Abstract
Background
When using botulinum toxin for the management of lateral epicondylitis, injection at a fixed distance from an anatomic landmark could result in inadequate paralysis of the intended muscle. We assessed the effectiveness of injection of botulinum toxin using precise anatomic measurement in individual patients.
Methods
In this randomized placebo-controlled trial, 48 patients with chronic refractory lateral epicondylitis were randomly assigned to receive a single injection of either botulinum toxin (60 units) or placebo (normal saline). The site of injection was chosen as a distance one-third the length of the forearm from the tip of the lateral epicondyle on the course of the posterior interosseus nerve. The primary outcome measure was intensity of pain at rest, measured with the use of a 100-mm visual analogue scale, at baseline and at 4, 8 and 16 weeks after injection.
Results
Compared with the placebo group, the group given botulinum toxin had significant reductions in pain at rest during follow-up (decrease at 4 weeks 14.1 mm, 95% confidence interval [CI] 5.8–22.3; at 8 weeks 11.5 mm, 95% CI 2.0–21.0; at 16 weeks 12.6 mm, 95% CI 7.7–17.8; p = 0.01). As for the secondary outcomes, the intensity of pain during maximum pinch decreased in the botulinum toxin group; there was no difference in pain during maximum grip or in grip strength between the two groups. All but one of the patients in the intervention group experienced weakness in the extension of the third and fourth fingers at week 4 that resolved by week 16. No serious adverse events were reported.
Interpretation
The use of precise anatomic measurement to guide injection of botulinum toxin significantly reduced pain at rest in patients with chronic refractory lateral epicondylitis. However, the transient extensor lag makes this method inappropriate for patients whose job requires finger extension. (ClinicalTrials.gov trial register no. NCT00497913.)
Botulinum toxin type A, a potent neuromuscular blocking agent, has been used for a variety of neuromuscular disorders, including cervical dystonia and blepharospasm.1 Recently, this drug has been used in the management of chronic lateral epicondylitis (tennis elbow).2–5 The extensor muscles of the wrist are paralyzed with the aim of releasing tension on the common extensor tendon and causing gradual healing of the epicondylitis. This approach is based on the same rationale as surgical release of the extensor carpi radialis brevis tendon.3
The most important factor in successfully paralyzing any muscle is the site of injection of the botulinum toxin.1 Injection at a fixed distance from anatomic landmarks, as was performed in previous clinical trials of botulinum toxin for the management of lateral epicondylitis,2–5 would result in inadequate paralysis of the intended muscle. It has been suggested that the extensor digitorum muscle, which is innervated by deep branches of the radial nerve, plays an important role in the development of lateral epicondylitis.6 We therefore chose to determine the injection site based on each patient’s forearm length and the course of the posterior interosseus nerve to achieve adequate paralysis of the extensor digitorum muscle. We conducted a randomized controlled trial to test whether this approach would be effective in reducing pain and improving grip strength.
Methods
Study design
The study was performed at the Imam Khomeini Hospital Complex, a 1230-bed tertiary care referral hospital affiliated with the Tehran University of Medical Sciences that serves patients from all over Iran. The study protocol was approved by the hospital’s ethical committee.
The participants of the study were chosen according to our inclusion and exclusion criteria (Box 1) from patients referred to our clinic from August 2007 to March 2008. All participants had to have a history of failed previous therapeutic interventions. The process and aim of the study as well as possible adverse effects of the drug injection were described to the patients. All of the enrolled patients signed a written informed consent.
Box 1. Inclusion and exclusion criteria
Inclusion criteria
-
Feeling pain on lateral epicondyle or on the course of extensor muscles in the forearm in at least two of the following situations:
- lateral epicondyle is pressed by physician
- passive flexion of wrist by physician
- active extension of wrist against counterforce applied by physician
- extension of third finger against counterforce applied by physician
Age 18–70 years
Duration of symptoms > 6 months
Previous complete course of physiotherapy or corticosteroid injection, or both
Exclusion criteria
Previous serious reaction to botulinum toxin injected for any purpose
Coexisting arthritis or arthralgia
Coexisting medial epicondylitis (golf elbow)
Bilateral lateral epicondylitis
Neurologic deficits*
Depression
Previous history of hand surgery
Use of any medicine or product containing corticosteroid in past 30 days
Current use of analgesic medications other than acetaminophen (especially nonsteroidal anti-inflammatory drugs)
Current enrolment in physiotherapy course
Pregnancy
Breastfeeding
Having a hobby or job that requires extension of fingers or wrist
Systemic disease (e.g., diabetes mellitus, collagen vascular disease)
Patients were randomly assigned to receive an injection of either botulinum toxin or normal saline. A computer-generated sequence with a block size of four patients was used for randomization. The patients were assigned consecutive numbers based on the order of enrolment in the study. A session for a pre-injection evaluation and injection was scheduled with the patients, and their assigned numbers were sent to a research assistant whose only role in this study was to prepare the solutions for the injection date. The research assistant was the only one with access to the randomization list. He prepared the injections according to the numbers received, and he delivered them in numbered envelopes to the clinic one hour before injection. Although both solutions were colorless, syringes were covered using opaque tape to prevent the physician and patients from guessing the contents. Injections were made by a physician who was not aware of the allocation status. Participants receiving botulinum toxin were given a single injection of 60 units of botulinum toxin A reconstituted in 1 mL of normal saline; those in the placebo group received an injection of 1 mL of 0.9% saline. Injections were performed using insulin syringes.
The injection site was individualized according to the length of each patient’s forearm (i.e., the distance between the lateral epicondyle and the tip of styloid process of radius). A distance of one-third the length of the forearm from the tip of the lateral epicondyle on the course of the posterior interosseus nerve (i.e., from the tip of the lateral epicondyle to the posterior midpoint of the wrist) was marked as the injection point. Based on previous cadaveric studies, the motor nerve branch enters the extensor digitorum and extensor carpi ulnaris muscles at a distance of 33% on average of the forearm length from the lateral epicondyle tip (range 17%–51% and 24%–43% of forearm length, respectively).7,8 Therefore, if one assumes an average forearm length of 27 cm,9 the site of injection should be 9 cm from the tip of the lateral epicondyle. Considering the range of anatomic variation for motor points7 and a diffusion radius of 3.5 cm for botulinum toxin,1 injection at this point should effectively paralyze the extensor digitorum muscle in 87.3% of patients and the extensor carpi ulnaris muscle in 94.1%.
Patients were allowed to take acetaminophen tablets (500 mg every four hours) for 48 hours after injection if they had any pain or discomfort associated with the injection. They were asked to avoid activities that required repetitive supination or forceful grasp.
Outcome measures
General characteristics of each participant, including age, sex, dominant hand, affected hand, duration of symptoms and forearm length, were recorded before the baseline evaluation. The primary outcome measure was pain at rest at four weeks after injection. Patients were asked to evaluate the intensity of pain using a 100-mm visual analogue scale, on which 0 represented no pain and 100 signified the most intense pain ever experienced.
Secondary outcome measures were pain reported by the patients during maximum grip and maximum pinch (pinching their index finger and thumb together with their elbow resting on a table), and the patient’s grip strength. Grip strength was recorded in kilograms using a calibrated hydraulic Jamar Hand Dynamometer (Sammons Preston, Bolingbrook, USA), according to the manufacturer’s instructions. The middle position of the dynamometer’s handle was used for all measurements. The physician recorded the average of three consecutive readings for data analysis. During each evaluation session, a research assistant evaluated the patient for the presence of extensor lag of the third and fourth fingers and asked if he or she had experienced any adverse reactions since the past follow-up. Open-ended questions with spontaneous reporting were used to detect adverse effects; all reports were evaluated for a possible association with the injection of botulinum toxin. The research assistant was not aware of the design of the study and was not involved in recording of other major and minor outcome measures of the study in order to preserve blinding. The patients were evaluated at baseline and at 4 weeks, 8 weeks and 16 weeks after injection.
We calculated the sample size using Stata software (version 8; StataCorp LP, College Station, USA). Wong and colleagues showed a decrease in intensity of pain at rest of 16 mm on a 100-mm visual analogue scale in the placebo group at week four compared with baseline.5 Because a generally accepted scale for the measurement of clinical outcomes in patients with lateral epicondylitis has not yet been reported, we considered results for patients with other rheumatologic diseases.10 From these data, we defined a clinically important therapeutic response in our study to be an improvement in pain intensity scores on a visual analogue scale of at least 20% in the treatment group over the improvement in the placebo group. By considering the standard deviation of four, we included 24 patients in each group to achieve a statistical power of 0.80 with a type I error of 0.01 for comparison of pain at rest between two groups four weeks after injections.
Statistical analysis
We report 95% confidence intervals (CIs) for differences between the botulinum toxin and placebo groups at baseline and p values for differences in primary and secondary outcome measures between the two groups over the study period. We used the Student t test and repeated-measurement analyses accordingly. The level of significance was set at a p value of 0.01 or less.
Funding
This study was funded by a contribution of the Vice Chancellor of Research at the Tehran University of Medical Sciences. Neither the vice chancellor nor the university had a role in the design, conduct of the study, data analysis or reporting of the results.
Results
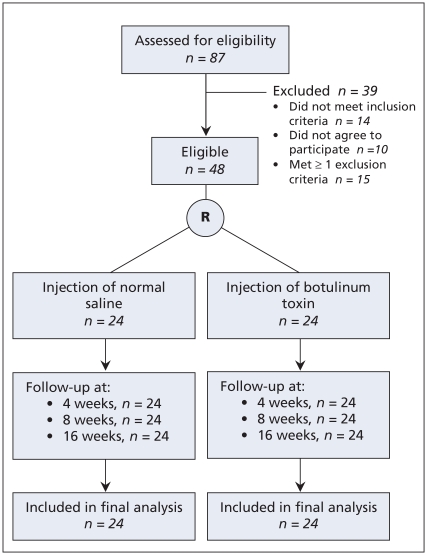
During the study period, all 87 patients with lateral epicondylitis who had been referred to our clinic were assessed for eligibility. We excluded 39 patients because they did not meet the inclusion criteria (n = 14), they did not wish to participate in the study (n = 10) or they met at least one of the exclusion criteria (n = 15). The remaining 48 patients were randomly assigned equally to the botulinum toxin group and the placebo group according to the randomization list. None of the patients were lost during follow-up, and the data for all 48 patients were included in the final analysis (Figure 1). The characteristics of the patients are provided in Table 1.
Figure 1.
Flow of participants through the study. R = randomization.
Table 1.
Characteristics of 48 patients with chronic lateral epicondylitis randomly assigned to receive injection of either botulinum toxin or placebo (saline)
| Characteristic | Botulinum toxin group n = 24 | Placebo group n = 24 |
|---|---|---|
| Age, yr, mean (SD) | 43.3 (7.8) | 44.2 (7.7) |
| Female sex, no. (%) | 22 (92) | 22 (92) |
| Right-hand dominant, no. (%) | 20 (83) | 18 (75) |
| Epicondylitis on right side, no. (%) | 8 (33) | 10 (42) |
| Length of forearm, cm, mean (SD) | 21.2 (1.8) | 21.5 (1.7) |
| Duration of symptoms, mo, mean (SD) | 12.5 (9.5) | 14.0 (8.4) |
Note: SD = standard deviation.
Pain
At baseline, the intensity of pain at rest was similar in the botulinum toxin and placebo groups (between-group difference in mean visual analogue score 2.3 mm, 95% CI −9.5 to 14.2). The intensity decreased significantly during follow-up in the botulinum toxin group compared with the placebo group (p = 0.01) (Table 2).
Table 2.
Mean pain intensity scores on 100-mm visual analogue scale and handgrip strength in botulinum toxin and placebo groups at baseline and at 4, 8 and 16 weeks after injection
| Outcome measure | Group; mean (SD) |
Difference in mean (95% CI) | p value (repeated measurement) | |
|---|---|---|---|---|
| Botulinum toxin n = 24 | Placebo n = 24 | |||
| Pain score at rest, mm | ||||
| Baseline | 48.8 (23.7) | 46.4 (16.2) | 2.3 (−9.5 to 14.2) | 0.010* |
| Week 4 | 20.4 (15.9) | 34.5 (12.2) | 14.1 (5.8 to 22.3) | |
| Week 8 | 17.9 (18.0) | 29.4 (14.5) | 11.5 (2.0 to 21.4) | |
| Week 16 | 3.9 (6.0) | 16.7 (10.5) | 12.6 (7.7 to 17.8) | |
| Pain score during maximum grip, mm | ||||
| Baseline | 65.8 (22.0) | 65.0 (18.3) | 0.8 (−10.9 to 12.6) | 0.22 |
| Week 4 | 52.0 (23.3) | 57.4 (18.2) | 5.3 (−6.8 to 17.5) | |
| Week 8 | 43.8 (23.1) | 51.5 (20.1) | 7.8 (−4.8 to 20.4) | |
| Week 16 | 18.8 (10.0) | 30.6 (15.6) | 11.8 (4.2 to 19.4) | |
| Pain score during maximum pinch, mm | ||||
| Baseline | 42.6 (26.5) | 45.1 (19.0) | 2.4 (−11.0 to 15.9) | 0.004* |
| Week 4 | 17.2 (16.8) | 32.7 (12.6) | 15.4 (6.8 to 24.1) | |
| Week 8 | 12.2 (14.9) | 26.6 (11.3) | 14.4 (6.8 to 22.2) | |
| Week 16 | 5.1 (9.7) | 13.6 (8.3) | 8.5 (3.1 to 13.9) | |
| Maximum grip strength, kg | ||||
| Baseline | 17.4 (5.2) | 18.8 (5.0) | 1.4 (−1.5 to 4.4) | 0.02 |
| Week 4 | 14.5 (4.5) | 19.0 (4.6) | 4.5 (1.8 to 7.1) | |
| Week 8 | 13.1 (4.4) | 18.4 (4.8) | 5.3 (2.6 to 8.0) | |
| Week 16 | 17.1 (5.4) | 18.8 (4.8) | 1.7 (−1.2 to 4.7) | |
Note: CI = confidence interval, SD = standard deviation.
Statistically significant difference.
The intensity of pain during maximum grip was also similar in the two groups at baseline (between-group difference in mean visual analogue score 0.8 mm, 95% CI −10.9 to 12.6). It decreased more in the botulinum toxin group than in the placebo group during follow-up; however, the difference was not statistically significant (p = 0.22) (Table 2).
The intensity of pain during maximum pinch was similar in the two groups at baseline (between-group difference 2.4 mm, 95% CI −11.0 to 15.9). As with pain at rest, it decreased significantly during follow-up in the botulinum toxin group compared with the placebo group (p = 0.004) (Table 2).
Grip strength
The mean maximum grip strength was similar in the two groups at baseline (between-group difference 1.4 mm, 95% CI −1.5 to 4.4). Although this value decreased in the botulinum toxin group during follow-up, the between-group differences were not significant (p = 0.02) (Table 2).
Extensor lag
Of the patients in the botulinum toxin group, all but one experienced weakness in the extension of the third and fourth fingers at week 4 that interfered with functioning at work. However, the paralysis resolved at week 8 in one patient and at week 16 in the rest of the patients. None of the patients in the placebo group experienced extensor lag.
Adverse reactions
The reported adverse reactions and their frequency in each group are summarized in Table 3.
Table 3.
Reported adverse reactions immediately after injection and during time frames between follow-up assessments
| Adverse reaction; time frame | Group; no. of patients | |
|---|---|---|
| Botulinum toxin | Placebo | |
| Pain at injection site | ||
| After injection | 3 | 2 |
| Weeks 0–4 | 7 | 2 |
| Weeks 4–8 | 0 | 0 |
| Weeks 8–16 | 0 | 0 |
| Tingling sensation around injection site | ||
| After injection | 0 | 0 |
| Weeks 0–4 | 5 | 0 |
| Weeks 4–8 | 0 | 0 |
| Weeks 8–16 | 0 | 0 |
| Subjective feeling of muscle spasm around injection site | ||
| After injection | 0 | 0 |
| Weeks 0–4 | 8 | 0 |
| Weeks 4–8 | 0 | 0 |
| Weeks 8–16 | 0 | 0 |
| Total | 23 | 4 |
Interpretation
We found that the intensity of pain at rest decreased significantly among patients with lateral epicondylitis after botulinum toxin was injected at a site based on precise anatomic measurement of each patient’s forearm length. The same was true for intensity of pain during maximum pinch. However, this treatment method caused a decline in grip strength and resulted in extensor lag.
If a 20% reduction in pain intensity is considered a clinically significant response in patients with rheumatoid arthritis,10 injection of botulinum toxin in our study provoked such a response. Plazeck and colleagues4 and Wong and colleagues5 reported a reduction in pain intensity similar to that in our study; however, they did not measure the intensity of pain during maximum grip or maximum pinch. Reports of pain in the extensor muscles of the forearm while the flexor muscles are used are common among patients with lateral epicondylitis;11 some patients experience intense pain during gripping while feeling minimal or no pain at rest. Therefore, we felt that measuring pain intensity during gripping would be a more precise measure of treatment. However, we did not observe a significant reduction in pain intensity during gripping in the botulinum toxin group. This might be because the participants in our study had chronic refractory epicondylitis, or because pain intensity following gripping could be more resistant to therapy and would generally take longer to respond to management.4 We did find a significant reduction in pain during maximum pinch in the botulinum toxin group.
The decrease in grip strength observed in the botulinum toxin group was greater than in the placebo group; however, the difference was not statistically significant and grip strength returned to its baseline level at week 16. Our findings were similar to those of Wong and colleagues,5 who reported no significant difference in grip strength between the botulinum toxin and placebo groups during the course of their study. Hayton and colleagues proposed that grip strength can be used as a precise objective measure indicative of pain intensity perceived by the patients.2 However, the reduction of grip strength in our study could not be explained only by the degree of pain experienced by the patient. We observed a high rate of extensor paralysis, which can result in decreased stabilization of the wrist during gripping12 and transient reduced grip strength in turn.13
Electrodiagnostically guided injection of botulinum toxin for the management of lateral epicondylitis has been reported to show high rates of success in producing paralysis and favourable clinical outcomes.14 However, this technique has limitations, including the need for local expertise and equipment, discomfort and pain during its application, and the multiplicity of motor points of a single muscle (only one of the nerve branches to the muscle may be detected with electrodiagnostic studies; if it is not the main branch to the muscle, the paralysis induced by botulinum toxin injection is not adequate). To overcome these limitations, we devised an anatomically based approach to determine the site of injection in individual patients that can easily be used in routine practice. The success rate of inducing paralysis in our study was 96%, as compared with less than 60% in previous studies, even in those that used considerably higher doses of botulinum toxin.2,3
Strengths and limitations
To preserve blinding of the physician who gave the injections and assessed pain and grip strength, extensor lag was assessed by a research assistant who was not aware of the study design. Also, the patients were asked to hold the dynamometer before the physician entered the room so he would not realize the existence of extensor lag.
As for the limitations of our study, we could not ensure that participants were blinded to the drug they received because of the high rate of extensor lag in the botulinum toxin group. Another limitation was that more than 90% of the participants were women. Most of the male participants did not meet the inclusion criteria, had at least one of the exclusion criteria or did not consent to participate in the study because they thought that development of extensor lag would affect their ability to work. Small sample size was another limitation. We calculated the sample size according to the primary outcome. The differences in secondary outcome measures between the two groups may have been significant with larger samples. Finally, our follow-up period was only 4 months. We are unable to draw conclusions about whether the effect of botulinum toxin injection would persist beyond that time.
Conclusion
Our trial showed that the use of anatomic measurement to guide injection of botulinum toxin can be effective in the management of chronic lateral epicondylitis. This approach is easily implemented and does not require complex methods. However, because of the high rate of transient extensor lag, it should be reserved for patients whose job does not require finger extension. Further research is needed to determine whether the pain-relieving effects of the treatment remain or diminish after four months.
Footnotes
Funding: This study was funded by a contribution of the Vice Chancellor of Research at the Tehran University of Medical Sciences. Neither the vice chancellor nor the university had a role in the design, conduct of the study, data analysis or reporting of the results.
Previously published at www.cmaj.ca
Competing interests: None declared.
Contributors: Ramin Espandar and Javad Mortazavi contributed to the study concept and, with Mahmood Farzan, the study design. Pedram Heidari, Mohsen Rostami and Shideh Yazdanian were responsible for the acquisition of data. Mohammad Reza Rasouli and Soheil Saadat contributed to the analysis and interpretation of the data. Pedram Heidari, Mohammad Reza Rasouli, Mohsen Rostami and Shideh Yazdanian drafted the manuscript. Ramin Espandar, Soheil Saadat, Mahmood Farzan and Javad Mortazavi critically revised the manucript. All of the authors approved the final version of the manuscript submitted for publication.
Includes entrapment of radial nerve, signs of compression of nerve root, decreased muscle force other than grip strength, multiple sclerosis and history of seizure.
This article has been peer reviewed.
REFERENCES
- 1.Seyler TM, Smith BP, Marker DR, et al. Botulinum neurotoxin as a therapeutic modality in orthopaedic surgery: more than twenty years of experience. J Bone Joint Surg Am. 2008;90(Suppl 4):133–45. doi: 10.2106/JBJS.H.00901. [DOI] [PubMed] [Google Scholar]
- 2.Hayton MJ, Santini AJA, Hughes PJ, et al. Botulinum toxin injection in the treatment of tennis elbow: a double-blind, randomized, controlled, pilot study. J Bone Joint Surg Am. 2005;87:503–7. doi: 10.2106/JBJS.D.01896. [DOI] [PubMed] [Google Scholar]
- 3.Keizer SB, Rutten HP, Pilot P, et al. Botulinum toxin injection versus surgical treatment for tennis elbow: a randomized pilot study. Clin Orthop Relat Res. 2002:125–31. doi: 10.1097/00003086-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Placzek R, Drescher W, Deuretzbacher G, et al. Treatment of chronic radial epicondylitis with botulinum toxin A: a double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89:255–60. doi: 10.2106/JBJS.F.00401. [DOI] [PubMed] [Google Scholar]
- 5.Wong SM, Hui AC, Tong PY, et al. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2005;143:793–7. doi: 10.7326/0003-4819-143-11-200512060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Fairbank SM, Corlett RJ. The role of the extensor digitorum communis muscle in lateral epicondylitis. J Hand Surg [Br] 2002;27:405–9. doi: 10.1054/jhsb.2002.0761. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Pho RWH, Pereira BP, et al. Distribution of primary motor nerve branches and terminal nerve entry points to the forearm muscles. Anat Rec. 1997;248:456–63. doi: 10.1002/(SICI)1097-0185(199707)248:3<456::AID-AR19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Linell EA. The distribution of nerves in the upper limb, with reference to variabilities and their clinical significance. J Anat. 1921;55:79–112. [PMC free article] [PubMed] [Google Scholar]
- 9.Karwowski W, Marras WS. The occupational ergonomics handbook. Boca Raton (FL): CRC Press; 1999. [Google Scholar]
- 10.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 11.Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38:1408–23. doi: 10.1080/00140139508925198. [DOI] [PubMed] [Google Scholar]
- 12.Snijders CJ, Volkers AC, Mechelse K, et al. Provocation of epicondylalgia lateralis (tennis elbow) by power grip or pinching. Med Sci Sports Exerc. 1987;19:518–23. [PubMed] [Google Scholar]
- 13.Mogk JP, Keir PJ. The effects of posture on forearm muscle loading during gripping. Ergonomics. 2003;46:956–75. doi: 10.1080/0014013031000107595. [DOI] [PubMed] [Google Scholar]
- 14.Morre HH, Keizer SB, van Os JJ. Treatment of chronic tennis elbow with botulinum toxin [letter] Lancet. 1997;349:1746. doi: 10.1016/s0140-6736(05)62958-3. [DOI] [PubMed] [Google Scholar]