Abstract
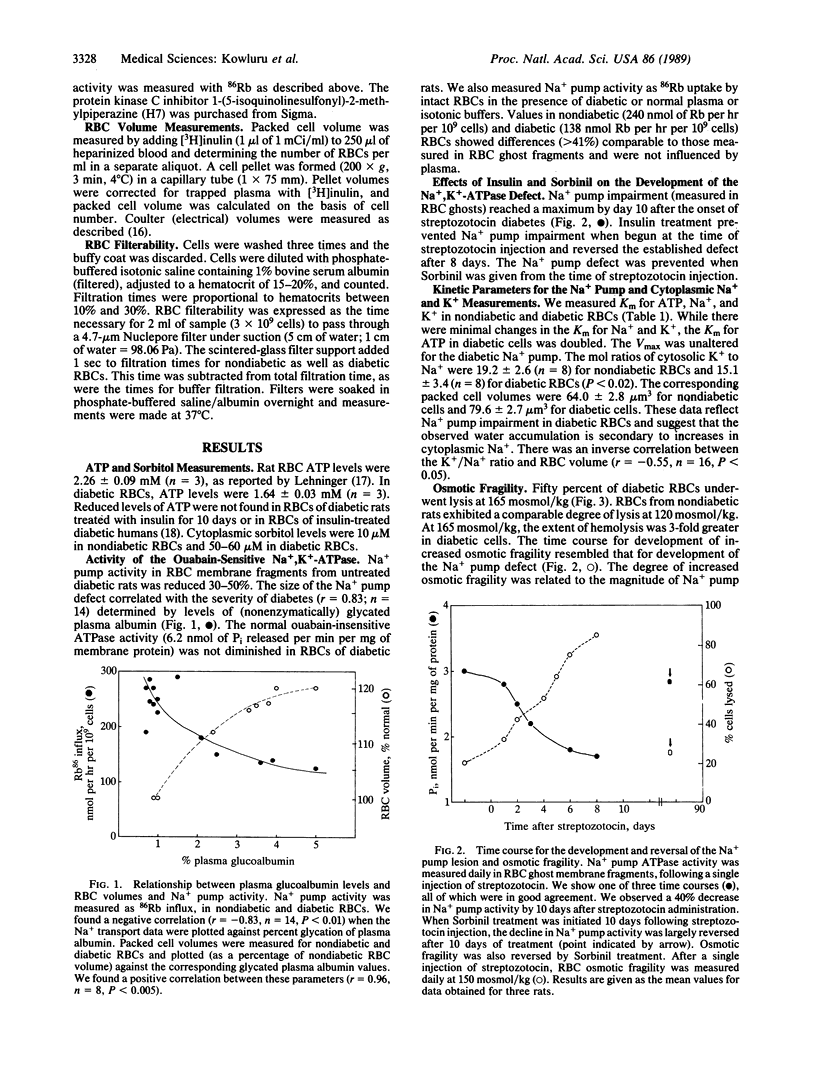
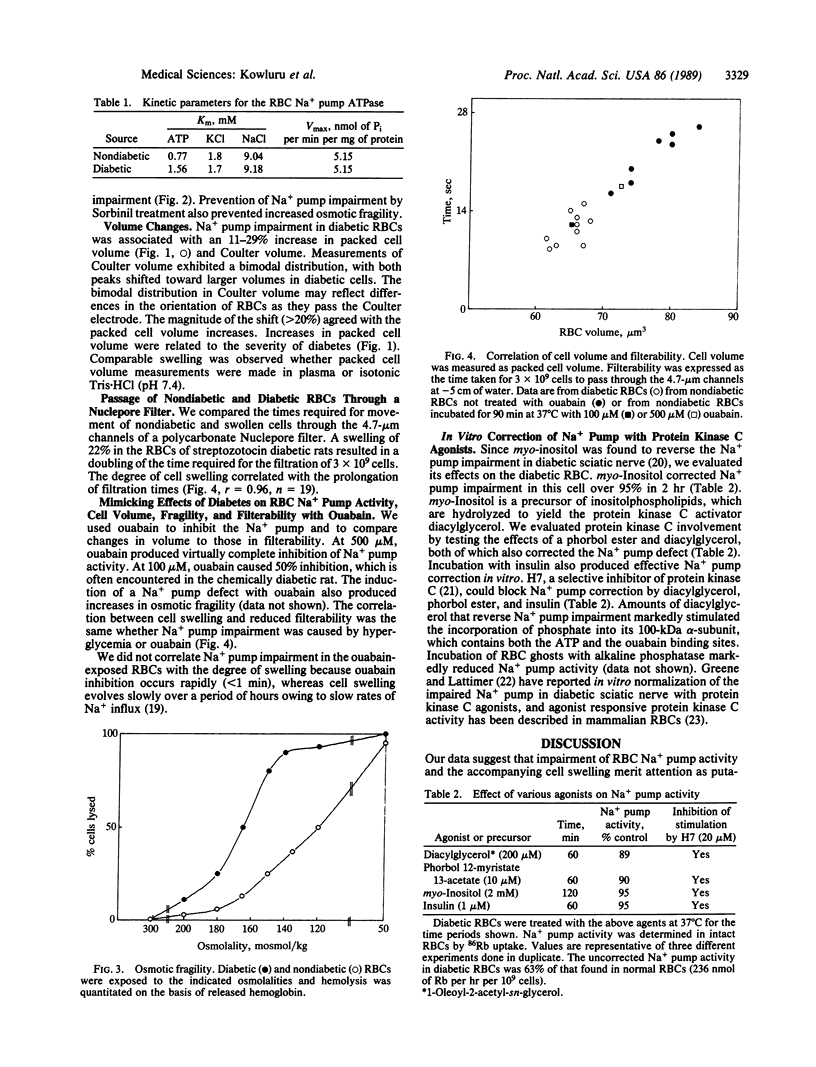
We have found a defect in the ouabain-sensitive Na+, K+-ATPase (Na+ pump, EC 3.6.1.37) of erythrocytes from streptozocin diabetic rats. This defect was accompanied by an increase in cell volume and osmotic fragility and a decrease in the cytosolic K+/Na+ ratio. There was also a doubling in the time needed for diabetic erythrocytes to pass through 4.7-micron channels in a polycarbonate filter. Our data are consistent with a primary defect in the erythrocyte Na+ pump and secondary changes in cell volume, osmotic fragility, K+/Na+ ratio, and cell filterability. All were reversed or prevented in vivo by insulin or the aldose reductase inhibitor Sorbinil. Protein kinase C agonists (phorbol ester and diacylglycerol) and agonist precursor (myoinositol) reversed the Na+ pump lesion, suggesting that protein kinase C-dependent phosphorylation of the 100-kDa subunit regulates Na+ pump activity and that insulin can influence erythrocyte protein kinase C activity. Ouabain inhibition of the erythrocyte Na+ pump also produced increases in cell size and reductions in rates of filtration. Theoretical treatment of the volume changes also predicts reduction in filterability as a consequence of cell swelling. We suggest that enlarged erythrocytes could play a role in the evolution of the microvascular changes of diabetes mellitus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal V. R., Rastogi A. K., Sahib M. K., Sagar P. In vitro insulin action on different ATPases of erythrocyte membranes in normal and diabetic rats. Acta Diabetol Lat. 1985 Apr-Jun;22(2):111–118. doi: 10.1007/BF02590784. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen M. P. Aldose reductase, glomerular metabolism, and diabetic nephropathy. Metabolism. 1986 Apr;35(4 Suppl 1):55–59. doi: 10.1016/0026-0495(86)90188-5. [DOI] [PubMed] [Google Scholar]
- De Luise M., Blackburn G. L., Flier J. S. Reduced activity of the red-cell sodium-potassium pump in human obesity. N Engl J Med. 1980 Oct 30;303(18):1017–1022. doi: 10.1056/NEJM198010303031801. [DOI] [PubMed] [Google Scholar]
- Decaux G., Efira A., Dhaene M., Unger J. Interference of serum tonicity with the measurement of red cell mean corpuscular volume. Acta Haematol. 1982;67(1):62–66. doi: 10.1159/000207026. [DOI] [PubMed] [Google Scholar]
- Finotti P., Palatini P. Reduction of erythrocyte (Na+-K+)ATPase activity in type 1 (insulin-dependent) diabetic subjects and its activation by homologous plasma. Diabetologia. 1986 Sep;29(9):623–628. doi: 10.1007/BF00869260. [DOI] [PubMed] [Google Scholar]
- Fung Y. C. Theoretical considerations of the elasticity of red cells and small blood vessels. Fed Proc. 1966 Nov-Dec;25(6):1761–1772. [PubMed] [Google Scholar]
- Goldin S. M. Active transport of sodium and potassium ions by the sodium and potassium ion-activated adenosine triphosphatase from renal medulla. Reconstitution of the purified enzyme into a well defined in vitro transport system. J Biol Chem. 1977 Aug 25;252(16):5630–5642. [PubMed] [Google Scholar]
- Greene D. A. A sodium-pump defect in diabetic peripheral nerve corrected by sorbinil administration: relationship to myo-inositol metabolism and nerve conduction slowing. Metabolism. 1986 Apr;35(4 Suppl 1):60–65. doi: 10.1016/0026-0495(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Biochemical alterations and complications in diabetes. Clin Chem. 1986 Oct;32(10 Suppl):B42–B47. [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve. Implications for (Na,K)-ATPase regulation and diabetic complications. Diabetes. 1986 Feb;35(2):242–245. doi: 10.2337/diab.35.2.242. [DOI] [PubMed] [Google Scholar]
- Kilo C., Vogler N., Williamson J. R. Muscle capillary basement membrane changes related to aging and to diabetes mellitus. Diabetes. 1972 Aug;21(8):881–905. doi: 10.2337/diab.21.8.881. [DOI] [PubMed] [Google Scholar]
- Kowluru A., Kowluru R., Bitensky M. W., Corwin E. J., Solomon S. S., Johnson J. D. Suggested mechanism for the selective excretion of glucosylated albumin. The effects of diabetes mellitus and aging on this process and the origins of diabetic microalbuminuria. J Exp Med. 1987 Nov 1;166(5):1259–1279. doi: 10.1084/jem.166.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling E., Sapirstein V. Phorbol ester stimulates the phosphorylation of rabbit erythrocyte band 4.1. Biochem Biophys Res Commun. 1984 Apr 16;120(1):291–298. doi: 10.1016/0006-291x(84)91447-5. [DOI] [PubMed] [Google Scholar]
- Luthra M. G., Hildenbrandt G. R., Hanahan D. J. Studies on an activator of the (Ca2+ plus Mg2+)-ATPase of human erythrocyte membranes. Biochim Biophys Acta. 1976 Jan 8;419(1):164–179. doi: 10.1016/0005-2736(76)90380-1. [DOI] [PubMed] [Google Scholar]
- Malone J. I., Leavengood H., Peterson M. J., O'Brien M. M., Page M. G., Aldinger C. E. Red blood cell sorbitol as an indicator of polyol pathway activity. Inhibition by sorbinil in insulin-dependent diabetic subjects. Diabetes. 1984 Jan;33(1):45–49. doi: 10.2337/diab.33.1.45. [DOI] [PubMed] [Google Scholar]
- Mayer K. D., Starkey B. J. Simpler flame photometric determination of erythrocyte sodium and potassium: the reference range for apparently healthy adults. Clin Chem. 1977 Feb;23(2 Pt 1):275–278. [PubMed] [Google Scholar]
- Nagamatsu S., Inoue N., Murakawa S., Matsui H. Evaluation of sodium and potassium pump activity and number in diabetic erythrocytes. Acta Endocrinol (Copenh) 1986 Jan;111(1):69–74. doi: 10.1530/acta.0.1110069. [DOI] [PubMed] [Google Scholar]
- Nair G. P., Standaert M. L., Pollet R. J., Cooper D. R., Farese R. V. Effects of insulin and phorbol esters on diacylglycerol generation and synthesis and hydrolysis of phosphatidylcholine in BC3H-1 myocytes. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1345–1349. doi: 10.1016/0006-291x(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Uemura Y., Hidaka H., Shirakawa S. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine(H-7), a potent inhibitor of protein kinases, inhibits the differentiation of HL-60 cells induced by phorbol diester. Life Sci. 1986 Sep 22;39(12):1101–1107. doi: 10.1016/0024-3205(86)90202-x. [DOI] [PubMed] [Google Scholar]
- O'Neill W. C., Mikkelsen R. B. The role of pump number and intracellular sodium and potassium in determining Na,K pump activity in human erythrocytes. Metabolism. 1987 Apr;36(4):345–350. doi: 10.1016/0026-0495(87)90205-8. [DOI] [PubMed] [Google Scholar]
- Rahmani-Jourdheuil D., Mourayre Y., Vague P., Boyer J., Juhan-Vague I. In vivo insulin effect on ATPase activities in erythrocyte membrane from insulin-dependent diabetics. Diabetes. 1987 Sep;36(9):991–995. doi: 10.2337/diab.36.9.991. [DOI] [PubMed] [Google Scholar]
- Reinila M., MacDonald E., Salem N., Jr, Linnoila M., Trams E. G. Standardized method for the determination of human erythrocyte membrane adenosine triphosphatases. Anal Biochem. 1982 Jul 15;124(1):19–26. doi: 10.1016/0003-2697(82)90214-7. [DOI] [PubMed] [Google Scholar]
- Suhail M., Rizvi S. I. Red cell membrane (Na+ +K+)-ATPase in diabetes mellitus. Biochem Biophys Res Commun. 1987 Jul 15;146(1):179–186. doi: 10.1016/0006-291x(87)90708-x. [DOI] [PubMed] [Google Scholar]
- Tegos C., Beutler E. Red cell glycolytic intermediates in diabetic patients. J Lab Clin Med. 1980 Jul;96(1):85–89. [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Yeh L. A., Rafford C. E., Goddu K. J., Ashton M. A., Beyer T. A., Hutson N. J. Na+-K+-ATPase pumping activity is not directly linked to myo-inositol levels after sorbinil treatment in lenses of diabetic rats. Diabetes. 1987 Dec;36(12):1414–1419. doi: 10.2337/diab.36.12.1414. [DOI] [PubMed] [Google Scholar]


