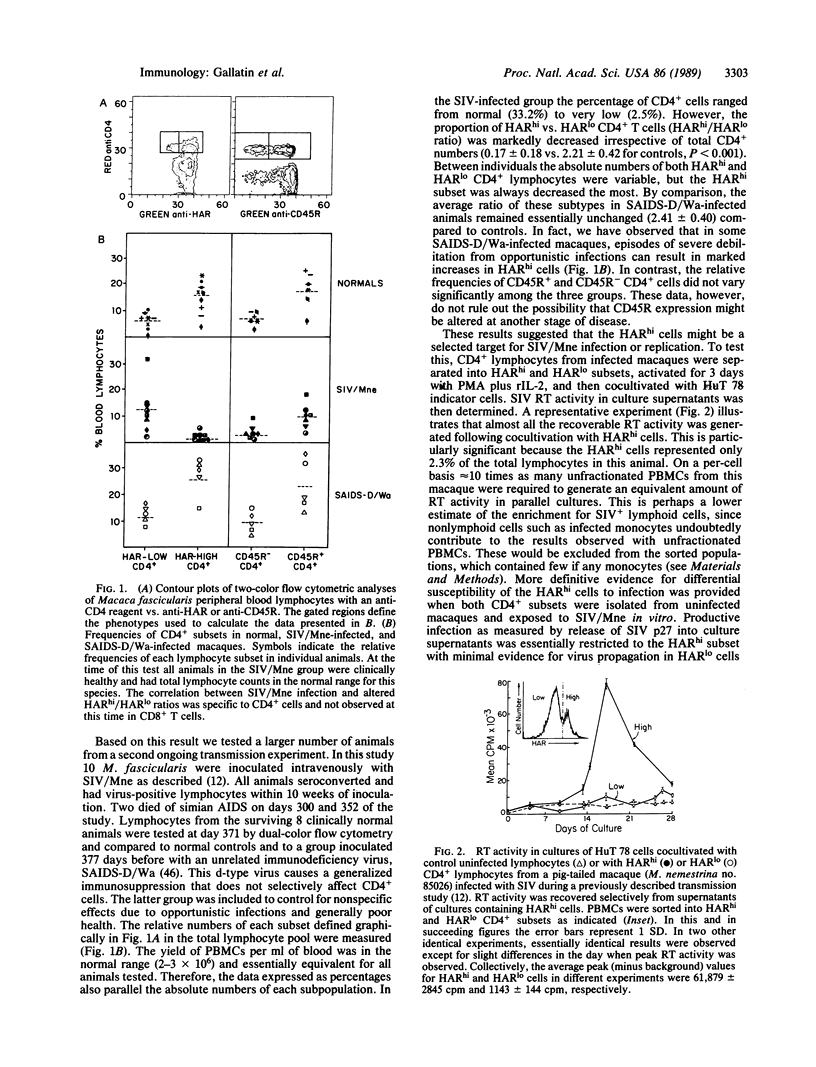
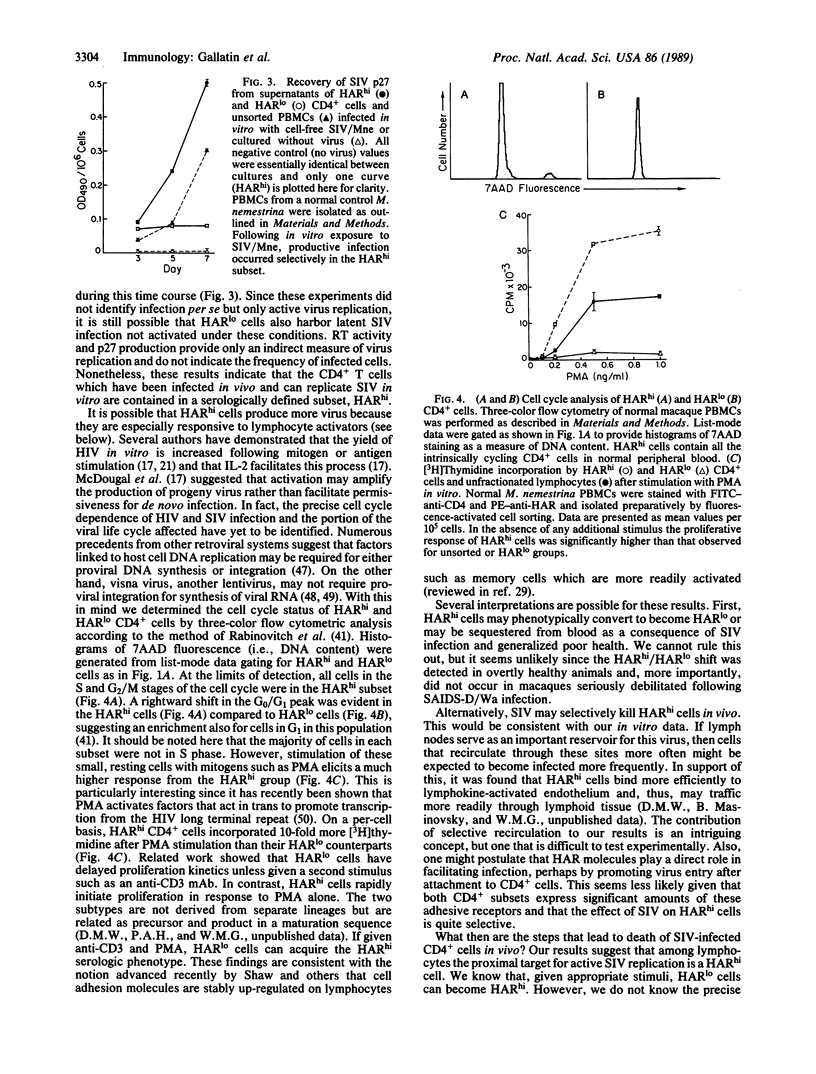
Although all CD4+ cells theoretically are at risk for infection by human immunodeficiency viruses or the related simian immunodeficiency viruses found in Old World monkeys, only a small proportion of CD4+ lymphocytes from infected individuals have detectable virus. This suggests that immunodeficiency viruses may replicate predominantly in a minor subset or activated form of CD4+ T cells, a possibility we examined in macaques infected with a simian immunodeficiency virus isolate, SIV/Mne. Macaque CD4+ lymphocytes could be divided into two subtypes that differed in their level [high (hi) or low (lo)] of expression of a class of heterotypic adhesion receptors (HARs). In blood from animals infected with SIV/Mne, HARhi CD4+ T cells were lost selectively compared to HARlo CD4+ cells and, when cultured, exhibited 50-fold more recoverable reverse transcriptase activity. The HARhi CD4+ subset was also markedly more susceptible to productive infection following exposure to SIV/Mne in vitro. Both subsets are composed primarily of small resting lymphocytes. However, HARhi cells respond differentially to mitogenic stimulation and may thus be more likely to provide the cellular factors necessary to initiate or enhance virus replication. Thus, HAR expression may prove useful both as a prognostic indicator in immunodeficiency virus infection and as a tool to analyze pathogenesis of immunodeficiency viruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Morton W. R., Clark E. A., Tsai C. C., Ochs H. D., Ward J. M., Kuller L., Knott W. B., Hill R. W., Gale M. J. Inoculation of baboons and macaques with simian immunodeficiency virus/Mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988 Jun;62(6):2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A., Holly R. C., Dinndorf P. A., Shu G. Polypeptides on human B lymphocytes associated with cell activation. Hum Immunol. 1986 May;16(1):100–113. doi: 10.1016/0198-8859(86)90039-x. [DOI] [PubMed] [Google Scholar]
- Clement L. T., Yamashita N., Martin A. M. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988 Sep 1;141(5):1464–1470. [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Gatenby P. A., Kansas G. S., Xian C. Y., Evans R. L., Engleman E. G. Dissection of immunoregulatory subpopulations of T lymphocytes within the helper and suppressor sublineages in man. J Immunol. 1982 Nov;129(5):1997–2000. [PubMed] [Google Scholar]
- Giorgi J. V., Fahey J. L., Smith D. C., Hultin L. E., Cheng H. L., Mitsuyasu R. T., Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987 Jun 1;138(11):3725–3730. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Gupta S. Subpopulations of CD4+ (T4+) cells in homosexual/bisexual men with persistent generalized lymphadenopathy. Clin Exp Immunol. 1987 Apr;68(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Blum H., Scott J., Traynor B., Ventura P., Haase A. Slow virus visna: reproduction in vitro of virus from extrachromosomal DNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7212–7215. doi: 10.1073/pnas.81.22.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F. Human T lymphocyte antigens as defined by monoclonal antibodies. Immunol Rev. 1981;57:127–161. doi: 10.1111/j.1600-065x.1981.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Yetz J. M., Letvin N. L. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Li Y., Naidu Y. M., Butler C. V., Ochs M. F., Jaenel G., King N. W., Daniel M. D., Desrosiers R. C. Comparison of simian immunodeficiency virus isolates. Nature. 1988 Feb 18;331(6157):619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld H., Riedel N., Viglianti G. A., Hirsch V., Mullins J. I. Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature. 1987 Apr 9;326(6113):610–613. doi: 10.1038/326610a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Sattentau Q. J., Beverley P. C., Hearn J. P., Fitzgerald A. K., Zuckerman A. J., Weiss R. A. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature. 1987 Dec 3;330(6147):487–489. doi: 10.1038/330487a0. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Sattentau Q. J., Beverley P. C., Hearn J. P., Fitzgerald A. K., Zuckerman A. J., Weiss R. A. HIV infection of primate lymphocytes and conservation of the CD4 receptor. Nature. 1987 Dec 3;330(6147):487–489. doi: 10.1038/330487a0. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Cort S. P., Kennedy M. S., Cabridilla C. D., Feorino P. M., Francis D. P., Hicks D., Kalyanaraman V. S., Martin L. S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV). J Immunol Methods. 1985 Jan 21;76(1):171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Echenberg D. F., Jones B. M., Jaffe H. W., Feorino P. M., McDougal J. S. T-cytotoxic/suppressor cell phenotypes in a group of asymptomatic homosexual men with and without exposure to HTLV-III/LAV. Clin Immunol Immunopathol. 1986 Sep;40(3):505–514. doi: 10.1016/0090-1229(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Jones B. M., Echenberg D. F., Spira T. J., McDougal J. S. Phenotypic distribution of T cells of patients who have subsequently developed AIDS. Clin Immunol Immunopathol. 1987 Apr;43(1):82–87. doi: 10.1016/0090-1229(87)90159-0. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Masuda T., Tsujimoto H., Ishikawa K., Kodama T., Morikawa S., Nakai M., Honjo S., Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int J Cancer. 1988 Jan 15;41(1):115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Torres R. M., Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser excitation: applications to study of cell activation and the cell cycle of murine Ly-1 B cells. J Immunol. 1986 Apr 15;136(8):2769–2775. [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Fitzgerald K. A., Hussey R. E., Daley J. F., Schlossman S. F. Heterogeneity of human T4+ inducer T cells defined by a monoclonal antibody that delineates two functional subpopulations. J Immunol. 1982 Jan;128(1):463–468. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Benveniste R. E., Arthur L. O., Rabin H., Giddens W. E., Jr, Ochs H. D., Morton W. R., Tsai C. C. Characterization of exogenous type D retrovirus from a fibroma of a macaque with simian AIDS and fibromatosis. Science. 1984 Apr 20;224(4646):289–282. doi: 10.1126/science.6200929. [DOI] [PubMed] [Google Scholar]
- Terao K., Rose L. M., Sackett G. P., Clark E. A. Development of lymphocyte subsets in pigtailed macaques. Hum Immunol. 1988 Jan;21(1):33–48. doi: 10.1016/0198-8859(88)90079-1. [DOI] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Vuillier F., Lapresle C., Dighiero G. Comparative analysis of CD4-4B4 and CD4-2H4 lymphocyte subpopulations in HIV negative homosexual, HIV seropositive and healthy subjects. Clin Exp Immunol. 1988 Jan;71(1):8–12. [PMC free article] [PubMed] [Google Scholar]



