Abstract
Anionic phospholipids (APs) present a variety of lipids in the cytoplasmic leaflet of the plasma membrane, including phosphatidylinositol (PI), PI-4-phosphate (PI(4) P), phosphatidylserine (PS), PI-4,5-bisphosphate (PI(4,5) P2), PI-3,4,5-trisphosphate (PI(3,4,5)P3), and phosphatidic acid (PA). We previously showed that PI(4,5)P2 and PI(3,4,5)P3 upregulate the renal epithelial sodium channel (ENaC). Further studies from others suggested that PI(4,5) P2 and PI(3,4,5)P3 respectively target β- and γ-ENaC subunit. To determine whether PI(4,5)P2 and PI(3,4,5)P3 selectively bind to β and γ subunit, we performed lipid-protein overlay experiments. Surprisingly, the results reveal that most APs, including PI(4)P, PS, PI(4,5)P2, PI(3,4,5)P3, and PA, but not PI, non-selectively bind to not only β and γ but also α subunit. To determine how these APs regulate ENaC, we performed inside-out patch-clamp experiments and found that PS, but not PI or PI(4)P, maintained ENaC activity, that PI(4,5)P2 and PI(3,4,5)P3 stimulated ENaC, and that PA, however, inhibited ENaC. These data together suggest that APs differentially regulate ENaC by physically interacting with α-, β-, and γ-ENaC. Further, the data from cell-attached patch-clamp and confocal microscopy experiments indicate that PA, a product of phospholipase D, may provide one of the pathways for inhibition of ENaC by endothelin receptors.
Keywords: Sodium transport, Confocal microscopy, Electrophysiology, Phospholipid, Signal transduction
Introduction
It has long been known that anionic phospholipids (APs) are normally located in the inner leaflet of the plasma membrane. However, whether they could regulate the function of transmembrane proteins such as ion channels and transporters remained unknown until 1996 when PI(4,5)P2 was found to regulate Na+-Ca2+ exchangers and ATP-sensitive K+ (KATP) channels [10]. As a negatively charged molecule, PI(4,5)P2 stimulates several types of inward rectifier K+ channels by directly interacting with their proximal COOH terminal regions [11]. Other studies indicate that not only PI(4,5)P2 but also other APs such as PI(4)P and PI(3,4,5)P3 also regulate KATP channels [4, 28]. Compared to the Kir2.1 channel which is selectively activated by PI(4,5)P2, the Kir6.2 channel is also non-selectively activated by PI(4,5)P2, PI-3,4-bisphosphate (PI(3,4)P2), and PI(3,4,5)P3 [26].
Our studies show that like KATP channels, ENaC is also stimulated by both PI(4,5)P2 and PI(3,4,5)P3 [18]. Such regulations are very important because a decrease in PI(4,5) P2 mediates inhibition of ENaC by purinergic P2Y and epidermal growth factor (EGF) receptors [12, 17, 21, 32], while an increase in PI(3,4,5)P3 accounts for stimulation of ENaC by insulin and steroid receptors [5, 9, 20, 25, 30, 31, 34]. As discussed in our recent review articles [15, 16], inhibition of ENaC by P2Y and EGF receptor-mediated decrease in PI(4,5)P2 may facilitate cyst development in polycystic kidney diseases; acute stimulation of ENaC by steroid receptor-mediated increase in PI(3,4,5)P3 may contribute to blood volume recovery from hypovolemic hypotension. Our recent studies also suggest that phosphatidylserine (PS) externalization, a reduction of PS in the inner leaflet of the cell membrane, mediate down-regulation of ENaC by the inflammatory cytokine, tumor necrosis factor (TNF) α [3]. Therefore, further determination of the mechanisms of ENaC regulation by APs has pathological significance. In terms of the sites for lipid targeting, we show that APs including PI(3,4,5)P3 interact with γ-ENaC [9]. Recent studies suggest that PI(4,5)P2 and PI(3,4,5)P3 may target different ENaC subunits: PI(4,5)P2 stimulates ENaC by directly interacting with the distal region of the N-terminus of the β subunit [12] while PI(3,4,5)P3 stimulates ENaC by directly interacting with the proximal region of the C-terminus of the γ subunit [23]. However, whether ENaC subunits selectively bind to PI(4,5)P2 and PI(3,4,5)P3 over other APs has not been clarified. Moreover, besides PI(4,5)P2 and PI(3,4,5)P3, how other APs regulate ENaC also remains unknown.
In the present study, using patch-clamp techniques combined with lipid-protein overlay and confocal microscopy imaging, we show that α-, β-, and γ-ENaC non-selectively bind to most APs. However, unlike PI(4,5)P2 and PI(3,4,5)P3 that stimulate ENaC, phosphatidic acid (PA) strongly inhibits ENaC, providing a pathway for endothelin receptor signaling in distal nephron principal cells.
Materials and methods
Cell culture
H441 human lung epithelial cells, HEK293 cells, and A6 distal nephron principal cells were purchased from American Type Culture Collection (Rockville, MD, USA). H441 and HEK293 cells were cultured in plastic flasks respectively in either Roswell Park Memorial Institute medium 1640 or Dulbecco's Modified Eagle's minimal essential medium (DMEM) with 2 mM L-glutamine and 10% fetal bovine serum. A6 cells were cultured in plastic flasks in a modified media containing three parts of DMEM/F-12 (1:1) medium (Invitrogen), one part of H2O, 2 mM L-glutamine, 10% fetal bovine serum (Invitrogen), 25 U/ml penicillin, 25 U/ml streptomycin, and 1 μM aldosterone at 26°C and 4% CO2. After the cells became 70% confluent in plastic flasks, H441 and HEK293 were removed from the flasks and plated on permeable supports attached to Transwell inserts (Corning Costar Co.) for lipid-protein overlay experiments. H441 cells were used because antibodies directed against human ENaC are commercially available. HEK293 cells were used for expressing tagged ENaC subunits; they were plated with a density to allow them to be 80% confluent within 48 h before the transfection. H441 and A6 cells were cultured for 10–14 days to allow them to be fully polarized before each experiment. A6 cells were plated on permeable supports attached to Snapwell inserts (Corning Costar Co.) and used for patch-clamp experiments because the ENaC in this cell line has been well characterized.
Expression of fluorescent protein-tagged ENaC subunits
HEK293 cells were respectively transfected with cDNA constructs of enhanced cyan fluorescent protein-tagged rat α-ENaC (α-rENaC-pECFP-N1), enhanced yellow fluorescent protein-tagged rat β-ENaC (β-rENaC-pEYFP-N1), and pDsRed2-protein-tagged rat γ-ENaC (γ-rENaC-pDsRed2-N1). These constructs were kindly provided by Dr. Douglas C. Eaton (Emory University, Atlanta, GA, USA) and were generated as we reported previously [9]. Lipofectamine 2000 (Invitrogen) was used for the transfection. Briefly, 80% confluent HEK293 cells on day 2 after the plating were used for the transfection. DNA constructs were diluted with serum-free Opti-MEM (1 μg DNA/50 μl Opti-MEM), mixed with Lipofectamine 2000, which was also diluted with serum-free Opti-MEM (1 μl Lipofectamine/50 μl medium), and then incubated at room temperature for 15 min. Finally, the transfection medium containing DNA and Lipofectamine 2000 was applied to the cells and allowed to be in the medium for 6 h at 26°C before the transfection medium was replaced with regular growth medium.
Lipid-protein overlay
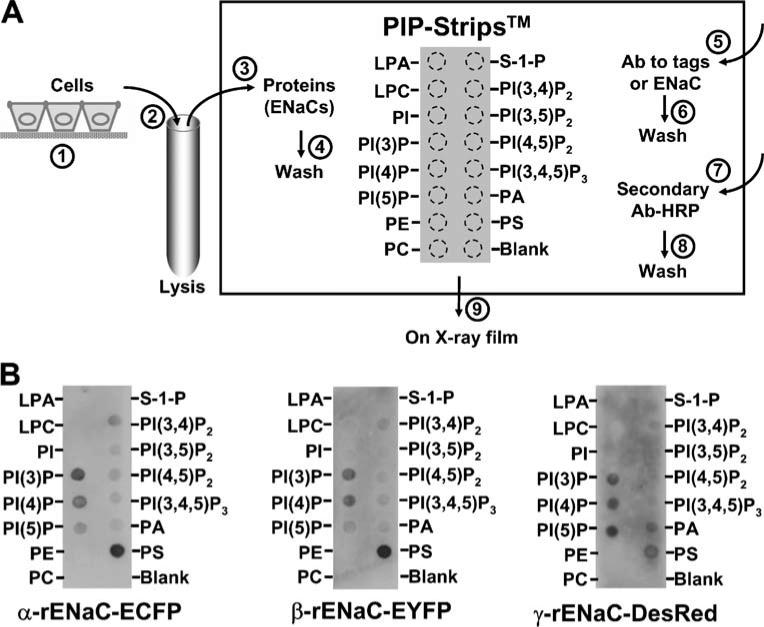
To test the lipid-binding properties of ENaC subunit, a lipid-protein overlay was performed using the so-called PIP Strips™, commercially available from Invitrogen (Chicago, IL, USA). The strip is a piece of nitrocellulose membrane on which 15 phospholipids at 100 pM and a blank sample were loaded by the manufacturer, as labeled beside the dashed circles in Fig. 1a. These lipids, which were dotted on the membrane, are lysophosphatidic acid, lysophosphatidylcholine, PI, PI-3-phosphate (PI(3)P), PI(4)P, PI-5-phosphate (PI(5)P), phosphatidylethanolamine, phosphatidylcholine (PC), sphingosine-1-phosphate, PI(3,4) P2, PI-3,5-bisphosphate (PI(3,5)P2), PI(4,5)P2, PI(3,4,5)P3, PA, and PS. H441 cells expressing native ENaC subunits and HEK293 cells transfected with plasma DNA constructs of fluorescence protein-tagged ENaC subunits (α-rENaC-pECFP-N1, β-rENaC-pEYFP-N1, or γ-ENaC-pDsRed2-N1) were cultured on Transwell filters (step 1, as shown in Fig. 1a). These cells were thoroughly rinsed with phosphate-buffered saline before lysing with hypotonic lysis buffer containing 10 mM Tris HCl, 10 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 0.5% Triton-X100, and freshly prepared 1X protease inhibitor cocktail (pH 7.5; step 2). The PIP Strips were blocked in Tris-buffered saline (TBS) buffer (10 mM Tris HCl, 140 mM NaCl, and 5% fatty acid-free bovine serum albumin (BSA; Equitech Bio Inc., Kerrville, TX, USA), pH 7.5) for 1 h at room temperature. Then, approximately 26 μg/mL protein from either H441 or HEK293 cell lysate was incubated with the Strips in TBS with 1% fatty acid-free BSA at 4°C overnight (step 3). The Strips were then washed with TBS-T buffer (TBS containing 0.05% Tween 20) three times (5 min for each wash) with gentle agitation (step 4). To detect the possible binding of each ENaC subunit to spotted phospholipids, the Strips were respectively incubated with rabbit polyclonal antibodies directed against α-, β-, or γ-ENaC (Abcam Inc., Cambridge, MA, USA; 1:1,000 dilution), green-fluorescent protein (Invitrogen; 1:4,000), or Ds-Red (1:2,000; BD Bioscience, Palo Alto, CA, USA) in TBS-T with 1% fatty acid-free BSA at 4°C overnight (step 5). Then, the membrane was washed three times (step 6) and incubated with horseradish peroxide-conjugated donkey anti-rabbit secondary antibody (Santa Cruz Biotechnology; 1:5,000 in TBS-T) for 1 h at room temperature (step 7). After thorough washes (step 8), the chemiluminescence was developed on X-ray film (step 9).
Fig. 1.
Each individual ENaC subunit expressed in HEK293 cells binds to most anionic phospholipids. a A diagram for lipid-protein overlay experiments, as described in “Materials and methods”. b HEK293 cells were transiently transfected with ECFP-tagged rat α-ENaC (α-rENaC-ECFP), EYFP-tagged rat β-ENaC (β-rENaC-EYFP), or DsRed-tagged rat γ-ENaC (γ-rENaC-DsRed). PIP Strips were respectively incubated with protein extracts from cells expressing each construct. α-rENaC-ECFP (left) and β-rENaC-EYFP (middle) were detected with anti-green-fluorescent protein antibody, while γ-rENaC-Red (right) was detected with anti-DsRed antibody. The data represent three experiments showing similar results
Patch-clamp recordings
Inside-out and cell-attached recordings of ENaC single-channel current from A6 distal nephron cells were carried out using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). As we previously described [35], prior to the experiments, A6 cells cultured on the Snapwell inserts were thoroughly washed with NaCl solution containing (in millimolar) 100 NaCl, 3.4 KCl, 1 CaCl2, 1 MgCl2, and 10 HEPES (pH was adjusted to 7.4 with NaOH). The glass micropipette was filled with NaCl solution (the pipette resistance is 7–10 MΩ). In inside-out experiments, KCl solution containing (in millimolar) 100 KCl, 5 NaCl, 1 MgCl2, and 10 HEPES (50 nM free Ca2+ after titration with 1 mM ethylene glycol tetraacetic acid; pH was adjusted to 7.2 with KOH) was used for the “cytoplasmic” bath. In cell-attached experiments, NaCl solution was used for both the luminal and the basolateral bath. Single-channel currents were obtained with applied pipette potentials of either +60 mV for inside-out recordings or 0 mV for cell-attached recordings, filtered at 1 kHz, and sampled every 50 μs with Clampex 8.0 software. Experiments were conducted at room temperature. The total numbers of functional channels in the patch were estimated by observing the number of peaks detected on the current amplitude histogram during at least 10 min recording period. The open probability (PO) of ENaC before (−3 to 0 min) and after (0–3 min and 3–6 min) each experimental manipulation was calculated using Clamfit 9.0.
Confocal microscopy imaging
Confocal microscopy XY scanning of A6 cell monolayer on the edge of a folded filter, which is the so-called filter-fold technique [33], was used to achieve a high-resolution side view of A6 cell monolayer. Briefly, the cells cultured on the polyester filter membrane of Transwell inserts were washed with NaCl solution. The cells were either in control conditions or basolaterally exposed to 20 nM endothelin-1 (ET-1) for 5 min, fixed with 2% paraformaldehyde for 10 min, and permeabilized with 0.1% triton-X100 in NaCl solution for 15 min, incubated with a rabbit polyclonal antibody directed against phospholipase D (PLD)-1 (Abcam; ab50695) at room temperature for 1 h, and then incubated with a secondary antibody (Alexa Fluor® 488 goat anti-rabbit IgG, 5 μg/ml) at room temperature for 30 min. Incubation of the cells with the secondary antibody alone served as a control. The nuclei were stained with Hoechst 33258 trihydrochloride (10 μg/ml) for 3 min. Each step described above was followed by washing the cells twice. Then, the polyester filter membrane was excised, folded with A6 cell monolayer facing out, and mounted on a glass slide. Optical sections of the edge of the folded filter (side view of A6 cell monolayer) were performed with a Leica confocal microscopy. In each set of experiments, images were taken using the same parameter settings.
Chemicals
Most chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). PI, PI(4)P, PS, PA, and PI(4,5)P2 were purchased from Avanti Polar Lipids, INC (Alabaster, AL, USA). PI(3,4,5)P3 was from Calbiochem.
Statistical analysis
Data is reported as mean values±SE. Statistical analysis was performed with SigmaPlot and SigmaStat software (Jandel Scientific, CA, USA). Student t test was used between two groups. Analysis of variance was used for multiple comparisons. Results were considered significant if P<0.01.
Results
α-, β-, and γ-ENaC bind to most anionic phospholipids (APs)
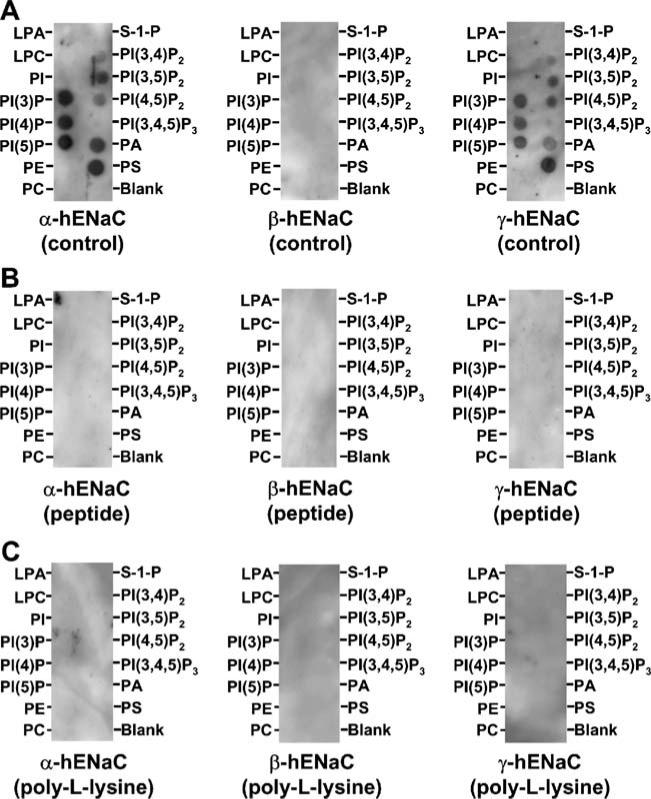
Previous studies suggest that PI(4,5)P2 and PI(3,4,5) P3 stimulate ENaC by respectively interacting with β- and γ-ENaC subunits [12, 23]. To determine whether ENaC subunits selectively bind to PI(4,5)P2 and PI(3,4,5)P3 over other APs, lipid-protein overlay experiments by using PIP Strips™ were performed as described in the “Materials and methods” and shown in Fig. 1a. We expressed fluorescent protein-tagged rat α-, β-, or γ-ENaC in HEK293 cells and detected each ENaC subunit with antibody against the attached fluorescent protein, and found that all three ENaC subunit could bind to most APs with a similar binding pattern (Fig. 1b). We have previously shown that the fluorescent proteins do not bind to these lipids [9, 15]. Since it is known that the phosphoinositide recognition domains not only require a lysine- and/or arginines-rich motif but also require a β-sandwich structure [13], the nonselective binding may be caused either by the tags or the separation of a subunit from the αβγ ENaC complex. Therefore, in the following experiments, we used non-denaturing protein extract from H441 cells in which the αβγ ENaC complex is natively expressed to detect how the αβγ ENaC complex interacts with these lipids with antibodies to human α-, β-, and γ-ENaC. Non-selectively bindings to most APs were detected by antibodies to human α- and γ-ENaC (Fig. 2a). No binding was observed by using the antibody to human β-ENaC. This may be due to a possible inefficient access of the antibody to β-ENaC. Among these APs, it seemed that PI(3,4,5)P3 had the lowest binding affinity to both α- and γ-ENaC. These bindings were abolished by pretreatment of the Strips with either the peptide, which was used for generating the antibody (Fig. 2b), or 10 μg/ml poly-L-lysine for 10 min (Fig. 2c). These data, together with the results by using ENaC subunits individually expressed in a cell model, reveal that all three ENaC subunits can bind to most APs, but with an observable preference to the APs with less negative charges, even though the negative charges are required for the binding.
Fig. 2.
The αβγ ENaC complex natively expressed in H441 cells also binds to most anionic phospholipids. PIP Strips™ were incubated with protein extract from H441 cells endogenously expressing ENaC subunits (α-, β-, and γ-hENaC) either in the absence (a) or in the presence of either the antigen peptide (b) or 10 μg/ml poly-L-lysine (c), and then detected with antibodies to human α-(left), β-(middle), and γ-ENaC (right) subunits, respectively. The data represent four experiments showing similar results
PI, PI(4)P, and PS do not stimulates ENaC
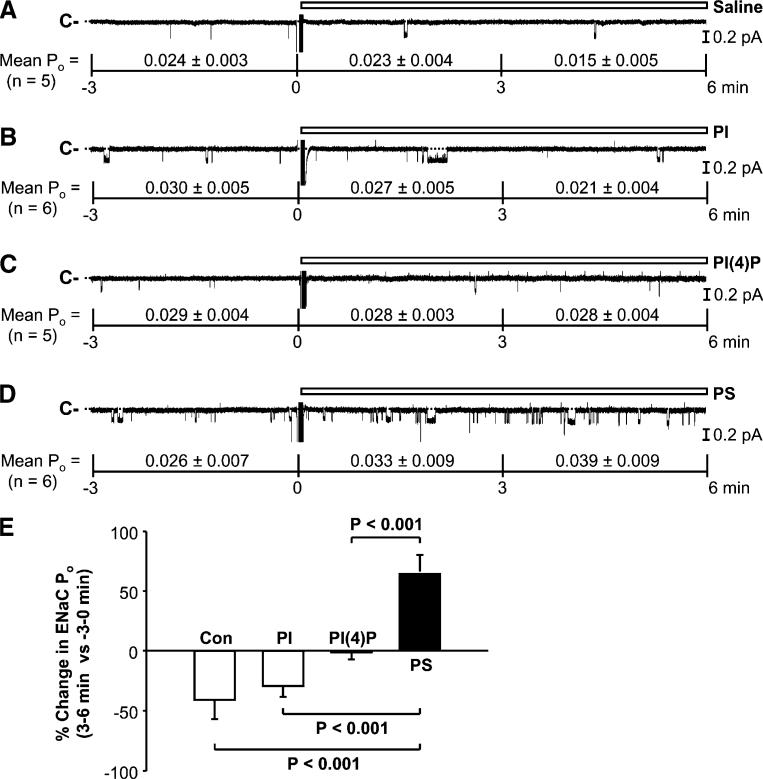
We [18] and others [24] have previously reported that PI(4,5)P2 and PI(3,4,5)P3 directly stimulate ENaC. The lipid-protein overlay data showed that PI(4)P and PS have an even higher binding affinity to ENaC than PI(4,5)P2 and PI(3,4,5)P3, leading to a hypothesis that the physical interactions of PI(4)P and PS with ENaC might also affect ENaC activity. To test this hypothesis, we performed inside-out patch-clamp experiments in A6 cells. To better observe a putative stimulatory effect, the patches containing low basal ENaC activity were used for the experiments. Serving as a control, addition of saline to the “cytoplasmic” bath did not affect ENaC activity (Fig. 3a). Mean ENaC POs from five inside-out patches were 0.024±0.003 (before addition), 0.023±0.004 (0–3 min after addition of saline; P=0.10), and 0.015±0.005 (3–6 min after addition of saline; P=0.05). Addition of PI(50 μM) to the “cytoplasmic” bath did not affect ENaC activity either; mean ENaC POs from six inside-out patches were 0.030±0.005 (before addition), 0.027±0.005 (0–3 min after addition of PI; P=0.22), and 0.021±0.004 (3–6 min after addition of PI; P=0.15; Fig. 3b). PI(4)P (50 μM) seemed to abolish the reduction of ENaC activity, which normally occurred in control conditions; mean ENaC POs from five inside-out patches were 0.029±0.004 (before addition), 0.028±0.003 (0–3 min after addition of PI(4); P=0.38), and 0.028±0.004 (3–6 min after addition of PI(4)P; P=0.33; Fig. 3c). PS (50 μM) either maintained or slightly enhanced ENaC activity. Mean ENaC POs from six inside-out patches ENaC POs were 0.026±0.007 (before addition), 0.033±0.009 (0–3 min after addition of PS; P=0.10), and 0.039±0.009 (3–6 min after addition of PS; P=0.08; Fig. 3d). Although none of these lipids significantly affected the mean ENaC POs, the percent change in ENaC PO (3 to 6 min versus −3 to 0 min) induced by PS was significantly different from those after addition of saline (control), PI, or PI(4)P (Fig. 3e). Since among APs, PS and PI(4)P have the highest binding affinity to ENaC, these results suggest that physical interaction between APs and ENaC does not promise an ability to regulate ENaC.
Fig. 3.
Phosphatidylserine (PS) maintains ENaC activity in inside-out patches. a ENaC single-channel current before and after addition of saline (as a control) to the “cytoplasmic” bath. b–d ENaC single-channel current before and after addition of phosphatidylinositol (PI) (50 μM), PI(4)P (50 μM), or PS (50 μM) to the “cytoplasmic” bath, respectively. In this figure and the following figures, mean ENaC Pos before (−3 to 0 min) and after each experimental manipulation (0–3 min and 3–6 min) were listed under each representative single-channel trace. e Percent change in ENaC Po (3–6 min after additions of saline (Con), PI, PI(4)P, or PS versus −3 to 0 min before each addition)
PI(4,5)P2 and PI(3,4,5)P3 stimulate ENaC via negative charges
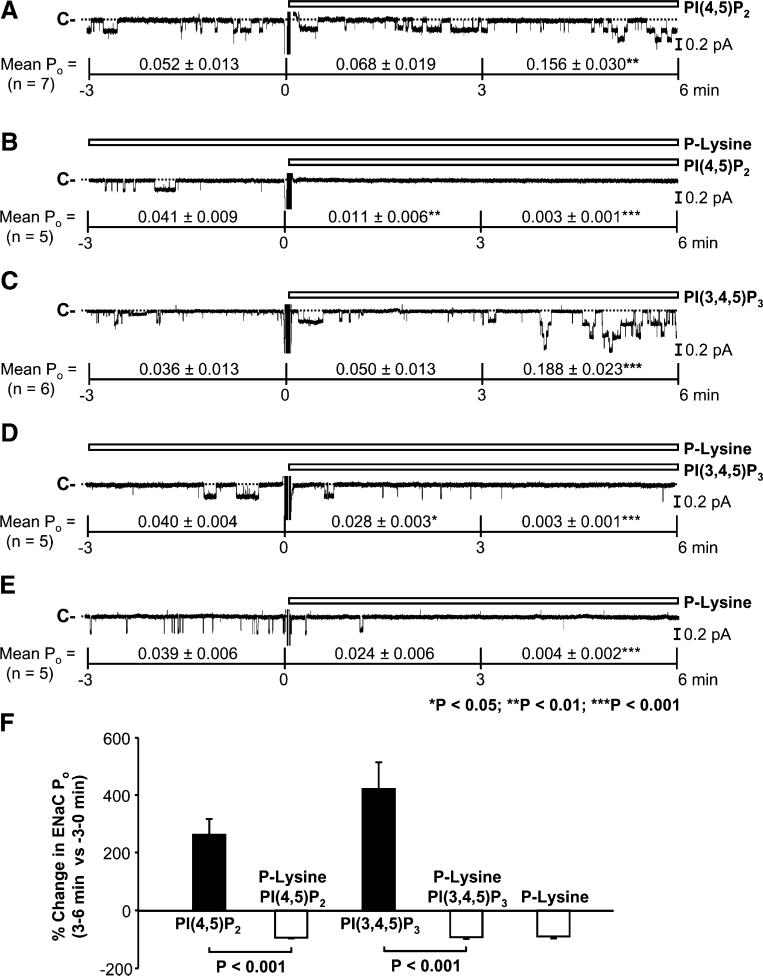
We previously showed that PI(4,5)P2 and PI(3,4,5) P3 at 5 μM maintained the activity of highly active ENaC [18]. To determine if they could enhance rather than just maintain ENaC activity, in the present study, we used a much higher concentration (50 μM) of PI(4,5)P2 and PI(3,4,5)P3 and patches containing ENaC with lower basal activity. The data demonstrated that addition of PI(4,5)P2 (50 μM) to the “cytoplasmic” bath strongly enhanced ENaC activity. Mean ENaC PO from seven inside-out patches was significantly increased, from 0.052±0.013 (before addition) to 0.068±0.019 (0–3 min after addition of PI(4,5)P2; P=0.07) and 0.156±0.030 (3–6 min after addition of PI(4,5)P2; P<0.01; Fig. 4a). However, in the presence of poly-L-lysine (10 μg/ml), PI(4,5)P2 (50 μM) was unable to stimulate ENaC. Mean ENaC POs from five inside-out patches were 0.041±0.009 (before addition; in the presence of poly-L-lysine), 0.011±0.006 (0–3 min after addition of PI(4,5)P2 in the presence of poly-L-lysine; P<0.01), and 0.003±0.001 (3–6 min after addition of PI(4,5) P2 in the presence of poly-L-lysine; P<0.001; Fig. 4b). In six inside-out patches, PI(3,4,5)P3 (50 μM) also increased mean ENaC PO from 0.036±0.013 (before addition) to 0.050±0.013 (0–3 min after addition of PI(3,4,5)P3; P=0.09) and 0.188±0.023 (3–6 min after addition of PI(3,4,5) P3; P<0.001; Fig. 4c). Similarly, in the presence of poly-L-lysine (10 μg/ml), PI(3,4,5)P3 (50 μM) was also unable to stimulate ENaC; mean ENaC POs from five inside-out patches were 0.040±0.004 (before addition; in the presence of poly-L-lysine), 0.028±0.003 (0–3 min after addition of PI(3,4,5)P3 in the presence of poly-L-lysine; P<0.01), and 0.003±0.001 (3–6 min after addition of PI(3,4,5)P3 in the presence of poly-L-lysine; P<0.001; Fig. 4d). In contrast, poly-L-lysine (10 μg/ml) alone even reduced basal ENaC activity. Mean ENaC POs from five inside-out patches were 0.039±0.006 (before addition), 0.024±0.006 (0–3 min after addition of poly-Lysine; P=0.09), and 0.004±0.002 (3–6 min after addition of poly-L-lysine; P<0.001; Fig. 4e). Poly-L-lysine abolished the percent increase in ENaC PO (3 to 6 min versus −3 to 0 min) induced by PI(4,5)P2 and PI(3,4,5)P3 (Fig. 4f). These data suggest that negative charges are required for the stimulatory effect of PI(4,5)P2 and PI(3,4,5)P3 on ENaC activity.
Fig. 4.
Stimulation of ENaC by phosphatidylinositol (PI) (4,5)P2 and PI(3,4,5)P3 in inside-out patches is dependent on negative charges. a, b ENaC single-channel current before and after addition of PI(4,5)P2 (50 μM) to the “cytoplasmic” bath either alone or in the presence of 10 μg/ml poly-L-lysine. c, d ENaC single-channel current before and after addition of PI(3,4,5)P3 (50 μM) to the “cytoplasmic” bath either alone or in the presence of 10 μg/ml poly-L-lysine. e ENaC single-channel current before and after addition of poly-L-lysine alone. f Percent change in ENaC Po (3–6 min after additions of PI(4,5)P2 and PI(3,4,5)P3 in the absence or presence of poly-L-lysine (poly-L-lysine alone serves as a control) versus −3 to 0 min before each addition)
PA inhibits ENaC
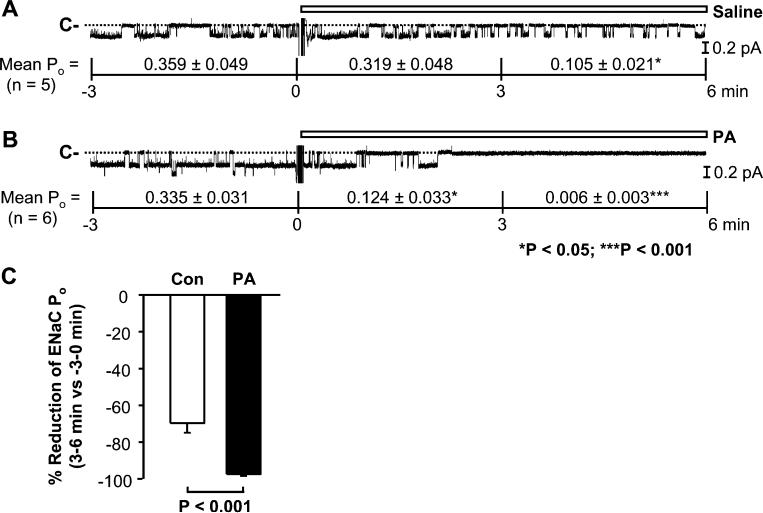
Since PA also physically interacts with ENaC, whether PA could regulate ENaC was examined. The preliminary data indicated that PA appeared to reduce ENaC activity. In order to clearly see the reduction, we used patches containing ENaC with high basal activity for this set of experiments. ENaC activity in these inside-out patches gradually ran down, which is consistent with our previous report [18]. Again, control saline did not affect the channel activity rundown; in five inside-out patches, ENaC POs were reduced, from 0.359±0.049 (before addition) to 0.319±0.048 (0–3 min after addition of saline; P=0.17) and 0.105±0.021 (3–6 min after addition of saline; P<0.05; Fig. 5a). In contrast, in six inside-out patches, addition of PA (50 μM) to the “cytoplasmic” bath rapidly reduced mean ENaC PO from 0.335±0.031 (before addition) to 0.124±0.033 (0–3 min after addition of PA; P<0.05) and 0.006±0.003 (3–6 min after addition of PA; P<0.001; Fig. 5b). Compared to control conditions, PA significantly enhanced the percent reduction of ENaC PO (3 to 6 min versus −3 to 0 min; Fig. 5c). These data suggest that unlike PI(4,5)P2 and PI(3,4,5)P3 that directly stimulate ENaC via a physical interaction, PA paradoxically inhibits ENaC probably also via a physical interaction.
Fig. 5.
Phosphatidic acid (PA) inhibits ENaC in inside-out patches. a, b ENaC single-channel current recorded before and after addition of either saline (as a control) or PA (50 μM) to the “cytoplasmic” bath. c Percent reduction of ENaC Po (3 to 6 min after addition of PA versus −3 to 0 min before the addition)
Endothelin-1 (ET-1) inhibits ENaC via activation of phospholipase D (PLD)
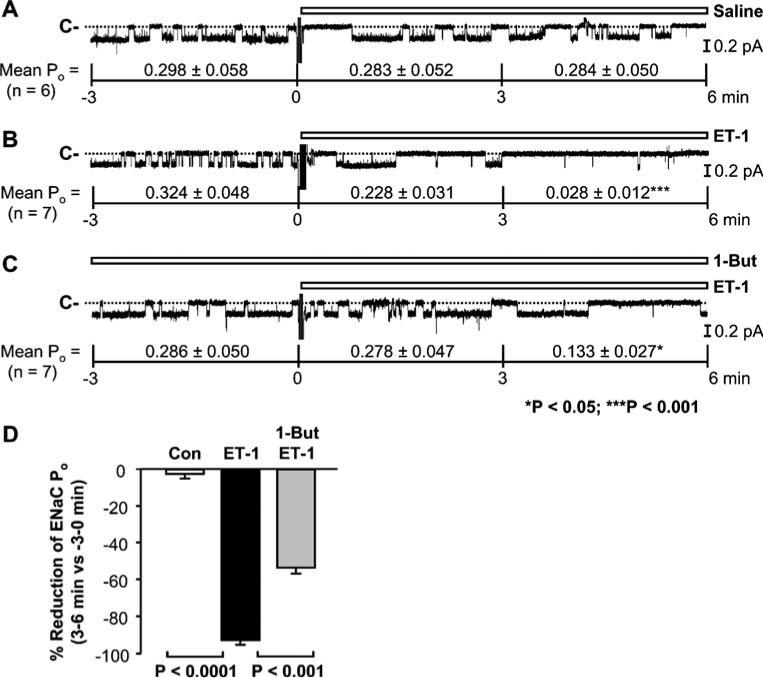
In a variety of cell types, endothelin receptors activate PLD [1, 2, 7, 14], an enzyme which uses PC as a substrate to produce PA [2, 14]. To determine whether PLD mediates inhibition of ENaC by endothelin, we performed cell-attached experiments. The basal ENaC activity in cell-attached patches was much more stable than that in inside-out patches. Addition of saline to the basolateral bath did not affect ENaC activity; mean ENaC POs from six cell-attached patches were 0.298±0.058 (before addition), 0.283±0.052 (0–3 min after addition of saline; P=0.97), and 0.284±0.050 (3–6 min after addition of saline; P=0.97; Fig. 6a). In contrast, addition of ET-1 (20 nM) to the basolateral bath significantly inhibited ENaC; mean ENaC PO from seven cell-attached patches was decreased from 0.324±0.048 (before addition) to 0.228± 0.031 (0–3 min after addition of ET-1; P<0.05) and 0.028± 0.012 (3–6 min after addition of ET-1; P<0.01; Fig. 6b). The inhibition was obviously attenuated by pretreatment of the cells with 1% (v/v) 1-butanol (1-But), a PLD inhibitor which has been recently used by others [19]. In the presence of 1-But, the reduction of ENaC PO induced by ET-1 was less significant; mean ENaC POs from seven cell-attached patches were 0.286±0.050 (before addition), 0.278±0.047 (0–3 min after addition of ET-1), and 0.133±0.027 (3–6 min after addition of ET-1; P<0.05). These data suggest that PLD participates in endothelin-induced inhibition of ENaC.
Fig. 6.
Endothelin-1 (ET-1) induces phospholipase D (PLD)-dependent inhibition of ENaC in cell-attached patches. a, b ENaC single-channel current before and after addition of either saline (as a control) or ET-1 (20 nM) to the basolateral bath. c ENaC single-channel current before and after addition of ET-1 (20 nM) to the basolateral bath in the presence of 1% 1-butanol (1-But). d Percent reduction of ENaC Po (3 to 6 min after additions of either saline or ET-1 in the absence or presence of 1% 1-But versus −3 to 0 min before each addition)
ET-1 induces translocation of PLD to the apical membrane
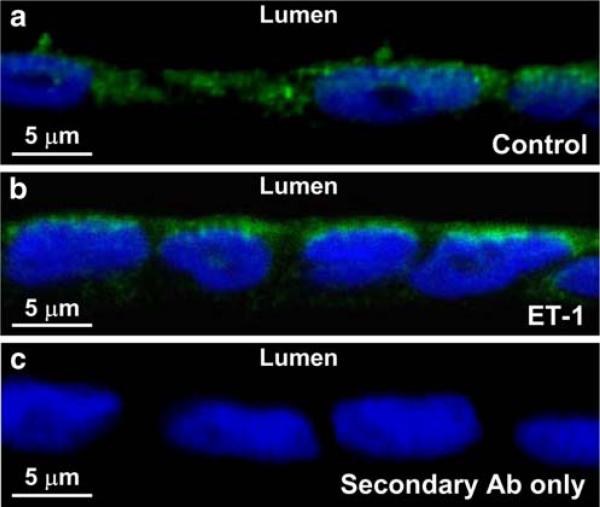
To further determine the mechanism for ET-1-induced stimulation of PLD in polarized A6 distal nephron cells, confocal microscopy was used to optically cross-section A6 cell monolayer. We found that under control conditions, PLD was sporadically localized in the cytoplasm (Fig. 7a). In contrast, after treatment with ET-1, PLD accumulated near the apical membrane (Fig. 7b). Direct incubation of the cells with the fluorescent secondary antibody showed no detectable fluorescence (Fig. 7c). These data suggest that ET-1 stimulates PLD at least in part by promoting translocation of PLD to the apical membrane.
Fig. 7.
Endothelin-1 (ET-1) induces translocation of phospholipase D (PLD) to the apical membrane. a Under control conditions, PLD (green fluorescence) was sporadically localized in the cytoplasm of A6 cells. b After the cells were basolaterally treated with 20 nM ET-1, PLD accumulated near the apical membrane. c In contrast, no green fluorescence was detected when the cells were incubated with the fluorescent secondary antibody alone. In a–c, nuclei were stained with Hoechst 33258 trihydrochloride, shown in blue. Confocal microscopy XY scanning was performed across A6 cell monolayer on the edge of folded filter (see “Materials and methods”). The figure represents three experiments showing similar results
Discussion
Previous studies showed that PI(4,5)P2 stimulates ENaC by interacting with β and γ subunits [36]. The data from mutagenesis experiments indicated that β-ENaC contains a PI(4,5)P2-binding site and that γ-ENaC contains a PI(3,4,5) P3-binding site [12, 23]. Recent studies suggested that the extreme N-terminus of β- and γ-ENaC were identified as being critical to down-regulation of ENaC activity and PO in response to depletion of membrane PI(4,5)P2, and that intracellular regions near the inner membrane interface just following the second transmembrane domains in β- and γ-but not α-ENaC as necessary for PI(3,4,5)P3 but not PI(4,5) P2 modulation [24]. The present study provides additional information suggesting that ENaC may contain different binding sites for different APs. Our data from lipid-protein overlay experiments showed that all three ENaC subunits can bind to most APs. The negative charges appear to be necessary because pretreatment of PIP Strips with positively charged poly-L-lysine was able to abolish ENaC binding to APs. However, the structure of lipid head group is also important because PI does not bind to ENaC, whereas PS, which has a head group different from PI, can significantly bind to ENaC, even though PS also contains one negative charge. However, the rank order of binding affinities of APs to ENaC does not always reflect their ability to regulate ENaC. One example is that among APs, PI(3,4,5)P3 has the lowest binding affinity to ENaC, but is the most potent AP in stimulating ENaC. Another is that among APs, PS has the highest binding affinity to ENaC, but only maintains ENaC activity. Moreover, the physical interaction between ENaC and PA can even produce an effect that is opposite to the effect of PI(4,5)P2 and PI(3,4,5)P3 on ENaC activity, as discussed below.
Like PS, PA also contains a negatively charged phosphoric residue and can significantly bind to ENaC. However, unlike PS, PA does not have a similar head group structure and strongly inhibits ENaC. Taking these data together with results from the lipid-protein overlay assay, we argue that the head groups of APs are very critical to their roles in regulating ENaC, even though negative charges are also required. Since PA binding domains are quite different from PS-binding domains [29], the possible different binding sites for PA and PS in ENaC subunits may also account for their opposite effects on ENaC activity. Previous studies reported that PA stimulates KATP channels [8]. This report should not dim the present finding that PA inhibits ENaC because PA stimulates KATP channels by reducing the sensitivity of the channel to ATP. Moreover, the direct effect of PA on KATP channel gating is to reduce its open time [8], which is actually consistent with the effect of PA on ENaC open probability.
Regulation of ENaC by these APs has both physiological and pathological significance. Inhibition of ENaC due to a decrease in membrane PI(4,5)P2 may account for a feed-back suppression of sodium absorption in distal nephrons via paracrine release of ATP [21]. As we have recently reviewed [15], excess suppression of ENaC activity via this pathway may participate in the pathogenesis of polycystic kidney diseases. In contrast, the stimulation of ENaC due to an increase in membrane PI(3,4,5)P3 may be involved in acute systemic volume recovery in response to hypovolemic challenge [15]. Although our results show that PS only maintains ENaC activity, such a maintenance is important because we have shown that TNF α inhibits ENaC by causing PS externalization from the inner leaflet of the apical membrane, which consequently reduces the interaction between ENaC and PS [3].
The present study provides the first evidence showing that PA, as a downstream signaling molecule of endothelin receptors, inhibits ENaC. Although activation of PLD produces not only PA but also choline [2, 14], we have shown that choline does not affect ENaC activity [3]. The inhibition should not be caused by a reduction of PC itself because we have already shown that PC does not affect ENaC activity [18]. Thus, it is the formation of PA that mediates inhibition of ENaC by endothelin receptor signaling. The present study also shows that inhibition of PLD with 1-But can only attenuate but not abolish the effect of ET-1 on ENaC activity, indicating that other pathways is also involved. This is not surprising because in addition to PLD, ET-1 also activates phospholipase C [1, 2], an enzyme which inhibits ENaC by reducing the levels of PI(4,5)P2 [17, 18, 22]. Stockand and colleagues have recently shown that ET-1 inhibits ENaC also via a pathway associated with SRC and MAPK1/2 [6]. Furthermore, it has also been suggested that nitric oxide is involved in ET-1 regulation of sodium absorption in distal nephrons [27]. These pathways together promise a very potent inhibition of ENaC by endothelin receptor signaling in distal nephrons.
Acknowledgments
We thank Albert Tousson in the Imaging Facilities at the University of Alabama at Birmingham for his technical assistance on confocal microscopy experiments.
Grants This research was supported by the Natural Science Foundation of the People's Republic of China (30871007 to Z-R Zhang), Natural Science Foundation of Heilongjiang Province (ZD200807-01 and ZD2008-08 to Z-R Zhang), Grant from Educational Office of Heilongjiang Province (1154HZ11 to Z-R Zhang), Department of Health and Human Services (National Institutes of Health Grant R01-DK067110 to H-P Ma), and American Society of Nephrology (M. James Scherbenske Grant to H-P Ma).
Contributor Information
Zhi-Ren Zhang, Departments of Pharmacy and Cardiology, The 2nd Affiliated Hospital, Key Laboratories of Education Ministry for Myocardial Ischemia Mechanism and Treatment, Harbin Medical University, Harbin, Heilongjiang Province 150086, People's Republic of China zhirenz@yahoo.com.
Chu-Fang Chou, Department of Medicine, Division of Nephrology, University of Alabama, 1530 Third Avenue South, Birmingham, AL 35294, USA.
Jing Wang, Department of Medicine, Division of Nephrology, University of Alabama, 1530 Third Avenue South, Birmingham, AL 35294, USA.
You-You Liang, Department of Medicine, Division of Nephrology, University of Alabama, 1530 Third Avenue South, Birmingham, AL 35294, USA.
He-Ping Ma, Department of Medicine, Division of Nephrology, University of Alabama, 1530 Third Avenue South, Birmingham, AL 35294, USA hepingma@uab.edu.
References
- 1.Ambar I, Sokolovsky M. Endothelin receptors stimulate both phospholipase C and phospholipase D activities in different cell lines. Eur J Pharmacol. 1993;245:31–41. doi: 10.1016/0922-4106(93)90166-7. [DOI] [PubMed] [Google Scholar]
- 2.Baldi E, Musial A, Kester M. Endothelin stimulates phosphatidylcholine hydrolysis through both PLC and PLD pathways in mesangial cells. Am J Physiol. 1994;266:F957–F965. doi: 10.1152/ajprenal.1994.266.6.F957. [DOI] [PubMed] [Google Scholar]
- 3.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-α through protein kinase C. Am J Physiol. 2007;293:F1178–F1186. doi: 10.1152/ajprenal.00153.2007. [DOI] [PubMed] [Google Scholar]
- 4.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 5.Blazer-Yost BL, Paunescu TG, Helman SI, Lee KD, Vlahos CJ. Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am J Physiol. 1999;277:C531–C536. doi: 10.1152/ajpcell.1999.277.3.C531. [DOI] [PubMed] [Google Scholar]
- 6.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–F1070. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desagher S, Cordier J, Glowinski J, Tence M. Endothelin stimulates phospholipase D in striatal astrocytes. J Neurochem. 1997;68:78–87. doi: 10.1046/j.1471-4159.1997.68010078.x. [DOI] [PubMed] [Google Scholar]
- 8.Fan Z, Gao L, Wang W. Phosphatidic acid stimulates cardiac KATP channels like phosphatidylinositols, but with novel gating kinetics. Am J Physiol. 2003;284:C94–C102. doi: 10.1152/ajpcell.00255.2002. [DOI] [PubMed] [Google Scholar]
- 9.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3, 4, 5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with γ-ENaC. J Biol Chem. 2005;280:40885–40891. doi: 10.1074/jbc.M509646200. [DOI] [PubMed] [Google Scholar]
- 10.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 11.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 12.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 13.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 14.Liu GL, Shaw L, Heagerty AM, Ohanian V, Ohanian J. Endothelin-1 stimulates hydrolysis of phosphatidylcholine by phospholipases C and D in intact rat mesenteric arteries. J Vasc Res. 1999;36:35–46. doi: 10.1159/000025624. [DOI] [PubMed] [Google Scholar]
- 15.Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch. 2007;455:169–180. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- 16.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol. 2005;16:3182–3187. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 17.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch-activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol. 2002;282:F501–F505. doi: 10.1152/ajprenal.00147.2001. [DOI] [PubMed] [Google Scholar]
- 18.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed ENaC. J Biol Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 19.Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem. 2007;282:3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- 20.Paunescu TG, Blazer-Yost BL, Vlahos CJ, Helman SI. LY-294002-inhibitable PI 3-kinase and regulation of baseline rates of Na+ transport in A6 epithelia. Am J Physiol. 2000;279:C236–C247. doi: 10.1152/ajpcell.2000.279.1.C236. [DOI] [PubMed] [Google Scholar]
- 21.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4, 5)P2 levels sets ENaC activity in principal cells. Am J Physiol. 2008;294:F38–F46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 23.Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3, 4, 5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem. 2005;280:37565–37571. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- 24.Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4, 5) P2 and PI(3, 4, 5)P3 regulation of the epithelial Na+ channel. J Gen Physiol. 2007;130:399–413. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Record RD, Froelich LL, Vlahos CJ, Blazer-Yost BL. Phosphatidylinositol 3-kinase activation is required for insulin-stimulated sodium transport in A6 cells. Am J Physiol. 1998;274:E611–E617. doi: 10.1152/ajpendo.1998.274.4.E611. [DOI] [PubMed] [Google Scholar]
- 26.Rohacs T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K+ channels for phosphoinosi-tides. J Biol Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- 27.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension. 2008;51:1605–1610. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 29.Stace CL, Ktistakis NT. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta. 2006;1761:913–926. doi: 10.1016/j.bbalip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol. 2007;18:1652–1661. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- 31.Tong Q, Booth RE, Worrell RT, Stockand JD. Regulation of Na+ transport by aldosterone: signaling convergence and cross talk between the PI3-K and MAPK1/2 cascades. Am J Physiol. 2004;286:F1232–F1238. doi: 10.1152/ajprenal.00345.2003. [DOI] [PubMed] [Google Scholar]
- 32.Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na+ channel inhibition by epidermal growth factor. Am J Physiol. 2005;288:F150–F161. doi: 10.1152/ajprenal.00261.2004. [DOI] [PubMed] [Google Scholar]
- 33.Tousson A, Fuller CM, Benos DJ. Apical recruitment of CFTR in T-84 cells is dependent on cAMP and microtubules but not Ca2+ or microfilaments. J Cell Sci. 1996;109:1325–1334. doi: 10.1242/jcs.109.6.1325. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Knight ZA, Fiedler D, Williams O, Shokat KM, Pearce D. Activity of the p110-α subunit of phosphatidylinositol-3-kinase is required for activation of epithelial sodium transport. Am J Physiol. 2008;295:F843–F850. doi: 10.1152/ajprenal.90348.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Zhang ZR, Chou CF, Liang YY, Gu Y, Ma HP. Cyclosporine stimulates the renal epithelial sodium channel by elevating cholesterol. Am J Physiol. 2008;296:F284–F290. doi: 10.1152/ajprenal.90647.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4, 5-bisphosphate (PIP2) stimulates sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]