Abstract
Purpose
Gastrointestinal (GI) symptoms are prevalent, often persistent, and detrimental to patients’ quality of life. This review discusses evaluation of GI symptoms as patient-reported outcomes (PROs), and presents an information technology (IT)-based system for symptom monitoring and management. The electronic PRO (ePRO) system is then placed within the larger context of rapid learning healthcare, a concept currently under development in which data obtained through both research and clinical care continuously build large datasets for analysis, seed future research, fuel expansion of the evidence base, and support clinical decision-making.
Recent findings
PROs are increasingly recognized as valid measures of symptoms, functional status, and quality of life. They have demonstrated prognostic significance and are being developed as a component of toxicity reporting in clinical trials. Recent studies have validated an IT-based approach for collecting ePROs in routine clinical care. The system is feasible and acceptable; electronic and paper-based data, collected on validated assessment instruments, are equivalent; ePRO collection supports real-time symptom monitoring and management. The ePRO system represents a first step toward implementing rapid learning healthcare at the clinic level.
Summary
EPROs provide a rich source of information to support monitoring and clinical management of troubling symptoms such as GI complaints.
Keywords: signs and symptoms, digestive (MeSH), palliative care (MeSH), terminal care (MeSH), patient-reported outcomes, rapid learning healthcare
Introduction
Resulting from medication side effects or underlying disease, gastrointestinal (GI) symptoms are among the most common and unpleasant side effects experienced by advanced cancer patients. Good symptom management involves routine assessment and monitoring of response to treatment over time. This review article describes: (1) recent development of methodologies for collecting and using patient-reported outcomes (PROs) to guide palliative symptom management; (2) the emergence of the rapid learning healthcare concept, and of the rapid learning cancer clinic as a prototype for its implementation in actual clinical environments; and, (3) the use of PRO methodologies to manage GI symptoms in a rapid learning cancer clinic model.
Background
Gastrointestinal (GI) symptoms plague a large proportion of patients in the palliative phase of disease. These symptoms can arise either from the disease itself or as side effects of treatment, particularly cancer chemotherapy. While this review article does not focus on recent approaches to GI symptom management, we note here the magnitude of the problem and point to evidence-based clinical practice guidelines to support clinical management of GI symptoms.
Prevalence and variety of gastrointestinal symptoms
Cancer patients with advanced life-limiting illness present a host of GI symptoms which may arise from medication side effects or the underlying disease. Untreated, nausea and/or vomiting occur in 21-68% of all advanced cancer patients,1, 2 and in 70-80% of cancer patients receiving chemotherapy.3 A systematic review of 44 studies, pooling data from over 25,000 patients with incurable cancer, reported a 31% prevalence of nausea and 20% prevalence of vomiting.4 Potentially leading to serious metabolic disturbance, malnutrition, electrolyte disturbances, and other physiological repercussions, nausea and vomiting can also impair functionality and erode the patient’s quality of life.2
Diarrhea is a common side effect of certain chemotherapeutic regimens; symptom prevalence ranges from 50-80% depending on the medication.5 Among heterogeneous cancer patients, the prevalence of moderate to severe diarrhea has been reported at 14%.6 As well as induced by chemotherapy, this symptom can be directly caused by certain cancer types, notably GI tract tumors and neuroendocrine tumors. If not properly managed, diarrhea and its complications can be debilitating and life-threatening. Risks of persistent or severe diarrhea include acute dehydration, renal insufficiency, dramatic weight loss, weakness, electrolyte imbalances, and infection. Diarrhea also compromises quality of life, and may interfere with adherence to treatment schedules.7
Constipation, a common symptom among elderly patients in general, afflicts 50-87% of terminally ill cancer patients.8-11 It can be physically, emotionally, and socially stressful, and can be associated with an array of troubling concerns: headache, fatigue, abdominal swelling and pain, nausea and vomiting, anorexia, hemorrhoids, and urinary complications.8, 12 Constipation can be more distressing to patients than pain,13, 14 and can cause some patients to decline further analgesic treatment.8, 11, 15
Other GI symptoms – including anorexia, heartburn, dysphagia, and pain – are frequently encountered in patients with advanced cancer. Like nausea/vomiting, diarrhea, and constipation, these complaints are highly subjective and often cause patients considerable distress. Accurate and regular assessment, to closely monitor their occurrence, severity, and progression, is the critical first step in medical management of these symptoms.
Medical management of GI symptoms
Clinical practice guidelines form the foundation of evidence-based management for GI symptoms in the advanced cancer patient. While it is not the purpose of this article to describe or review current guidelines, the reader can locate these resources through professional organizations such as the Multinational Association for Supportive Care in Cancer (MASCC) and National Comprehensive Cancer Network (NCCN). Although clear instructions are outlined for the management of common problems like nausea and vomiting, not all symptoms have their own guideline. In these cases, reference to a basic palliative and supportive care guideline or handbook provides a solid foundational approach.
Basic symptom management fundamentally includes: (a) routine assessment of the symptom, and (b) iterative adjustment of treatment based upon assessment results. While longitudinal assessment is recognized as central to symptom management, the exigencies of day-to-day clinical practice often compromise providers’ ability to gather, on a regular basis, a comprehensive report on the patient’s symptoms. Further complicating routine symptom assessment is the at least partially subjective nature of many GI symptoms, making them best described by patients themselves. However, methods of data collection that directly capture patients’ experiences have, until recently, been lacking.
Electronic patient-reported outcomes: A new approach to symptom assessment
Although physicians have typically solicited symptom-related information from patients during clinical visits, the patient’s report of symptoms is increasingly becoming (a) formalized as a valid clinical measure, and (b) standardized and tested as research data.
Patient-reported outcomes
The term, “patient-reported outcomes” (PROs) refers to a wide range of potential types of measurement, all of which gather data directly from patients, typically through questionnaires which they complete. An umbrella term, PROs are now generally considered to include any endpoint derived from patient reports, whether the data be collected at the clinic, in a diary, or by other means; methods of PRO data collection include single-item outcome measures, event logs, symptom reports, and formal assessment instruments.16 The most common domains of PRO assessment are symptoms, functionality/impairment, health-related quality of life (HRQOL), and quality of life (QOL).17
PROs have increasingly gained acceptance as important and valid measures of patients’ symptoms, experiences, and QOL.18-20 Some studies suggest that patient-reported measures may more accurately portray the patient’s experience than do physician-recorded symptom reports. In a Dutch prospective observational study, the severity of eight GI complaints was independently scored by both patients and general practitioners (GPs) before and after patients were treated with esomeprazole. Weighted kappa values indicated poor to moderate agreement on symptom severity, with similarly poor agreement on symptom presence/absence. Certain systematic differences in scoring were found: the GPs tended to underestimate the severity of belching, nausea, early satiety, vomiting, and abdominal pain; additionally, GPs tended to overestimate the treatment effect for belching and lower abdominal pain, but to underestimate the treatment effect for nausea.21 An earlier (2005) United States (US) study found better concordance between clinician and patient scoring. Lung and genitourinary cancer patients, and their clinicians, completed 400 paired surveys using a questionnaire comprising 11 Common Toxicity Adverse Criteria Adverse Event (CTCAE)-reported symptoms. For most symptoms, agreement between patient and clinician was high; most discrepancies fell within a one-point grade difference. Agreement was higher for directly observable symptoms, such as vomiting and diarrhea, than for more subjective symptoms, such as fatigue and dyspnea. Although differences in symptom reporting rarely would have changed treatment decisions or dosing, patients generally assigned greater severity to symptoms than did clinicians.22
Propelling the movement toward collection and use of PROs, in both clinical care and research, is a growing recognition that traditional medical outcomes (particularly survival and progression) do not fully capture the patient’s experience of health, disease, or healthcare. This recognition reflects a desire to align patient assessment with the principles of patient-centered care – that is, to measure outcomes that have meaning and importance to patients themselves. Palliative care has always maintained this orientation toward assessment, in that the goals of palliative care have been reduction of symptom burden and optimization of QOL rather than increase in survival time or time to progression. Across other areas of medicine, acceptance of PROs reflects an acknowledgement that patients’ self-perceived well-being is a valid outcome in itself, and constitutes legitimate data to guide clinical decisions. In fact, evidence suggests that PRO assessment as part of routine clinical care improves communication and patient well-being.23 PROs are also being developed for use in toxicity reporting. A PRO version of the CTCAE, the predominant system for describing adverse events in oncology clinical trials24, is currently under development; preliminary studies showed that online patient self-reporting is a feasible long-term strategy for toxicity symptom monitoring during chemotherapy, even among patients with advanced cancer and high symptom burden.25
Prognostic significance of PROs
Several recent studies have drawn attention to the relationship between patient-reported measures and more traditional outcomes, notably survival. Multiple population- or intervention-specific studies have examined the prognostic significant of PROs, and particularly of QOL scores. One example is a well-designed, placebo-controlled, randomized trial of antioxidant supplements (beta carotene, vitamin E), in which Meyer et al. report on QOL measurement as an independent prognostic indicator for survival in patients with head and neck cancer. In pooled analysis of the randomized groups, multivariate statistics were used to determine the independent contributions of different factors to prognosis. The investigators reported that, for every 10-point increase in baseline HRQOL score, there was a corresponding 13% decrease in risk of mortality. At six months, the physical functioning domain of the HRQOL instrument was the only change score that independently predicted survival.26, 27
With respect to GI cancers, McKernan et al. examined the relationship between QOL, measured by the EORTC QLQC30, clinico-pathological characteristics, and survival in patients with gastro-esophageal cancer. In univariate analysis, EORTC QLQ-C30, global QOL, functionality (physical, role, cognitive, social), and several symptoms (fatigue, nausea/vomiting, pain, dyspnea, appetite loss, constipation) were statistically significantly associated with cancer-specific survival.28 In a clinical study of gemcitabine and infliximab, among 86 pancreatic cancer patients with involuntary significant weight loss (cachexia), Robinson et al. explored the relationship between PROs and survival. PRO endpoints included scores from the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Functional Assessment of Anorexia/Cachexia Therapy (FAACT), Brief Pain Inventory (BPI), and Short-Form 36 general health survey (SF-36). Baseline fatigue and physical-functioning scores predicted survival as well as, or better than, baseline Karnofsky Performance Status or hemoglobin level. A cut-point in the FACIT-F score strongly predicted mortality; patients with greater fatigue had a lower median overall survival than did those with less fatigue.29
To evaluate the cumulative evidence regarding PROs’ prognostic value, Gotay et al. performed a comprehensive review of studies examining the prognostic capacity of PROs in the context of cancer clinical trials. Eligible studies were: identified through MEDLINE, the Cochrane database, American Society of Clinical Oncology/European Society for Medical Oncology abstracts, and hand searches; published in English; and used multivariate analyses of PROs that controlled for one or more clinical factors. In 36 of 39 studies (n=13,874), at least one PRO was significantly associated with survival in multivariate analysis (p<0.05), with varying effect sizes. The most commonly assessed PRO was QOL, measured by the EORTC QLQ C30 in 56% of studies. Results indicated that PROs provide distinct prognostic information beyond standard clinical measures in cancer clinical trials.30 A year later, Quinten et al. performed a meta-analysis of randomized controlled trials that measured baseline HRQOL using the EORTC QLQ-C30, in order to definitively determine whether this QOL measure carries prognostic value. Included studies were initiated between 1986 and 2004, involved 11 cancer sites, were mostly Phase 3 trials, and measured QOL as a secondary outcome. In multivariate Cox regression analysis, HRQOL parameters that significantly predicted survival were physical functioning, pain, and appetite loss; predictive socio-demographic parameters were age, sex, and distant metastases. WHO performance status did not significantly predict survival.31
Development of electronic patient-reported outcome (ePRO) methodologies
Historically, PRO data have been collected using cumbersome paper questionnaires – a process which interrupts work flow and incurs personnel expense (e.g., for data entry), thereby introducing inefficiency and extra cost into clinical and/or research operations. Over recent years, new information technologies have been tested in the US for electronic collection of PROs; technologies for this sort of data collection include handheld devices (e.g., palm pilots, digital pens, tablet personal computers, interactive voice response systems, web interfaces). These electronic methods make it possible to gather clinical and research data in a cost-effective manner, at a diversity of locations including point of care (i.e., clinic, hospital), home, or other facility.32, 33 Efforts to develop PRO data collection methods are also underway in Europe, where the European Palliative Care Research Collaborative (EPCRC), a project funded by the European Union, is developing a computerized assessment and classification tool for pain, depression, and cachexia. A systematic approach will be applied for the tool development, with emphasis on multicultural and multilingual challenges.34
Are ePROs valid?
Any new assessment methodology requires validation prior to its use for research or clinical purposes. The Medical Outcomes Trust, a not-for-profit US organization that promotes the science of outcomes measurement, recommends examination of multiple attributes of new PRO assessment instruments: reliability (internal consistency, test-retest reliability, inter-rater reliability), validity (content validity, construct validity), responsiveness, interpretability, burden and alternate modes of administration, cultural and language adaptation.35 Multiple paper-based PRO instruments have been validated with respect to these attributes. Certain well-recognized and widely-accepted paper PRO assessment instruments are now frequently incorporated into the analyses of research studies; the Functional Assessment of Cancer Therapy (FACT) series 36, for example, has been used in numerous studies in the US to evaluate patients’ experiences with symptoms and their QOL. Reflecting the widespread acceptance of PROs and their incorporation into research, the US Food and Drug Administration issued a 2006 draft guidance on the use of PRO measures in clinical research, specifically in drug development to support medical product labeling claims.37 The FDA plans to release a final version of this guidance document in fall 2009.38
Studies that utilize ePRO methods either program existing, validated, assessment instruments onto the selected technology platform or develop new surveys for study purposes. In the former case, validation of the ePRO assessment hinges on evaluation of the equivalence of (a) data collected using the new electronic methodology, and (b) data collected using the original paper format. This validation provides assurance that the technology-based methodology for data collection does not compromise the integrity of the data collected using previously validated instruments.
Multiple studies have accomplished this paper vs. electronic validation task for PRO assessment instruments. A recent meta-analysis of 65 studies examined the equivalence of computer-based vs. paper versions of PROs used in clinical trials. The authors reported that computer- and paper-administered PROs are equivalent data collection methodologies; the very small mean differences found were neither statistically nor clinically significant.39 The authors cautioned, however, that their results directly compare the differences between assessments moved from paper to electronic versions, and cannot be generalized to all forms of electronic administration of PROs.39
Is it feasible to collect ePROs as a routine part of clinical care?
At Duke University Medical Center, we have developed a system for collecting ePROs in the course of routine clinical care. The Duke ePRO system is designed for use with diverse electronic data capture platforms, though the prototype system uses wireless tablet personal computers (e/Tablets). Surveys, programmed onto the tablet computers, appear one question per screen (Figure 1); the system can be set to queue up multiple surveys, selected for the specific patient from an on-screen menu, for seamless presentation to the patient (Figure 2).
Figure 1.
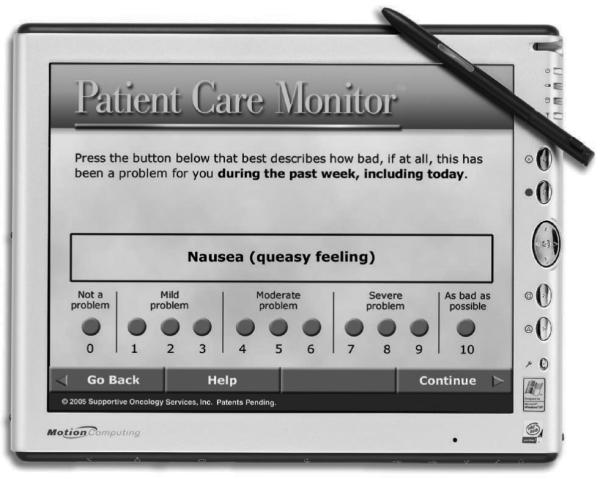
Screen shot of a sample symptom assessment item on the e/Tablet
With permission from Supportive Oncology Services, Inc. (SOS) Memphis, Tennessee.
Figure 2.
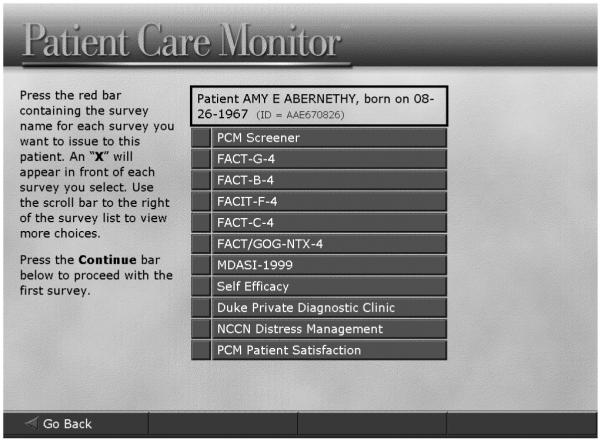
Customizable menu of surveys programmed onto an e/Tablet
With permission from Supportive Oncology Services, Inc. (SOS) Memphis, Tennessee.
In a feasibility study at Duke, we sought to determine whether cancer patients in the academic setting find a specific ePRO data collection technology (e/Tablets) logistically acceptable and satisfactory for communicating their symptoms to care providers. The study enrolled metastatic breast cancer patients with a prognosis of at least six months. E/Tablets were programmed with several well-recognized assessment instruments including the FACT – Breast Cancer,40 MD Anderson Symptom Inventory (MDASI),6 Functional Assessment of Chronic Illness Therapy – Fatigue subscale,41 and Self-Efficacy scale,42 as well as the Patient Care Monitor™ (PCM, a review of systems)43 and an eight-item satisfaction survey. Participants used e/Tablets to complete the surveys at four clinic visits within six months.
Participants’ (n=65) mean age was 54 years (standard deviation [SD] 12); 77% were Caucasian; 47% had less than a college degree. Regarding the e/Tablets, 94% reported that they were easy to read; 98%, easy to use to answer survey questions; 99%, easy to navigate; and 90%, a comfortable weight. Initially, 75% indicated satisfaction with the PCM for reporting symptoms; this proportion increased over time. By the last visit, 88% indicated willingness to recommend the PCM to other patients, and 74% felt that this system of ePRO reporting helped them remember symptoms to discuss with their doctors. Patients enjoyed the educational content which they could access after completing surveys. The e/Tablets thus proved to be a feasible and acceptable method of PRO data collection in an academic oncology clinic;44 results of this feasibility study were subsequently confirmed in two additional cancer populations – GI and lung cancer.
Validation study of an ePRO data collection approach across three cancer types
Further exploring the Duke ePRO data collection system, we next performed a validation study to determine whether data collected using the ePRO system were equivalent to data collected using standard paper-based assessment instruments. Sequential studies in three distinct clinics enrolled GI cancer (GIC, n=113), breast cancer (BC, n=65, see above), and lung cancer (LC, n=97) patients. At four visits within a six-month period, participants used e/Tablets to complete multiple assessment surveys including the FACT-General scale 36, MDASI, and PCM.
Additionally, at each visit, the participant completed a paper version of one of the electronic assessment instruments (e.g., FACT-G using e/Tablet and FACT-G on paper); paper instruments were completed before the electronic assessments. Cronbach’s alpha coefficients were calculated to verify internal consistency. Differences between electronic and paper scores were compared using raw scores and paired Student t-tests; results were verified with the Sign test.
All correlation coefficients were acceptable (all α>0.66). Responses collected via paper and electronic methods were statistically similar for both MDASI subscales (Severity, Interference), and for the FACT-G Physical and Emotional Wellbeing subscales. Paper and electronic scores on the FACT-G Social Wellbeing subscale differed statistically within BC and LC cohorts. We concluded from this study that ePRO data on most subscales were sufficiently similar to consider ePRO data as being “research quality.”45, 46 Implementation in three sites confirmed the logistical feasibility of this method of data collection: use of e/Tablets did not disrupt the clinic flow, and no internet or data security problems arose. The color-coded PCM reports, highlighting moderate and severe symptoms, were well-received by physicians, nurses, and social workers. (Figure 3)
Figure 3.
Report generated by the ePRO system, from Patient Care Monitor responses
With permission from Supportive Oncology Services, Inc. (SOS) Memphis, Tennessee.
Symptom assessment: Placing the patient’s experience at the center of new healthcare models
While reduction of symptoms has long been a mainstay of palliative care practice, healthcare delivery and research structures have not typically been constructed with patient-reported symptom assessment as a central purpose. Recent interest in the validity, prognostic capacity, and utility of PROs has prompted reconsideration of the driving forces behind clinical practice and research, and articulation of new models that emphasize the patient’s experience.
ePROs as the foundation of rapid learning healthcare
The concepts of “rapid learning healthcare” and the “learning healthcare system” have gained considerable momentum in the US since July 2006, when the Institute of Medicine Roundtable on Evidence-Based Medicine convened the Learning Healthcare System workshop. The resulting publication and ensuing national discussion have developed the vision of a new paradigm for healthcare, one that is driven by three fundamental purposes: (1) to generate and apply the best evidence relevant to each patient; (2) to propel scientific discovery “as a natural outgrowth of patient care;” and, (3) to support quality assessment and improvement, spark innovation, enhance patient safety, and allow payers to maximize healthcare value.47, 48
Initial formulations of rapid learning healthcare have described its development at the national (US) level, with the intention of facilitating overhaul of the research enterprise and update of Medicare reimbursement policies. Attention has focused on the use of new technologies and bioinformatic approaches to link the nation’s computerized clinical research databases, so as to support rapid, large-scale, collaborative, research projects. These studies, primarily examining the comparative effectiveness of treatments in sizeable real-world clinical populations, would make use of open-access biobanks with linked clinical, genomic, and environmental data.49 The US National Cancer Institute has actively advanced this vision through the Cancer Biomedical Informatics Grid (CaBIG®), part of a national Biomedical Informatics Grid that serves as an interoperable, interconnected, information technology platform to enable information sharing. Currently under construction, CaBIG® will connect over 60 NCI-designated Cancer Centers and numerous NCI community cancer centers in a national network. CaBIG® is the foundational infrastructure for a seamless clinical/research continuum, in which aggregated clinical outcomes data drive next-generation research; research results are then validated in clinical care to improve outcomes, while also contributing further to the evidence base. This “virtuous cycle” of clinical care informing research and research informing care lies at the heart of rapid learning healthcare.50
Although rapid learning healthcare discussions in the US emphasize national level coordination and processes, with primary focus on integrating large-scale datasets and connecting major institutions, there are multiple reasons why it is important to retain the patient’s perspective as paramount in the development of a rapid learning healthcare system. Patients represent the final decision-makers with respect to where they receive care, from which providers, and which treatments they agree to undergo. The research enterprise depends fundamentally on patients for participation in clinical trial; in those studies, it is patients who bear the risks and harms as well as benefits of new treatments.51 Patients alone possess certain critical data elements, such as satisfaction with care and the subjective components of PROs. And patients, ultimately, are the ones who will most directly experience the newly integrated clinical/research system, once developed.
To ensure that patients’ symptoms remain central outcomes of inquiry in rapid learning healthcare, and that the goals of learning are to improve indicators which patients value, Duke researchers propose that PROs be one of the fundamental building blocks of rapid learning healthcare. Routine collection of data directly from patients will support a system of data linkage that starts at the level of the individual – beginning with the patient’s chief complaint, incorporating additional information as it becomes available, and ultimately integrating a full range of patient-specific data in order to develop a comprehensive view of the patient’s status. The specificity to the individual patient, inherent in this approach, is the essence of personalized medicine and, indeed, of good clinical practice in palliative care.
An approach to rapid learning healthcare that begins at the patient level with ePROs is consistent with national initiatives in the US such as the Patient-Reported Outcomes Measurement Information System (PROMIS) Network, part of the National Institutes of Health Roadmap Initiative. PROMIS aims to improve the way in which PROs are selected and assessed in clinical research by establishing a publicly available resource of standardized, accurate, and efficient PRO measures. These measures capture major self-reported health domains (e.g., pain, fatigue, emotional distress, physical function, social function) that are relevant across chronic illnesses including cancer. PROMIS is also developing measures of self-reported health domains specifically targeted to cancer, such as sleep/wake function, sexual function, cognitive function, and the psychosocial impacts of the illness experience such as stress response and coping, shifts in self-concept, social interactions, and spirituality.52
A rapid learning healthcare model is well-suited to palliative care in several respects. First, rapid learning healthcare seeks to support each patient’s treatment with latest research evidence available from a broad range of sources (e.g., classic randomized controlled trials, observational studies, patient registries, database queries, population-based studies). This fundamentally evidence-based approach is particularly relevant to palliative medicine, where an historical paucity of traditional “evidence” (e.g., from randomized controlled trials) has impeded efforts to advance evidence-based practice. Second, in rapid learning healthcare, clinicians tailor care to the individual by using available evidence from a variety of sources, including large population-based datasets, to personalize treatments; the precision with which palliative care clinicians will be able to shape care to match individual needs and circumstances will increase as larger, more robust datasets become available to enable queries and support decision-making. Given the complexity of many clinical scenarios in palliative care, this tailoring capacity holds the promise of helping clinicians deliver more individually appropriate care to each patient, based on data obtained from other, similar, patients. Third, the rapid learning healthcare model values quality assessment and quality improvement as well as the evaluation of clinical and research outcomes; in palliative care, quality of care is a critical concern, one which stands to improve with a routine, technology-facilitated method for benchmarking quality and monitoring quality over time.
Rapid learning healthcare in operation: Use of an ePRO system to monitor GI symptoms
Within the Duke GI Oncology clinics, patients and care providers are regularly using an ePRO system to monitor GI and other symptoms. Completing a review of systems survey (PCM) programmed onto tablet computers, patients answer a comprehensive set of symptom-related questions with each visit. Once the patient has finished the survey, a report is printed for the clinician to aid him/her in rapidly identifying symptoms that might be changing over time. This real-time ePRO assessment contains several items specific to GI status and experiences; patients are queried regarding their symptoms related to indigestion, appetite changes, constipation, diarrhea, nausea, and vomiting. Responses to these questions inform the clinician and often impact treatment-related decision-making for GI symptom management.
Monitoring of GI symptoms through ePROs, in the framework of a rapid learning cancer clinic, can add greater responsiveness and attention to detail to the patient encounter. For example, a patient with lung cancer might not typically, or routinely, be asked if he is experiencing changes in appetite. This symptom is, however, one of the items in the review of systems survey which patients routinely complete in the Duke oncology clinics. If the patient’s responses to the ePRO survey reveal a worsening appetite, that score is highlighted in orange to draw the clinician’s attention. (Figure 3) The clinician can then discuss the appetite change with the patient, use the patient’s description to complement the ePRO system information, prescribe an intervention, and monitor the effectiveness of that intervention on subsequent visits based on changes in the patient’s scoring of his appetite. In this way, the ePRO system can help the clinician plan, personalize, and fine-tune longitudinal care using patients’ reports of their symptoms over time.
Generally, patients have adopted the ePRO practice with enthusiasm, both for the detailed assessment it provides and for the educational videos presented after the patient completes the survey(s). It also appears that patients may be more willing to initially report sensitive symptoms (e.g., sexual dysfunction) using the ePRO system rather than written or verbal communication; these symptoms, which clinicians may be less likely to address without prompting, can be of serious concern to patients.53
Plans to expand clinical implementation of the ePRO system are underway throughout the Duke Oncology clinics. Thus far, we have established the feasibility, acceptability, validity, and clinical utility of the system, and we have used it to gain a better understanding of our population’s symptom profile. In the March 2009 issue of Current Opinion in Supportive and Palliative Care, we reported on initial use of the ePRO system to describe and compare GI symptoms across cancer populations. In a study among breast, GI, and lung cancer patients, the incidence of severe patient-reported nausea was highest in breast cancer patients (17%, versus 14% and 4% in GI and lung cancer cohorts); GI patients reported more moderate to severe diarrhea (31%, versus 22% and 11% for breast and GI cancer cohorts); breast cancer patients reported more severe constipation (15%) and heartburn (11%) than did GI (7%, 5%) or lung (6%, 3%) cancer patients; and GI cancer patients experienced more moderate to severe decrease in appetite (36%) than did lung (30%) or breast (28%) cancer patients. These analyses illustrate one way in which ePRO data can help clinicians proactively anticipate GI symptoms, and then use the system to monitor patients’ experiences and the impact of interventions. 54
How is Duke moving from standard symptom reporting at the point of care to rapid learning in the GI oncology clinic? First, we use real-time reports to understand symptom prevalence and severity across the clinic population. Identified concerns are matched to patient education support materials, delivered to the patient using the e/Tablet in the waiting room. In certain areas, appropriate patient education materials are available; where they are not, we are working to develop them. Next, we focus on the most severe problems -- as indicated by high prevalence, intensity, or both -- and we conduct further analyses to determine strategies for alleviating the problem. For example, in the case of cancer-related anorexia/cachexia syndrome, we have partnered with drug developers, clinical trialists, and biostatisticians to develop and test a new suite of interventions for people with refractory symptoms. We use the ePRO system to identify individuals who potentially need these advanced interventions, and also use the ePRO system to track outcome measures for these patient, highlighting results of the intervention. Once we identify successful interventions, we will invest that new information back into the ePRO system through patient education and symptom triggers; this return of new knowledge to inform care for future patients represents the “learning” aspect of the rapid learning clinic, and fuels a cyclical process of assessment, intervention, monitoring, and learning.
Conclusion
GI symptoms represent an important class of PROs. Routine collection of patient-reported data on these symptoms can support good clinical care for advanced cancer patients while also helping to build an evidence base to support clinicians in managing these cancer-related symptoms. In this way, a PRO-based learning healthcare system, once fully implemented, could simultaneously advance the state of the science, improve patient outcomes, and enhance the patient-centeredness of care.
Acknowledgements
Components of the ePRO system at Duke were funded as follows: Development of the ePRO data collection system was funded through an Outcomes Research service agreement with Pfizer, Inc. Computer hardware was funded by Supportive Oncology Services (SOS), Inc. and subsequently by Duke University Hospital and Duke University Health System (DUHS). DUHS provided funds for the wireless system, some programming, and technical support. Research pilot studies were funded by the Duke Comprehensive Cancer Center and Duke Cancer Care Research Program.
Footnotes
Disclaimers: None
Competing interests: None
Complete Funding Declaration: No relevant funding to report
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NCI Nausea and Vomiting. 2008 September 9; http://www.cancer.gov/cancertopics/pdq/supportivecare/nausea/HealthProfessional/
- 2.Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Supportive Care in Cancer. 2006 Apr;14(4):348–353. doi: 10.1007/s00520-005-0897-1. [DOI] [PubMed] [Google Scholar]
- 3.Naeim A, Dy SM, Lorenz KA, Sanati H, Walling A, Asch SM. Evidence-based recommendations for cancer nausea and vomiting. Journal of Clinical Oncology. 2008 Aug 10;26(23):3903–3910. doi: 10.1200/JCO.2007.15.9533. [DOI] [PubMed] [Google Scholar]
- 4.Teunissen SCCM, Wesker W, Kruitwagen C, de Haes HCJM, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. Journal of Pain & Symptom Management. 2007 Jul;34(1):94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 5.NCI Gastrointestinal Complications. 2008 August 20; http://www.cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/HealthProfessional/
- 6.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000 Oct 1;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, 3rd, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. Journal of Clinical Oncology. 2004 Jul 15;22(14):2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 8.Choi YS, Billings JA. Opioid antagonists: a review of their role in palliative care, focusing on use in opioid-related constipation. Journal of Pain & Symptom Management. 2002 Jul;24(1):71–90. doi: 10.1016/s0885-3924(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 9.Mostafa SM, Bhandari S, Ritchie G, Gratton N, Wenstone R. Constipation and its implications in the critically ill patient. British Journal of Anaesthesia. 2003 Dec;91(6):815–819. doi: 10.1093/bja/aeg275. [DOI] [PubMed] [Google Scholar]
- 10.Smith S. Evidence-based management of constipation in the oncology patient. European Journal of Oncology Nursing. 2001;5:18–25. doi: 10.1054/ejon.2000.0119. [DOI] [PubMed] [Google Scholar]
- 11.Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliative Medicine. 1998;12(5):375–382. doi: 10.1191/026921698674125048. [DOI] [PubMed] [Google Scholar]
- 12.Kyle G. Constipation and palliative care - where are we now? International Journal of Palliative Nursing. 2007 Jan;13(1):6–16. doi: 10.12968/ijpn.2007.13.1.22776. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop G. A study of the relative frequency and importance of gastrointestinal symptoms and weakness in patients with far advanced cancer: Student paper. Palliative Medicine. 1989;4:37–44. [Google Scholar]
- 14.Holmes S. Use of a modified symptom distress scale in assessment of the cancer patient. International Journal of Nursing Studies. 1989;26(1):69–79. doi: 10.1016/0020-7489(89)90047-3. [DOI] [PubMed] [Google Scholar]
- 15.Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. Journal of Pain & Symptom Management. 2000 Feb;19(2):130–136. doi: 10.1016/s0885-3924(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 16.Wilke RJ, Burke LB, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Controlled Clinical Trials. 2004;25(6):535–532. doi: 10.1016/j.cct.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Doward LC, McKenna SP. Defining patient-reported outcomes. Value in Health. 2004 Sep-Oct;7(Suppl 1):S4–8. doi: 10.1111/j.1524-4733.2004.7s102.x. [DOI] [PubMed] [Google Scholar]
- 18.Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. European Journal of Cancer. 1997 Jun;33(7):1025–1030. doi: 10.1016/s0959-8049(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 19.Dancey J, Zee B, Osoba D, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Quality of Life Research. 1997 Mar;6(2):151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Webster K. Linking outcomes management to quality-of-life measurement. Oncology. 1997 Nov;11(11A):232–235. [PubMed] [Google Scholar]
- 21.*Fransen GAJ, Janssen MJR, Muris JWM, Mesters I, Knottnerus JA. Measuring the severity of upper gastrointestinal complaints: does GP assessment correspond with patients’ self-assessment? Family Practice. 2007 Jun;24(3):252–258. doi: 10.1093/fampra/cmm011. This study substantiates the claim that patient-reported measures represent valuable complementary information to the information on patients’ symptoms recorded by physicians. Indeed, for highly subjective symptoms, the patient’s report may even be more accurate and/or more clinically useful than documentation of the symptom using “objective” or physician-reported methods.
- 22.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncology. 2006 Nov;7(11):903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 23.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. Journal of Clinical Oncology. 2004 Feb 15;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 24.Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. Journal of Clinical Oncology. 2007 Nov 10;25(32):5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]
- 25.*Basch E, Iasonos A, Barz A, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. Journal of Clinical Oncology. 2007 Dec 1;25(34):5374–5380. doi: 10.1200/JCO.2007.11.2243. This study demonstrated that even patients with advanced cancer can use an ePRO system to report on their toxicity-related symptoms. Since adverse event reporting is a critical aspect of clinical trials, the potential to use the patient’s report in toxicity monitoring -- as well as in clinical decision-making -- implies a central role for ePROs in research, as well as clinical care.
- 26.*Meyer F, Fortin A, Gelinas M, et al. Health-related quality of life as a survival predictor for patients with localized head and neck cancer treated with radiation therapy. Journal of Clinical Oncology. 2009 Jun 20;27(18):2970–2976. doi: 10.1200/JCO.2008.20.0295. This study provides a useful example of the way in which investigators have demonstrated the prognostic value of PROs in specific cancer populations. Taken together, the data compellingly suggest that PROs can serve as a predictor of mortality. The association between fundamentally subjective and objective measures conjoins the new way of thinking about assessment (i.e., patient-reported, symptom- and QOL-focused) with traditional, objectively focused outcomes.
- 27.Browman GP, Berrang T, Smith S. Prognostic tools for cancer survival: a secondary role for quality-of-life measurement. Journal of Clinical Oncology. 2009 Jun 20;27(18):2902–2904. doi: 10.1200/JCO.2009.21.8438. [DOI] [PubMed] [Google Scholar]
- 28.*McKernan M, McMillan DC, Anderson JR, Angerson WJ, Stuart RC. The relationship between quality of life (EORTC QLQ-C30) and survival in patients with gastro-oesophageal cancer. British Journal of Cancer. 2008 Mar 11;98(5):888–893. doi: 10.1038/sj.bjc.6604248. This study, conducted in a GI cancer population, established statistically significant associations between QOL and survival, and between several severe symptoms (including GI symptoms -- nausea/vomiting, appetite loss, constipation) and survival. It reinforces, in the population of interest, the contention that patient-reported measures have prognostic value and correlate with objective outcomes.
- 29.*Robinson DW, Jr., Eisenberg DF, Cella D, Zhao N, de Boer C, DeWitte M. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. The Journal of Supportive Oncology. 2008 Jul-Aug;6(6):283–290. This interesting study found that certain PROs, notably fatigue and physical functioning, predicted survival as well as or better than the more “objective” indicators generally used to evaluate prognosis -- baseline Karnofsky Performance Status or hemoglobin level.
- 30.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. Journal of Clinical Oncology. 2008 Mar 10;26(8):1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 31.**Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncology. 2009 Sep;10(9):865–871. doi: 10.1016/S1470-2045(09)70200-1. Culminating a succession of studies exploring the prognostic significance of PROs, with particular focus on QOL measures, this meta-analysis found that certain patient-reported measures (physical functioning, pain, and appetite loss) significantly predicted survival whereas WHO performance status did not. Studies included in the meta-analysis were conducted in 11 cancers, and all measured QOL with the most widely used instrument for the purpose, the EORTC QLQ-C30. Recent and definitive, this study is likely to be referenced repeatedly as rationale for the use of PROs (and specifically QOL using the EORTC QLQ-C30) for predictive clinical and research purposes.
- 32.Ruland CM. Handheld technology to improve patient care: evaluating a support system for preference-based care planning at the bedside. Journal of the American Medical Informatics Association. 2002 Mar-Apr;9(2):192–201. doi: 10.1197/jamia.M0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood A, Pisters K, Chi M, et al. Evaluation of antiemetic guidelines and outcomes in patients receiving highly emetogenic chemotherapy utilizing the palm pilot. Proceedings of the American Society of Clinical Oncology. 2000;19 abst. 2512. [Google Scholar]
- 34.Kaasa S, Loge JH, Fayers P, et al. Symptom assessment in palliative care: a need for international collaboration. Journal of Clinical Oncology. 2008 Aug 10;26(23):3867–3873. doi: 10.1200/JCO.2007.15.8881. [DOI] [PubMed] [Google Scholar]
- 35.Trust SACotMO Assessing health status and quality-of-life instruments: attributes and review criteria. Quality of Life Research. 2002 May;11(3):193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 36.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology. 1993 Mar;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 37.United States Department of Health and Human Services. Food and Drug Administration . Draft Guidance. 2006. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Food and Drug Administration FDA to Release Final Guidance on Patient-Reported Outcomes. FDAnews Drug Daily Bulletin. 2009;6(179) [Google Scholar]
- 39.**Gwaltney CJ, Shields AL, Shiffman S. Equivalence of electronic and paper- and-pencil administration of patient-reported outcome measures: a meta-analytic review. Value in Health. 2008 Mar-Apr;11(2):322–333. doi: 10.1111/j.1524-4733.2007.00231.x. Establishing the validity of electronic data is of vital importance when considering substituting computer-based data collection methodologies for existing paper-based methods (e.g., paper questionnaires). The value of the ePRO system, and indeed of any data system built on electronic data capture, is only as good as the quality of the data it contains. Hence the finding of this meta-analysis, that electronically collected data are essentially equivalent to data collected using the same assessment instruments in paper format, is of fundamental importance as we move toward new models of care and research, such as the rapid learning cancer clinic, which are based upon electronic data capture.
- 40.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Journal of Clinical Oncology. 1997 Mar;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 41.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain & Symptom Management. 1997 Feb;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 42.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis & Rheumatism. 1989 Jan;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 43.Fortner B, Okon T, Schwartzberg L, Tauer K, Houts AC. The Cancer Care Monitor: psychometric content evaluation and pilot testing of a computer administered system for symptom screening and quality of life in adult cancer patients. Journal of Pain & Symptom Management. 2003 Dec;26(6):1077–1092. doi: 10.1016/j.jpainsymman.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 44.**Abernethy AP, Herndon JE, 2nd, Wheeler JL, et al. Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: pilot study of an e/Tablet data-collection system in academic oncology. Journal of Pain & Symptom Management. 2009 Jun;37(6):1027–1038. doi: 10.1016/j.jpainsymman.2008.07.011. This study demonstrated that an electronic data collection system used in community oncology to collect primarily clinical data could also be used in the academic oncology setting to collect data simultaneously for clinical and research purposes. The system did not disrupt the clinical flow in the busy academic setting, nor were logistical/technical issues insurmountable. Patients found the system helpful and acceptable; in fact, they anecdotally reported that they genuinely enjoyed the educational content available to them after completing the ePRO surveys.
- 45.**Herndon JE, Wheeler JL. Improving healthcare efficiency and quality using tablet personal computers to collect research-quality, patient-reported data. Health Services Research. 2008;43(6):1975–91. doi: 10.1111/j.1475-6773.2008.00887.x. AP. al. e. This paper reports the results of a validation study of the ePRO system at Duke University Medical Center, in which responses to multiple well-recognized research instruments were collected in two ways: on standard paper-based questionnaires, and electronically using tablet personal computers. The data were found to be equivalent on most scales and subscales, and electronic data were of comparable or better quality than paper-based data in terms of internal consistency. The significance of this finding is worth noting: it indicates that data routinely collected and used as part of clinical care can also be used for research purposes. This closing of the gap between clinical and research functions heralds a new efficiency in medicine, and an era in which routine clinical care can be data-driven as standard practice.
- 46.Abernethy AP, Zafar SY, Coeytaux R, Rowe K, Wheeler JL, Lyerly HK. Electronic patient-reported data capture as the foundation of a learning health care system. Journal of Clinical Oncology. 2009;27(15s) Abstract 6522. [Google Scholar]
- 47.**Institute of Medicine . The learning healthcare system: workshop summary (IOM Roundtable on Evidence-based Medicine) The National Academies Press; Washington, D.C.: 2007. This document prepared by the Institute of Medicine is seminal to the development of an emerging paradigm -- rapid learning healthcare. It is a “must read” for anyone interested in new and evolving models of integrated clinical care/research.
- 48.**Etheredge LM. A rapid-learning health system. Health Affairs. 2007 Mar-Apr;26(2):w107–118. doi: 10.1377/hlthaff.26.2.w107. This important manuscript provides a national-level view of the potential utility of a rapid learning healthcare system that uses large-scale electronic databases encompassing millions of people. Data linking and analyses on this scale could dramatically advance the evidence base available to support clinical care. The author describes how rapid learning could fill major knowledge gaps about the costs of care, the benefits/risks of interventions, disparities in outcomes based on location, race/ethnicity, gender, age, and other factors, environmental health influences, and personalized medicine. He also discusses the way in which, on a national level, policy-makers could use information generated in a rapid learning health care system to inform their activities. This vantage point is an important complement to the clinic- and patient-level development suggested by the ePRO system; both are essential approaches -- one being top-down, the other being ground-up, and both synergistically contributing to the development of a workable new clinical/research system.
- 49.Etheredge LM. Medicare’s future. Health Affairs. 2009 Jan-Feb;28(1):148–159. doi: 10.1377/hlthaff.28.1.148. [DOI] [PubMed] [Google Scholar]
- 50.**Buetow KH, Niederhuber J. Infrastructure for a learning health care system: CaBIG. Health Affairs. 2009 May-Jun;28(3):923–924. doi: 10.1377/hlthaff.28.3.923-a. author reply 924-925. This letter, written by John Niederhuber (Director, National Cancer Institute [NCI]) and Kenneth Buetow (Associate Director for Biomedical Informatics and Information Technology, NCI and a leader of the caBIG) explains the vital role of caBIG in developing a national, knowledge-based, biomedical system. Being rapidly developed at the present time, caBIG provides as a nationwide, interoperable, interconnected information technology platform that enables information sharing. Systems such as the ePRO system will interdigitate with and contribute to the national system, while drawing upon it to inform local practice.
- 51.**Slutsky JR. Moving closer to a rapid-learning health care system.[comment] Health Affairs. 2007 Mar-Apr;26(2):w122–124. doi: 10.1377/hlthaff.26.2.w122. While concerted efforts are being devoted to developing the learning healthcare system at the national level, through coordination of large-scale national datasets, it is of critical importance not to lose sight of the individual patient. This article returns the focus to the patient level, reminding us that patients play a central role in the evolution, and ultimate success, of the envisioned system. The author’s point is well-taken, and lies at the heart of the ePRO system which is developing a local model of rapid learning healthcare using data provided directly by patients as its first building blocks.
- 52.Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. Journal of Clinical Oncology. 2007 Nov 10;25(32):5106–5112. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 53.Dupont A, Wheeler J, Herndon JE, 2nd, et al. Use of tablet personal computers for sensitive patient-reported information. The Journal of Supportive Oncology. 2009 May-Jun;7(3):91–97. [PubMed] [Google Scholar]
- 54.Abernethy AP, Wheeler JL, Zafar SY. Detailing of gastrointestinal symptoms in cancer patients with advanced disease: new methodologies, new insights, and a proposed approach. Current Opinion in Supportive & Palliative Care. 2009 Mar;3(1):41–49. doi: 10.1097/SPC.0b013e32832531ce. [DOI] [PubMed] [Google Scholar]



