Abstract
Nicastrin and presenilin 1 are integral components of the high molecular weight γ-secretase complexes that regulate proteolytic processing of various type I membrane proteins including amyloid precursor protein and Notch. At present, there is little information regarding the cellular distribution of nicastrin in the developing or adult rat brain. We report here, using immunoblotting and immunohistochemical methods, that nicastrin in the adult rat brain is widely expressed and co-localized with presenilin 1 in select neuronal populations within all major areas, including the basal forebrain, striatum, cortex, hippocampus, amygdala, thalamus, hypothalamus, cerebellum and brainstem. We also observed dense neuropil labeling in many regions in the brain, suggesting that nicastrin gets transported to dendrites and/or axon terminals in the central nervous system. The levels of nicastrin are found to be relatively high at the early stages of postnatal development and then declined gradually to reach the adult profile. At the cellular level, nicastrin is localized predominantly in neuronal cell bodies at early postanatal stages, but is apparent both in cell bodies and dendrites/neuropil in all brain regions at the later stages. The regulation of nicastrin expression and localization during development and its distribution in a wide spectrum of neurons in the postnatal and adult rat brains provide an anatomical basis to suggest a multifunctional role for the γ-secretase complex in the developing and adult rat brains.
Keywords: Alzheimer’s disease, APP processing, β-amyloid, immunocytochemistry, presenilin, Western blotting
Introduction
An invariant feature associated with Alzheimer’s disease (AD) pathology is the presence of neuritic plaques containing a compact deposit of amyloid fibrils surrounded by dystrophic neurites, activated microglia and reactive astrocytes [8,39,42]. The amyloid filaments are composed of 39–43 amino acid amyloid β (Aβ) peptides, which are derived from sequential proteolysis of amyloid precursor protein (APP) by β-secretase and γ-secretase [15,24,42]. While the β-secretase has been identified as an aspartyl protease called β-APP cleaving enzyme (BACE), recent data suggest that γ-secretase activity resides in a high molecular weight multimeric protein complex composed of at least four core components, i.e., presenilin 1 or 2 (PS1 or PS2), nicastrin, anterior pharynx defective-1 (APH-1) and presenilin enhancer-2 (PEN-2) [9,46,50]. More recently cell surface type I transmembrane glycoprotein CD147, and a member of the p24 family of transmembrane proteins, p23/TMP21, involved in vesicle trafficking between the ER and Golgi apparatus, have been identified as regulatory subunits of the γ-secretase [5,57]. In addition to processing of APP, γ-secretase cleaves C-terminal stubs derived from at least 30 other type I membrane proteins including Notch, releasing cytosolic domains that in some cases migrate to the nucleus and regulate transcription of select target genes [10]. At present, however, the significance of various components of the γ-secretase and their role in regulating the biological activity of the secretase remain unclear.
Nicastrin, an integral component of the γ-secretase complex, is a transmembrane glycoprotein with a long hydrophilic N-terminal domain containing multiple glycosylation sites, a hydrophobic transmembrane domain and a short hydrophilic C-terminal tail [6,55]. Nicastrin was first identified in a C. elegans genetic screen as aph-2, an essential component of GLP-1/Notch signaling pathway in early embryos [16]. In the same year, the mammalian homolog of aph-2 was independently identified by biochemical methods as a protein bound to PS1, and was named nicastrin [55]. Nicastrin deficiency leads to lethal Notch or PS1/PS2 like phenotype with impaired γ-secretase cleavage of APP and Notch [20,31]. Additionally, fibroblasts derived from nicastrin knock-out mice failed to generate Aβ peptide as a consequence of destabilized γ-secretase complex, thus indicating significance of nicastrin in regulating the γ-secretase activity [31]. Studies from biochemical approaches such as RNAi and protein overexpression have indicated that nicastrin may first bind to APH-1 to form a nicastrin-APH-1 subcomplex prior to its association with PS1 and PEN-2 [11,23,56]. Nevertheless, biogenesis, maturation, stability and the steady-state levels of γ-secretase components are co-dependent. For example, the heavily glycosylated type I membrane protein nicastrin does not mature or exit the ER in cells lacking PS1 expression [30]. The function of nicastrin within the γ-secretase complex is beginning to emerge. Ectodomain shedding is a prerequisite for γ-secretase cleavage of substrates [47]. The extracellular domain of nicastrin binds to C-terminal stubs generated by ectodomain shedding of type I membrane protein substrates, thus recruiting substrates for cleavage by the γ-secretase [43].
Although much is known about the processing, assembly and intracellular trafficking of nicastrin, very little information is currently available on the anatomical localization of this protein either in the adult or in the developing mammalian brain. Additionally, it remains to be defined whether the cellular distribution profile of nicastrin overlaps or differs from that of other core components of the γ-secretase complex (PS1, APH-1 and PEN-2). While co-localization of nicastrin with other γ-secretase components will provide an anatomical basis for the site of γ-secretase activity, variations in their distribution may raise the possibility for a γ-secretase-independent function of nicastrin. Earlier studies have shown that nicastrin protein and/or its mRNA are constitutively expressed in a variety of cell lines and tissues including the brain [7,19,41]. Using mouse lines bearing targeted mutations in their PS1 and/or APP genes, nicastrin immunoreactivity has been demonstrated to be distributed predominantly in neurons throughout the neuroaxis [45]. More recently, the relative amount of cerebellar nicastrin located in active site of synaptogenesis was found to be altered during postnatal development suggesting a possible role for γ-secretase complex in synapse formation and maintenance [49]. However, cellular distribution and/or levels of nicastrin in postnatal or adult rat brains remain to be established. Here, using Western blotting and immunohistochemistry, we report that nicastrin immunoreactivity is widely distributed in all major regions of the developing and adult rat brains. Additionally, we have demonstrated that nicastrin immunoreactivity is co-localized with PS1 in all regions of the adult rat brain thus providing an anatomical basis for nicastrin’s function as an integral component of the γ-secretase complex.
Methods
Materials
Sprague-Dawley rats obtained from Charles River (St Constant, Quebec) were used in the study. Adult male rats (225–275 g) and postnatal rats from different developmental stages i.e., postnatal day 1 (P1), P7 and P21 were housed and maintained in accordance with the University of Alberta Policy on the handling and treatment of laboratory animals. The characterization of a polyclonal nicastrin antiserum raised against synthetic peptide corresponding to residues 689–709 (SP718) and a polyclonal PS1 antisera raised against N-terminal (PS1NT) fragment has been described previously [29,30,48]. Commercially available goat polyclonal nicastrin antiserum (N-19) was purchased from SantaCruz Biotechnology Inc. (California, USA). Polyacrylamide electrophoresis gels (4–20%) were from Invitrogen (Burlington, Canada), PVDF membranes were from BioRad (California, USA), anti-Glyceraldehyde-3-phosphate dehydrogenase (GADPH) antiserum was from Abcam Inc. (Cambridge, USA) and enhanced chemiluminescence (ECL) kit was from New England Nuclear (Mississauga, Canada). Donkey anti-goat Texas Red and donkey anti-rabbit fluorescein isothiocyanate (FITC) conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA, USA). Horseradish peroxidase-conjugated protein A and anti-actin antiserum were purchased from Sigma (St. Louis, USA), while elite Vectastain ABC kit was from Vector Laboratories (Burlingame, USA). All other chemicals of analytical grade were purchased from either Fisher Scientific or Sigma Chemical.
Immunoblotting
Six adult rats were decapitated, their brains removed immediately, areas of interest (i.e., frontal cortex, parietal cortex, septum, striatum, hippocampus, thalamus, hypothalamus, cerebellum and brainstem) dissected out and homogenized in Tris lysis buffer [50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 0.1% bovine serum albumin (BSA), 5 mM phenyl-methylsulfonyl fluoride, 10 μg/ml leupeptin and 10 μg/ml aprotinin]. In parallel, four rats from each of the following postnatal developmental stages i.e., P1, P7 and P21 were decapitated and brain regions of interest (i.e., cortex, hippocampus, cerebellum and brainstem) were dissected out and homogenized in Tris-lysis buffer. Tissues were then processed for immunoprecipitation by incubating with SP718 nicastrin antiserum as described earlier [30,51]. The immune complexes were precipitated by protein A/G PLUS-agarose, separated by 4–20% polyacrylamide gel electrophoresis for 90 min before being transferred to PVDF membranes. Membranes were then blocked for 1 hr in phosphate buffered saline supplemented with 10% non-fat milk and 0.2% Tween-20 (PBST) and incubated overnight at 4°C with N-19 nicastrin antibody (1:1000). Membranes were washed three times with PBST, incubated with horseradish peroxidase-conjugated protein A (1:2500) for 1 hr at room temperature and then exposed to X-ray films using an ECL detection kit. Blots depicting the presence of nicastrin in the adult rat brain were then stripped and sequentially reprobed with anti-PS1NT and anti-GADPH (1:1000) antibodies. Blots depicting the presence of nicastrin at different stages of developing brains were reprobed with anti-actin (1:500) antibodies and quantified using an MCID image analysis system as described [17]. The data from the developing rat brains which are presented as mean ± S.E.M. were analyzed using one way ANOVA followed by Tukey’s post-hoc analysis with significance set at p < 0.05.
Immunohistochemistry
Eight adult male rats were deeply anesthetized with 8% chloral hydrate (VWR Canlab, Montreal, Canada), prior to perfusion with 0.01M phosphate buffered saline (PBS, pH 7.2), followed by 4% paraformaldehyde (PFA) or Bouin’s solution. Six postnatal rats from each stages of development (i.e., P1, P7 and P21) were anesthetized by cooling on ice (P1) or with isoflurine gas (i.e., P7 and P21) and then perfused with 0.01M PBS followed by 4% PFA. Brains were removed, post fixed overnight in the same fixative and stored at 4°C in 30% PBS/sucrose. Coronal brain sections (20 and 40 μm) were cut on a cryostat and then processed following free-floating procedure as described earlier [17]. For enzyme-linked procedure, sections were first washed with PBS, boiled for 15 min in 10 mM sodium citrate buffer (pH 6) and then treated with 1% hydrogen peroxide for 30 minutes prior to overnight incubation with either rabbit/goat anti-nicastrin antibody (1:1000) or rabbit anti-PS1NT antibody (1:000) at room temperature. Subsequently, sections were exposed to appropriate secondary antiserum (1:200) for 1 hr at room temperature, rinsed with PBS and incubated with avidin-biotin reagents for an additional hr at room temperature. Finally, the sections were developed using glucose-oxidase-diaminobenzidine tetrahydrochloride-nickel enhancement method, as described earlier [17,22]. The specificity of the nicastrin antibody was determined by omission of the primary antibody and by pre-adsorption of the diluted antiserum with 10 μM peptide antigen. Nicastrin staining was not observed in sections in which the primary antibody was either omitted or preadsorbed with excess peptide antigen. Immunostained sections were examined under a Zeiss Axioskop-2 microscope and the photomicrographs were taken with a Nikon 200 digital camera and exported to the Adobe Photoshop 6.0 program. For double immunofluroscence staining, adult rat brain sections (20 μm) were incubated overnight at room temperature with goat anti-nicastrin (1:50) and rabbit anti- PS1NT (1:200) antisera. After incubation with primary antisera, sections were rinsed with PBS, exposed to Texas Red- or FITC-conjugated secondary antibodies (1:200) for 2 hr at room temperature, washed thoroughly with PBS and then cover-slipped with Vectashield mounting medium. Immunostained sections were examined under a Zeiss Axioskop-2 fluorescence microscope and the photomicrographs were taken with a Nikon 200 digital camera and exported to the Adobe Photoshop CS2 program for further processing [18]. The rat brain atlas of Paxinos and Watson [38] was used to define and name anatomical structures.
Results
Immunoblotting
We first performed immunoblot analysis to examine the distribution of nicastrin in the adult rat brain. As shown in Fig 1, the nicastrin antiserum recognized one band with apparent molecular weight of 120 kDa corresponding to the mature glycosylated nicastrin. The nicastrin, as evident from a representative immunoblot, was present at all major regions of the brain including frontal cortex, parietal cortex, hippocampus, thalamus, hypothalamus, striatum, septum, cerebellum and brainstem. The overall expression of nicastrin was found to be relatively lower in the septum and cerebellum compared to other regions of the brain (Fig. 1). Reprobing of the blots with polyclonal PS1 antibodies PS1NT revealed the presence of a 30 kD PS1 N-terminal fragment in all major regions of the brain such as frontal cortex, parietal cortex, hippocampus, thalamus, hypothalamus, striatum, septum, cerebellum and brainstem (Fig. 1).
Fig. 1.
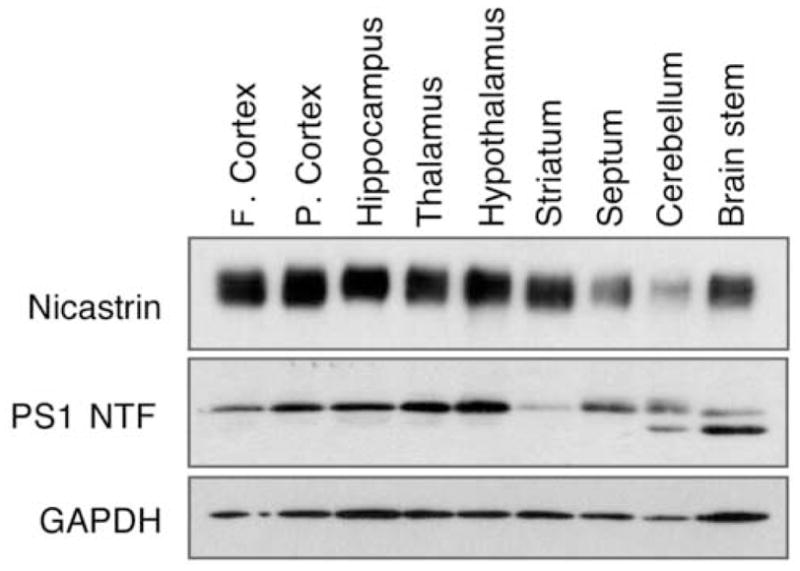
Immunoblot analysis of nicastrin in different brain regions of the adult rat. Nicastrin (NCT) antiserum reacts with one major band of approximately 120 kDa, corresponding to the mature complex glycosylated form of nicastrin. The same membrane was sequentially probed with PS1NT and Glyseraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies. F. cortex, frontal cortex; P. cortex, parietal cortex.
Distribution of nicastrin immunoreactivity in the adult rat brain
Nicastrin immunoreactivity, as observed with two different polyclonal nicastrin antisera (i.e., SP718 and N-19), is widely distributed throughout the adult rat brain in select neuronal populations within the basal forebrain, cerebral cortex, hippocampus, amygdala, thalamus, hypothalamus, cerebellum and brainstem (Figs. 2–4). However, the intensity of staining differed in a neuron- and region-specific manner. The specificity of the immunostaining was established by preadsorption of the antibody with excess antigen, which abolished the immunolabeling (see Fig. 4). In the following sections, we describe the general distribution profile of nicastrin immunoreactivity observed in specific brain regions.
Fig. 2.
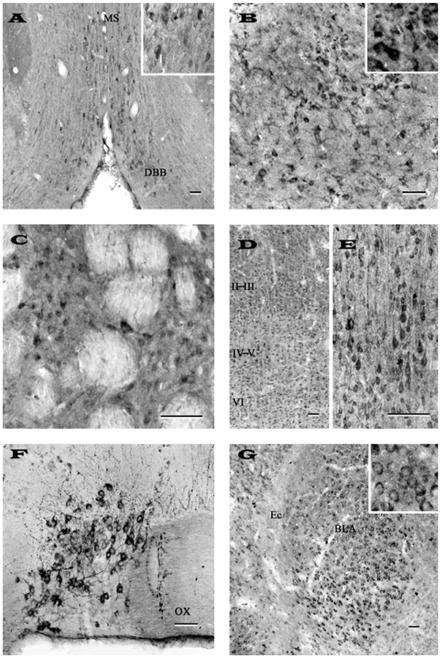
Photomicrographs of transverse sections of adult rat brain showing the distribution of nicastrin immunoreactive neurons and fibers in the diagonal band of Broca (A), globus pallidus (B), striatum (C), cortex (D, E), supraoptic nucleus (F) and amygdaloid nuclues (G). Note that neurons of the diagonal band of broca, globus pallidus and striatum are moderately labeled, whereas supraoptic and amygdaloid nuclei exhibited rather intense immunoreactivity. The staining intensity in the neocortex is variable: weak labeling in layer I, moderate staining in layers II-III (D) and strong immunoreactivity in deep layers IV-VI (D and E). Inset in (A), (B) and (G) show neuronal labeling at higher magnification. Representative photomicrographs of striatum (C) and cortex (D, E) were obtained with N-19 antiserum, whereas others (A, B, F, G) were acquired following labeling with SP718 antiserum. Scale bar = 50 μm. MS, medial septum; DBB, diagonal band complex; OX, optic chiasm; Ec, external capsule; BLA, basolateral amygdala.
Fig. 4.
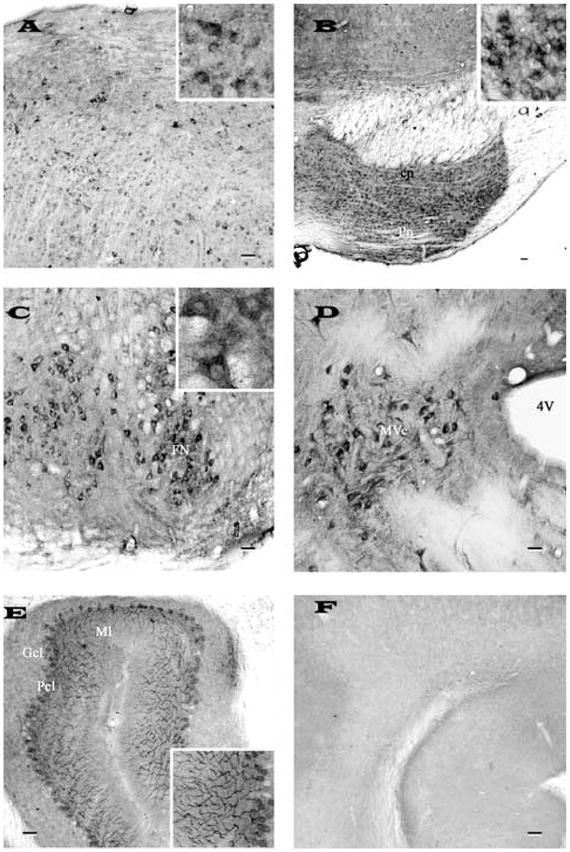
Photomicrographs of transverse sections of adult rat brain showing the distribution of nicastrin immunoreactivity in the inferior colliculi (A), pontine nucleus (B), facial nucleus (C), vestibular nucleus (D) of the brainstem and in the cerebellum (E). Note intense labeling of the brainstem neurons and the Purkinje cells of the cerebellum. F, represents a cerebellar section processed following preabsorption of the antibody with 10 μM purified rat nicastrin. Inset in (A), (B), (C) and (E) show neuronal labeling at higher magnification. All photomicrographs were acquired following labeling with SP718 antiserum. Scale bar = 50 μm. cp, cerebral peduncle; Pn, pontine nuclei; FN, facial nucleus; Mve, medial vestibular nucleus; Gcl, granular cell layer; Pcl, Purkinje cell layer; Ml, molecular layer.
Basal forebrain and basal ganglia
Nicastrin immunoreactivity was observed in all subfields of the basal forebrain including the septum, diagonal band complex (Fig. 2A) and nucleus baslis of Meynert. In septal nuclei, a group of multipolar cells were moderately labeled, whereas in the diagonal band complex, some weakly labeled neurons were found intermingled with moderately labeled neurons (Fig. 2A). Few nicastrin-immunoreactive neurons were also seen in the bed nucleus of the stria terminalis and nucleus basalis of Meynert. A population of small neurons was also encountered in the globus pallidus (Fig. 2B) and ventral pallidum, whereas the entopeduncular nucleus displayed few nicastrin immunoreactive cells. A number of nicastrin positive neurons were found scattered throughout the caudate putamen (Fig. 2C). These neurons, which were usually located in between unstained myelinated fascicles, were multipolar with short processes.
Cerebral cortex
Nicastrin immunoreactive neurons were detected in most layers of the neocortex with varying degrees of staining intensity. Characteristically, the labeling was high in layers IV-VI, moderate in layers II-III and relatively low in layer I (Figs. 2D, E). The laminar distribution of nicastrin labeled neurons was particularly striking in cingulate cortex and in the frontoparietal cortex. In general, a number of moderately stained smaller multipolar neurons were visible in layers II-III, whereas intensely labeled pyramidal neurons with vertically oriented apical dendrites were seen in layers IV and V of the cortex (Fig. 2E). Layer VI, on the other hand, was characterized by some scattered multipolar neurons with moderate somatodendritic labeling. In the piriform cortex, intensely labeled nicastrin immunoreactive neurons were common in both the pyramidal and polymorphic layers, intermingled with a smaller population of weakly labeled neurons.
Amygdala
Several groups of moderately labeled nicastrin-immunoreactive cell bodies were evident in the cortical, medial and basolateral amygdaloid nuclei (Fig. 2G). Additionally, some multipolar cells exhibiting rather weak immunoreactivity were also apparent in the anterior amygdaloid area and central amygdaloid nucleus. Most of the immunostaining in the amygdaloid nuclei was found to be associated predominantly with cell bodies (see Fig. 2G inset).
Hippocampus
The hippocampal formation showed some of the most intense nicastrin immunoreactivity in the brain (Fig. 3A–C). Within the Ammon’s horn, strong labeling was apparent in the CA1-CA3 pyramidal cells and their apical dendrites (Fig. 3A, B), but occasional multipolar neurons were also found scattered in the strata oriens and stratum radiatum. Within the dentate gyrus, granule cell somata were moderately labeled (Fig. 3C), whereas little nicastrin immunoreactivity was evident in the adjacent molecular layer. A number of nicastrin-positive polymorphic neurons were also observed in the hilus region of the hippocampus (Fig. 3C).
Fig. 3.
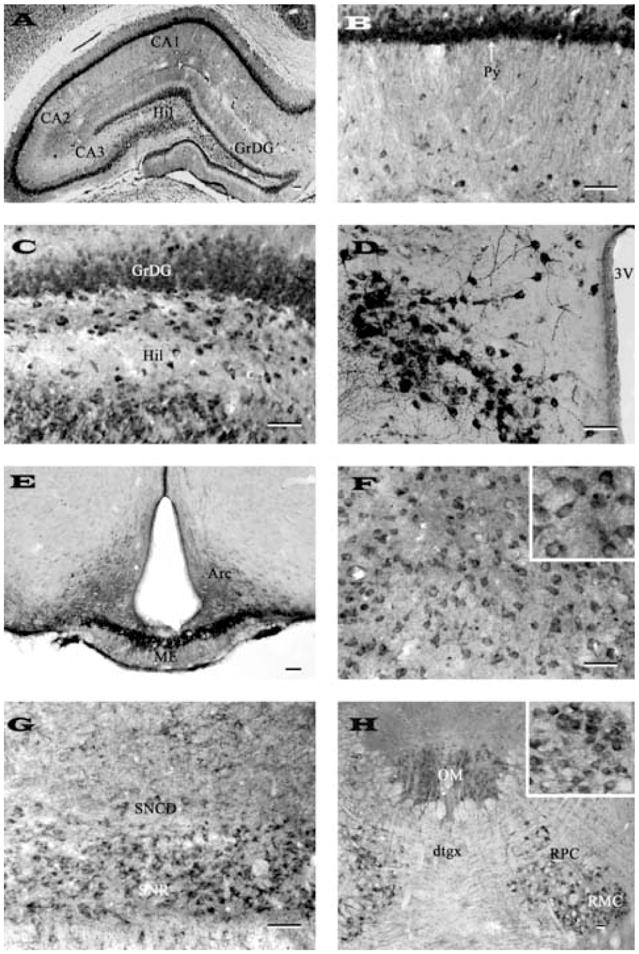
Photomicrographs of transverse sections of adult rat brain showing the distribution of nicastrin immunoreactive neurons and fibers in the hippocampus (A), CA1 pyramidal cell layer of the Ammon’s horn (B), granule cell layer and hilus of the dentate gyrus (C), paraventricular nucleus of the hypothalamus (D), arcuate nucleus/median eminence (E), medial thalamic nucleus (F), substantia nigra (G) and in oculomotor and red nuclei (H). Note intense labeling in neurons of the pyramidal cell layer, hilus and paraventricular nucleus and fibers in the median eminence. Inset in (F) and (G) show neuronal labeling at higher magnification. Representative photomicrographs of medial thalamic nucleus (F) and substantia nigra (G) were obtained with N-19 antiserum, whereas others (A, B, C, D, E, H) were acquired following labeling with SP718 antiserum. Scale bar = 50 μm. Hil, hilus; GrDG, granular cell layer of the dentate gyrus; Py, pyramidal cell layer; 3V, third ventricle; Arc, arcuate nucleus; ME, median eminence; SNCD, substantia nigra compact part; SNR, substantia nigra reticular part; OM, oculomotor nucleus; dtgx, dorsal tegmental decussation; RPC, red nucleus pervicellular part; RMC, red nucleus magnocellular part.
Hypothalamus and Thalamus
In the hypothalamus rather strong neuronal labeling was observed in the supraoptic (Fig. 2F) and paraventricular (Fig. 3D) nuclei, whereas neurons located in the ventromedial nucleus, dorsolateral hypothalamic areas and arcuate nucleus (Fig. 3E) showed rather weak to moderate labeling. The immunoreactivity in the median eminence was confined exclusively to neuropil (Fig. 3E). A number of medium-sized nicastrin immunostained neurons were observed throughout the thalamus (Fig. 3F). These neurons which were moderately labeled were evident in ventral and lateral portions of the thalamus (Fig. 3F) and in the habenular nucleus.
Midbrain
Moderate somatodendritic labeling was observed in the superficial gray layers of the superior colliculus and in the central gray matter. The substantia nigra pars reticulata was characterized by multipolar neurons with moderate nicastrin immunoreactivity (Fig. 3G), whereas the pars compacta exhibited rather weak labeling. Neurons of the red and oculomotor nuclei displayed rather intense immunoreactivity (Fig. 3H). Moderately labeled neurons were also apparent in the intermediate gray layer of superior colliculus and mesencephalic trigeminal nucleus.
Brainstem
Nicastrin immunoreactivity was visible at all brainstem levels. A population of large multipolar neurons was encountered in the pontine reticular nucleus, whereas numerous moderately labeled neurons were seen in the inferior colliculus (Fig. 4A), abducens nucleus and reticulotegmental nucleus of pons. However, strong labeling was evident particularly in the motor trigeminal nucleus, pontine nucleus (Fig. 4B) and in the facial (Fig. 4C) as well as vestibular nuclei (Fig. 4D).
Cerebellum
A common pattern of intense nicastrin immunoreactivity prevailed throughout the cerebellum (Fig. 4E, F). In the cortex, Purkinje cells were heavily stained and often seen in continuity with their stained dendritic shafts extending into the molecular layer (Fig. 4E). The granule cells exhibited rather weak staining, whereas a number of deep cerebellar nuclei showed numerous moderately labeled immunoreactive cell bodies and dendrites.
Co-localization of nicastrin and PS1 in the adult rat brain
To determine the possible co-localization of nicastrin and PS1 in the adult rat brain, we first established the normal distribution profile of PS1 immunoreactivity using a single labeling procedure and then performed double labeling experiments in sections from different brain regions. In agreement with earlier reports [3,12,27,28,29,53], PS1-immunoreactive neurons and fibers were distributed throughout the brain including the septal/diagonal band complex, nucleus basalis of Meynert, striatum, cerebral cortex, hippocampus, amygdala, thalamus, hypothalamus, median eminence, brainstem motor nuclei and cerebellar Purkinje cells (Figs. 5 and 6). Dual-labeling experiments revealed that nicastrin is co-localized with PS1-positive neurons in virtually all regions of the brain including vertical and horizontal limbs of the diagonal band complex (Fig. 5B, C), striatum (Fig. 5E, F), cerebral cortex (Fig. 5I, J), hippocampal formation (Fig. 5L, M), median eminence (Fig. 6B, C), thalamus (Fig. 6E, F), brainstem motor nuclei (Fig. 6H, I) and cerebellar Purkinje cells (Fig. 6K, L). It is apparent from our double labeling experiments that nicastrin and PS1 are co-localized not only in cell bodies but also in dendrites/neuropil in discrete regions of the brain.
Fig. 5.
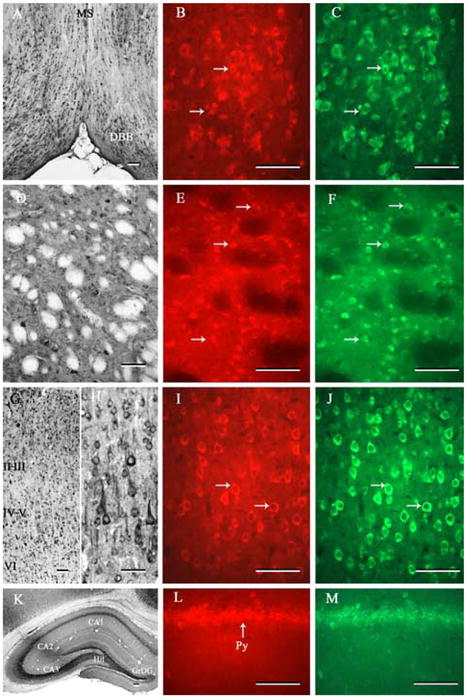
Photomicrographs of transverse sections of adult rat brain showing the distribution of PS1 immunoreactivity and its co-localization with nicastrin in the diagonal band of Broca (A-C), striatum (D-F), cortex (G-J) and hippocampal formation (K-M). Note the widespread distribution of immunoreactive PS1 in various regions of the adult rat brain (A, D, G, H, K). Double labeling experiment showed that PS1 is co-localized with nicastrin in all neurons located in the diagonal band of Broca (B, C), striatum (E, F), cortex (I, J) and hippocampus (L, M) of the adult rat brain. Scale bar = 50 μm. MS, medial septum; DBB, diagonal band complex; Hil, hilus; GrDG, granular cell layer of the dentate gyrus; Py, pyramidal cell layer.
Fig. 6.
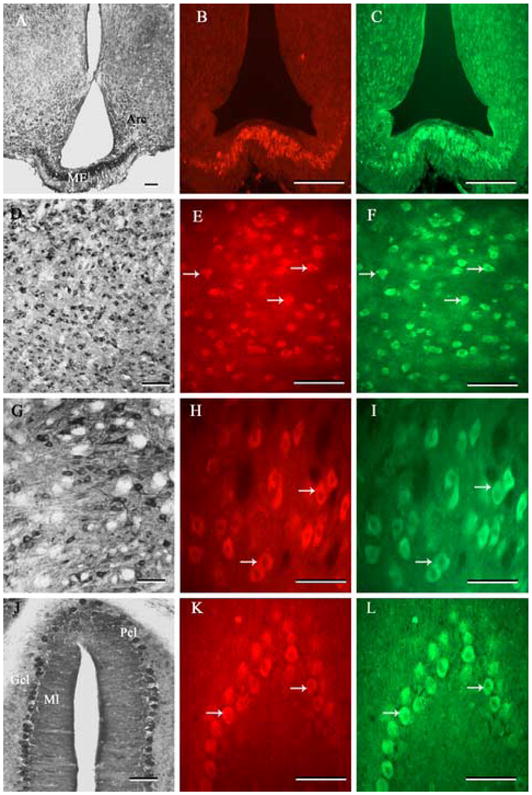
Photomicrographs of transverse sections of adult rat brain showing the distribution of PS1 immunoreactivity and its co-localization with nicastrin in the median eminence (A-C), thalamus (D-F), brainstem (G-I) and cerebellum (J-L). Note the widespread distribution of immunoreactive PS1 in various regions of the adult rat brain (A, D, G, J). Double labeling experiment showed that PS1 is co-localized with nicastrin in all neurons and/or nerve terminals located in the median eminence (B, C), thalamus (E, F), brainstem (I, J) and cerebellum (L, M) of the adult rat brain. Scale bar = 50 μm. ARC, arcuate nucleus; ME, median eminence; Gcl, granular cell layer; Pcl, Purkinje cell layer; Ml, molecular layer.
Nicastrin immunoreactivity in the postnatal developing rat brain
Earlier studies have indicated that PS1 expression is relatively high at early stages of development and then declined gradually to reach adult profile of distribution [13,25,34,49,52]. To establish whether nicastrin expression level and distribution are also developmentally regulated, we performed immunoblotting and immunocytochemical analyses of the cortex, hippocampus, cerebellum and brainstem obtained from P1, P7 and P21 brains (Fig. 7A–L). The relative levels of nicastrin in all these brain regions were found to be highest at P1 followed by a gradual decline to attain the levels found in adult. However, the chronological alterations were more evident in hippocampal and brainstem regions than in the cortex or cerebellum (see Fig. 7A, D, G, J). At the cellular levels, nicastrin immunoreactivity is widely distributed at all stages of the postnatal developing brains (Fig. 7B, C, E, F, H, I, K, L). At P1 and P7, nicastrin immunoreactivity was localized predominantly in neuronal cell bodies, whereas at P21 immunoreactivity was apparent not only in the cell bodies but also in dendrites/neuropil of all brain regions. The cortical regions of P1 and P7 brains exhibited rather homogenous nicastrin immunoreactivity in all layers without any laminar distinction, whereas cortex from P21 brain showed subtle variation in labeling intensity in different layers (Fig. 7B, C). In the hippocampus, intense nicastrin immunoreactivity at P1 and P7 was evident primarily in the CA1-CA2 pyramidal cell layers and granular cell layers of the dentate gyrus. The CA3 pyramidal cell layer showed relatively lower intensity of immunoreactivity. The distribution profile of nicastrin did not exhibit significant alteration during the course of development but its intensity, as evident from P21 hippocampal formation, was found to be drastically decreased (Fig. 7E, F). As for the brainstem motor nuclei, nicastrin immunoreactivity was apparent mostly in cell bodies at P1 and P7, whereas a number of dendrites/neuropil, in addition to motoneuron cell bodies, were also labeled at P21 stage (Fig. 7H, I). Interestingly, the cerebellum of P1 and P7 brains showed robust nicastrin immunoreactivity in cell bodies of Purkinje cells and some punctate staining in the external granular layer. At P21, labeling intensity was relatively lower but often seen in dendritic shafts of the Purkinje cells (Fig. 7K, L).
Fig. 7.
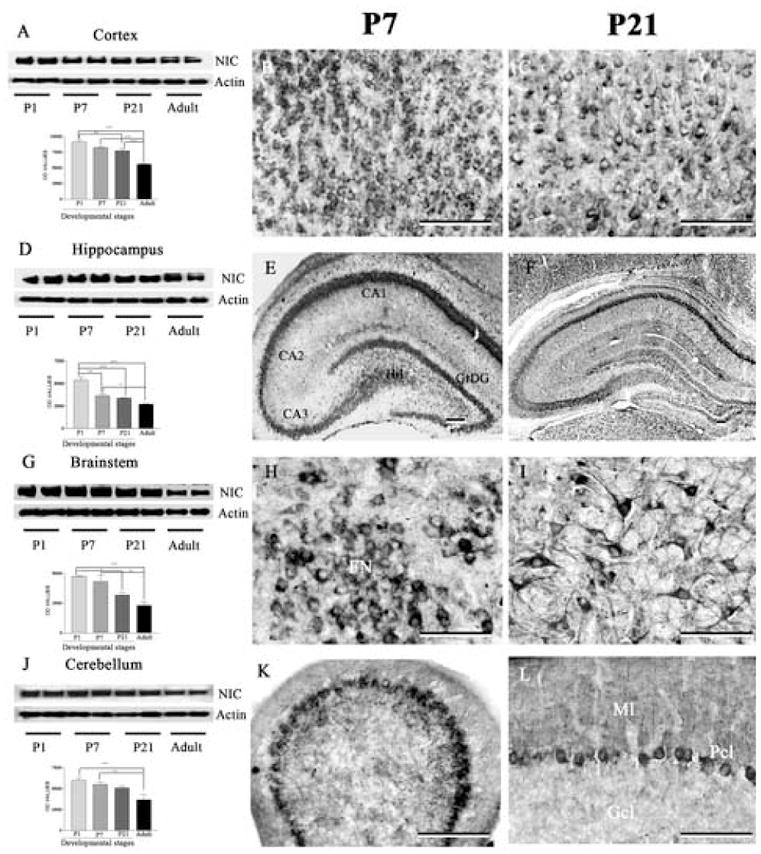
Immunoblotting and immunohistochemical staining showing the levels and expression of nicastrin in the cortex (A-C), hippocampus (D-F), brainstem (G-I) and cerebellum (J-L) of the postnatal rat brains at different stages of development. Note the gradual decrease in nicastrin levels at all four regions of the brain with the progress of the development (A, D, G, J). Immunohistochemical staining revealed that nicastrin expression is relatively high in the cortex (B, C), hippocampus (E, F), brainstem (H, I) and cerebellum (K, L) at P7 (B, E, H, K) compared to P21 (C, F, I, L) postnatal brains. All immunocytochemical photomicrographs were acquired following labeling with SP718 antiserum. Scale bar = 50 μm. * p < 0.05, ** p < 0.01, *** p < 0.001. Hil, hilus; GrDG, granular cell layer of the dentate gyrus; FN, facial nucleus; Gcl, granular cell layer; Pcl, Purkinje cell layer; Ml, molecular layer.
Discussion
The present study provides the first comprehensive cellular distribution of nicastrin, an integral subunit of γ-secretase complex, in the postnatal developing and adult rat brains. As evident from our immunoblotting and immunohistochemical analyses, nicastrin is widely expressed throughout the brain and the level of expression is developmentally regulated during postnatal stages. Additionally, nicastrin immunoreactivity extensively overlaps with PS1 distribution in various regions of the adult rat brain, thus providing an anatomical basis for a physiological role for γ-secretase complex in a wide-spectrum of neurons located throughout the brain.
A potential concern regarding any immunological study is the specificity of the antiserum employed for the immunolabeling. The nicastrin and PS1 antisera used in the present study have been previously characterized [29,30,51]. The specificity of antisera is further confirmed by our immunoblotting experiment, which showed that nicastrin antiserum essentially reacted with one major band of approximately 120 kDa corresponding to the mature complex glycosylated forms of the protein in all brain regions examined. The anti-PS1NT antibody visualized a single band with a molecular weight of approximately 30 kDa, equivalent to human PS1-NTF. For immunohistochemistry, standard immunological controls, including omission of the primary antiserum and preincubation of the antisera with excess antigen, eliminated staining, thus indicating that the nicastrin and PS1 antisera specifically recognize the endogenous nicastrin and PS1, respectively. As for nicastrin, this is further substantiated by evidence that two nicastrin antisera reproducibly stained similar groups of neurons and fibers in the rat brain.
Our results clearly indicate that nicastrin is expressed in all major areas of the adult rat brain. At the cellular level, nicastrin immunoreactivity predominantly appears to be associated with neurons and their processes. Although no positive staining was readily apparent in morphologically identifiable glial cells, the expression of nicastrin in astrocytes and/or microglia in normal adult rat brain cannot be formally ruled out based on the present findings. Interestingly, in addition to the neuronal soma, neuropil labeling was apparent in many brain regions, suggesting that nicastrin is localized in dentrites and/or axon terminals. However, the intensity of immunoreactivity in select neuronal populations varies distinctly among different regions of the brain. Areas that express relatively high levels of nicastrin include the cortex (layers IV and V), pyramidal and granule cell layers of the hippocampus, selected hypothalamic nuclei, Purkinje cells of the cerebellum, pontine nucleus and motoneurons of the brainstem. Moderate neuronal labeling was apparent in the basal forebrain areas, amygdala, thalamus, superior colliculus, midbrain areas and granule cells of the cerebellum.
In keeping with the adult distributional profile, analysis of postnatal rats showed widespread distribution of nicastrin in various brain regions including cerebral cortex, hippocampus, brainstem and cerebellum. However, nicastrin expression decreased with the progress of postnatal development. It is also of interest to note that nicastrin expression was apparent predominantly in cell bodies at the early stages of development, whereas during later stages immunoreactivity was evident both in the cell bodies as well as dendrites/neuropil. Furthermore, nicastrin expression markedly decreased in the hippocampus and brainstem as compared with the level of expression in cortex and cerebellum, thus suggesting that developmental profile of nicastrin is possibly regulated differentially in various brain regions.
To the best of our knowledge, the previous immunocytochemical report regarding nicastrin distribution in the developing and adult rat brain is mostly restricted to the analysis of cerebellum [49]. Consistent with this study, our results show nicastrin expression is developmentally regulated and it is localized in both the Purkinje cells as well as granule cell layer of the cerebellum. Furthermore, the present study extends previous findings on two accounts by revealing that i) the levels and distribution of nicastrin are developmentally regulated not only in the cerebellum but also in the cerebral cortex, hippocampus and brainstem regions, and ii) nicastrin immunoreactivity in the adult rat brain is distributed throughout the neuroaxis including the basal forebrain, cortex, amygdala, thalamus, hypothalamus, substantia nigra and brainstem. These results are very much compatible with the findings reported in the adult mouse brain [45]. However, variation in the intensity of labeling is discernible in certain brain areas such as the cortex where a gradient of immunoreactivity was evident in the adult rat brain in contrast to the uniform labeling across the cortical layers in the mouse brain. Additionally, neuropil of stratum radiatum and stratum lacunosum moleculare of the adult rat hippocampal formation, unlike the mouse hippocampus, did not exhibit significant nicastrin immunoreactivity [45]. These apparent discrepancies could relate either to the animal species or to the epitope specificity of nicastrin antisera used in both the studies. At present, the distribution profile of nicastrin mRNA has not been thoroughly investigated at the cellular levels in the adult rat brain, but high levels of nicastrin transcripts have been detected in the cortex, hippocampus and cerebellum of the adult rat brain by RT-PCR assay. Interestingly, in addition to the full length nicastrin mRNA, the expression of an alternative spliced variant lacking exon 3 has also been found to be expressed preferentially in embryonic and adult nervous system [7].
Earlier studies have shown that nicastrin can be co-immunoprecipitated and co-localized with PS1 in a variety of cells/neurons [1,30,37,44,49,55]. Consistent with previous reports [3,12,27,28, 29,53], we find that PS1 is distributed widely in the adult rat brain. Additionally, our double labeling experiments showed that PS1 is coexpressed with nicastrin in almost all brain regions investigated in the present study, leading to the suggestion that nicastrin most likely acts as an integral component of the γ-secretase complex in the brain rather than mediating any effects of its own. However, it is of interest to determine whether nicastrin and PS1 are localized at the same subcellular site within neurons along with other components of the γ-secretase complex. At present, no report is available on the cellular distribution of APH-1 or PEN-2 in the adult rat brain, but a recent immunocytochemical study showed that four components of the γ-secretase complex exhibit a coincident anatomical localization in the adult transgenic mouse brain [45]. Some subtle variations have been reported in the cellular distribution profile among the four γ-secretase subunits in selected brain regions as well as in certain peripheral tissues. For example, PEN-2 immunoreactivity, compared to other γ-secretase components, was found to be rather intense in mossy fiber terminal zone of the hippocampus [45], whereas nicastrin, but not PS1, was expressed at relatively high levels in the muscle membranes [21]. Whether these differences reflect unique functions of PEN-2 or nicastrin independent of the γ-secretase remains to be established.
Nicastrin was initially identified as a cofactor of PS1 within the γ-secretase complex that cleaves several membrane proteins including APP and Notch [10]. It has recently been shown that the extracellular domain of nicastrin is involved in the substrate recognition, whereas the transmembrane domain of the protein is critical in the assembly and trafficking of the γ-secretase complex to the cell surface [4,43]. The significance of this protein is further highlighted by RNAi and gene knock out studies, which clearly demonstrated that nicastrin is essential for the assembly of PS1/γ-secretase complex and secretion of Aβ peptide [20,30,31,54,56]. Since Aβ-related peptides are produced constitutively [42], it is likely that distribution of nicastrin observed in a variety of neuronal populations represents cellular sites of active γ-secretase complex, which mediates APP processing in the developing as well as adult rat brain. Interestingly, we find that nicastrin is expressed both in the brain regions that are vulnerable in AD pathology such as cortex and hippocampus, and also in relatively unaffected regions such as striatum and cerebellum. This is somewhat compatible with a recent study which showed that γ-secretase subunits are expressed at similar levels in amyloid-rich (e.g., deep entorhinal cortex) and amyloid-poor (superficial entorhinal cortex) brain regions of the mice bearing targeted mutations in the PS1 and APP genes [45]. Thus, it is likely that neuronal vulnerability or amyloid deposition in AD, may depend on factors other than the differential distribution of γ-secretase complex in the brain.
As a component of γ-secretase complex, nicastrin is also involved in the recognition and cleavage of a variety of other integral membrane proteins such as Notch, Nectin-1a, E-cadherin and ErbB-4 receptor [26,32,35,50]. There is evidence that peptides generated from these cleavage are capable of modulating functions in the developing as well as the adult brain [10]. Earlier studies have shown that neurogenesis in the rat brain is mostly completed by birth, with neuronal differentiation, migration and synaptogenesis continuing for several weeks [2,33]. The pronounced expression of nicastrin in the early postnatal brains, together with the evidence that γ-secretase plays a critical role in development [14,40], raise the possibility that nicastrin as an integral component of γ-secretase complex may be involved in regulating neuronal differentiation, maturation and/or synaptogenesis during development. Additionally, it is likely that widespread nicastrin expression in the adult brain may also contribute, at least in part, in the γ-secretase processing of substrates to regulate normal synaptic function in the adult brain [36]. In conclusion, the present study demonstrates clearly that nicastrin is widely distributed in the adult rat brain which may provide underlying basis to define precisely the site of γ-secretase activity and other function of the protein, if any, in normal and AD brains.
Acknowledgments
This work is supported in part by grants from Canadian Institutes of Health Research (S.K.), National Institutes of Health/National Institute on Aging (G.T.), Alzheimer’s Association (K.S.V and G.T.), and funding from Ms. Irene Godberson (S.K.). S.K. is a recipient of Canada Research Chair (Tier-II) in Neurodegenerative Diseases and a Senior Scholar award from the Alberta Heritage Foundation for Medical Research.
Footnotes
Disclosure statement: We would like to indicate that none of the authors included in this manuscript has any actual or potential conflict of interest including financial, personal or other relationships with other people or organizations at any time that could inappropriately influence (bias) the work. Additionally, Sprague-Dawley rats used in the study were handled in accordance with protocol approved by the University of Alberta Policy on the handling and treatment of laboratory animals.
References
- 1.Baulac S, LaVoie MJ, Kimberly WT, Strahle J, Wolfe MS, Selkoe DJ, Xia W. Functional γ-secretase complex assembly in Golgi/trans-Golgi network: interactions among presenilin, nicastrin, Aph1, Pen-2 and γ-secretase substrates. Neurobiol Dis. 2003;14:194–204. doi: 10.1016/s0969-9961(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 2.Bayer SA. Development of the hippocampal region in the rat II: morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard V, Czech C, Bonici B, Clavel N, Gohin M, Dalet K, Revah F, Pradier L, Imperato A, Moussaoui S. Immunohistochemical analysis of presenilin 2 expression in the mouse brain: distribution pattern and co-localization with presenilin 1 protein. Brain Res. 1997;758:209–17. doi: 10.1016/s0006-8993(97)00231-x. [DOI] [PubMed] [Google Scholar]
- 4.Capell A, Kaether C, Edbauer D, Shirotani K, Merkl S, Steiner H, Haass C. Nicastrin interacts with γ-secretase complex components via the N-terminal part of its transmembrane domain. J Biol Chem. 2003;278:52519–23. doi: 10.1074/jbc.C300435200. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St George-Hyslop P, Fraser P. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–12. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Yu G, Arawaka S, Nishimura M, Kawarai T, Yu H, Tandon A, Supala A, Song YQ, Rogaeva E, Milman P, Sato C, Yu C, Janus C, Lee J, Song L, Zhang L, Fraser PE, St George-Hyslop P. Nicastrin binds to membrane-tethered. Notch Nat Cell Biol. 2001;3:751–4. doi: 10.1038/35087069. [DOI] [PubMed] [Google Scholar]
- 7.Confaloni A, Crestini A, Albani D, Piscopo P, Campeggi LM, Terreni L, Tartaglia M, Forloni G. Rat nicastrin gene: cDNA isolation, mRNA variants and expression pattern analysis. Mol Brain Res. 2005;136:12–22. doi: 10.1016/j.molbrainres.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Cordell B. β-amyloid formation as a potential therapeutic target for Alzheimer’s disease. Annu Rev Pharmacol Toxicol. 1994;34:69–89. doi: 10.1146/annurev.pa.34.040194.000441. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 10.De Strooper B. Nicastrin: gatekeeper of the gamma-secretase complex. Cell. 2005;122:318–20. doi: 10.1016/j.cell.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Edbauer D, Winkler E, Haass C, Steiner H. Presenilin and nicastrin regulate each other and determine amyloid β-peptide production via complex formation. Proc Natl Acad Sci USA. 2002;99:8666–71. doi: 10.1073/pnas.132277899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder GA, Tezapsidis N, Carter J, Shioi J, Bouras C, Li HC, Johnston JM, Efthimiopoulos S, Friedrich VL, Robakis NK. Identification and neuron specific expression of the S182/presenilin I protein in human and rodent brains. J Neurosci Res. 1996;45:308–20. doi: 10.1002/(SICI)1097-4547(19960801)45:3<308::AID-JNR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Fakla I, Kovacs I, Yamaguchi H, Geula C, Kasa P. Expressions of amyloid precursor protein, synaptophysin and presenilin-1 in the different areas of the developing cerebellum of rat. Neurochem Int. 2000;36:143–151. doi: 10.1016/s0197-0186(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa DJ, Morris JA, Ma L, Kandpal G, Chen E, Li YM, Austin CP. Presenilin-dependent gamma-secretase activity modulates neurite outgrowth. Neurobiol Dis. 2002;9:49–60. doi: 10.1006/nbdi.2001.0447. [DOI] [PubMed] [Google Scholar]
- 15.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 16.Goutte C, Hepler W, Mickey KM, Priess JR. aph-2 encodes a novel extracellular protein required for GLP-1-mediated signaling. Development. 2000;127:2481–92. doi: 10.1242/dev.127.11.2481. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes C, Kar S. Insulin-like growth factor-II/mannose-6-phosphate receptor: widespread distribution in neurons of the central nervous system including those expressing cholinergic phenotype. J Comp Neurol. 2003;458:113–27. doi: 10.1002/cne.10578. [DOI] [PubMed] [Google Scholar]
- 18.Hawkes C, Kabogo D, Amritraj A, Kar S. Up-regulation of cation-independent mannose 6-phosphate receptor and endosomal-lysosomal markers in surviving neurons after 192-IgG-saporin administrations into the adult rat brain. Am J Pathol. 2006;169:1140–1154. doi: 10.2353/ajpath.2006.051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebert SS, Serneels L, Dejaegere T, Horre K, Dabrowski M, Baert V, Annaert W, Hartmann D, De Strooper B. Coordinated and widespread expression of γ-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol Dis. 2004;17:260–72. doi: 10.1016/j.nbd.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Ye Y, Fortini ME. Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2:69–78. doi: 10.1016/s1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 21.Ilaya NT, Evin G, Masters CL, Culvenor JG. Nicastrin expression in mouse peripheral tissues is not co-ordinated with presenilin and is high in muscle. J Neurochem. 2004;91:230–37. doi: 10.1111/j.1471-4159.2004.02718.x. [DOI] [PubMed] [Google Scholar]
- 22.Jafferali S, Dumont Y, Sotty F, Robitaille Y, Quirion R, Kar S. Insulin-like growth factor-I and its receptor in the frontal cortex, hippocampus and cerebellum of normal human and Alzheimer’s disease brains. Synapse. 2000;38:450–9. doi: 10.1002/1098-2396(20001215)38:4<450::AID-SYN10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Kaether C, Lammich S, Edbauer D, Ertl M, Rietdorf J, Capell A, Steiner H, Haass C. Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol. 2002;158:551–61. doi: 10.1083/jcb.200201123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J, Lemaire GH, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell surface receptor. Nature. 1987;325:733–6. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 25.Keino H, Kishikawa M, Satoh M, Shimada A. Expression of presenilin 1 and synapse-related proteins during postnatal development is not different between accelerated senescence-prone and -resistant mice. Neuropathology. 2003;23:16–24. doi: 10.1046/j.1440-1789.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim DY, Ingano LA, Kovacs DM. Nectin-1α, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem. 2002;277:49976–81. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 27.Kim KS, Wegiel J, Sapienza V, Chen J, Hong H, Wisniewski HM. Immunoreactivity of presenilin-1 in human, rat and mouse brain. Brain Res. 1997;757:159–63. doi: 10.1016/s0006-8993(97)00243-6. [DOI] [PubMed] [Google Scholar]
- 28.Lah JJ, Heilman CJ, Nash NR, Rees HD, Yi H, Counts SE, Levey AI. Light and electron microscopic localization of presenilin-1 in primate brain. J Neurosci. 1997;17:1971–80. doi: 10.1523/JNEUROSCI.17-06-01971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MK, Slunt HH, Martin LJ, Thinakaran G, Kim G, Gandy SE, Seeger M, Koo E, Price DL, Sisodia SS. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J Neurosci. 1996;16:7513–23. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leem JY, Vijayan S, Han P, Cai D, Machura M, Lopes KO, Veselits ML, Xu H, Thinakaran G. Presenilin 1 is required for maturaiuon and cell surface accumulation of nicastrin. J Biol Chem. 2002;277:19236–40. doi: 10.1074/jbc.C200148200. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J Neurosci. 2003;23:3272–7. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marambaud P, Shioi J, Serben G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–56. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marty S, Wehrle R, Alvarez-Leefmans FJ, Gasnier B, Sotelo C. Postnatal maturation of Na+, K+, 2Cl− cotransporter expression and inhibitory synaptogenesis in the rat hippocampus: an immunohistochemical analysis. Eur J Neurosci. 2002;15:233–245. doi: 10.1046/j.0953-816x.2001.01854.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Flores MT, Medina M, Wandosell F. Expression of presenilin 1 in nervous system during rat development. J Comp Neurol. 1999;410:556–570. [PubMed] [Google Scholar]
- 35.Ni CY, Murphy MP, Golde TE, Carpenter G. γ-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 36.Parent AT, Barnes NY, Taniguchi Y, Thinakaran G, Sisodia SS. Presenilin attenuates receptor-mediated signaling and synaptic function. J Neurosci. 2005;25:1540–9. doi: 10.1523/JNEUROSCI.3850-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasternak SH, Bagshaw RD, Guiral M, Zhang S, Ackerley CA, Pak BJ, Callahan JW, Mahuran DJ. Presenilin-1, nicastrin, amyloid precursor protein, and γ-secretase activity are co-localized in the lysosomal membranes. J Biol Chem. 2003;278:26687–94. doi: 10.1074/jbc.m304009200. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- 39.Price DL, Tanzi RE, Borchelt DR, Sisodia S. Alzheimer’s disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–93. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar SN, Das HK. (2003) Regulatory roles of presenilin-1 and nicastrin in neuronal differentiation during in vitro neurogenesis. J Neurochem. 2003;87:333–343. doi: 10.1046/j.1471-4159.2003.02006.x. [DOI] [PubMed] [Google Scholar]
- 41.Satoh J, Kuroda Y. Nicastrin, a key regulator of presenilin function, is expressed constitutively in human neural cell lines. Neuropathology. 2001;21:115–22. doi: 10.1046/j.1440-1789.2001.00378.x. [DOI] [PubMed] [Google Scholar]
- 42.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 43.Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, Sudof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–47. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Siman R, Velji J. Localization of presenilin-nicastrin complexes and γ-secretase activity to the trans-Golgi network. J Neurochem. 2003;84:1143–53. doi: 10.1046/j.1471-4159.2003.01616.x. [DOI] [PubMed] [Google Scholar]
- 45.Siman R, Salidas S. Gamma-secretase subunit composition and distribution in the presenilin wild-type and mutant mouse brain. Neuroscience. 2004;129:615–28. doi: 10.1016/j.neuroscience.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Sisodia SS, St George-Hyslop P. γ-secretase, Notch, Aβ and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–90. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- 47.Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–36. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 48.Thinakaran G, Regard JB, Bouton CM, Harris CL, Price DL, Borchelt DR, Sosodia SS. Stable association of presenilin derivatives and absence of presenilin interactions with APP. Neurobiol Dis. 1998;4:438–53. doi: 10.1006/nbdi.1998.0171. [DOI] [PubMed] [Google Scholar]
- 49.Uchihara T, Sanjo N, Nakamura A, Han K, Song SY, St George-Hyslop P, Fraser PE. Transient abundance of presenilin 1 fragments/nicastrin complex associated with synaptogenesis during development in rat cerebellum. Neurobiol Aging. 2006;27:88–97. doi: 10.1016/j.neurobiolaging.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Vetrivel KS, Thinakaran G. Amyloidogenic processing of beta-amyloid precursor protein in intracellular compartments. Neurology. 2006;66(2 Suppl 1):S69–73. doi: 10.1212/01.wnl.0000192107.17175.39. [DOI] [PubMed] [Google Scholar]
- 51.Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–54. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wines-Samuelson M, Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- 53.Yan XX, Li T, Rominger CM, Prakash SR, Wong PC, Olsen RE, Zaczek R, Li YW. Binding sites of γ-secretase inhibitors in rodent brain: distribution, postnatal development, and effect of deafferentiation. J Neurosci. 2004;24:2942–52. doi: 10.1523/JNEUROSCI.0092-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang DS, Tandon A, Chen F, Yu G, Yu H, Arawaka S, Hasegawa H, Duthie M, Schmidt SD, Ramabhadran TV, Nixon RA, Mathews PM, Gandy SE, Mount HT, St George-Hyslop P, Fraser PE. Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J Biol Chem. 2002;277:28135–42. doi: 10.1074/jbc.M110871200. [DOI] [PubMed] [Google Scholar]
- 55.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song Y, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang D, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith A, Janus C, Zhang Y, Aebersold R, Farrer L, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Luo W, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H. Nicastrin is critical for stability and trafficking but not association of other presenilin/γ-secretase components. J Biol Chem. 2005;280:17020–6. doi: 10.1074/jbc.M409467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer’s disease amyloid beta-peptide production. Proc Natl Acad Sci USA. 2005;102:7499–504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]


