Abstract
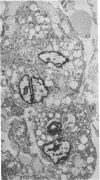
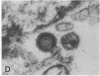
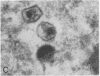
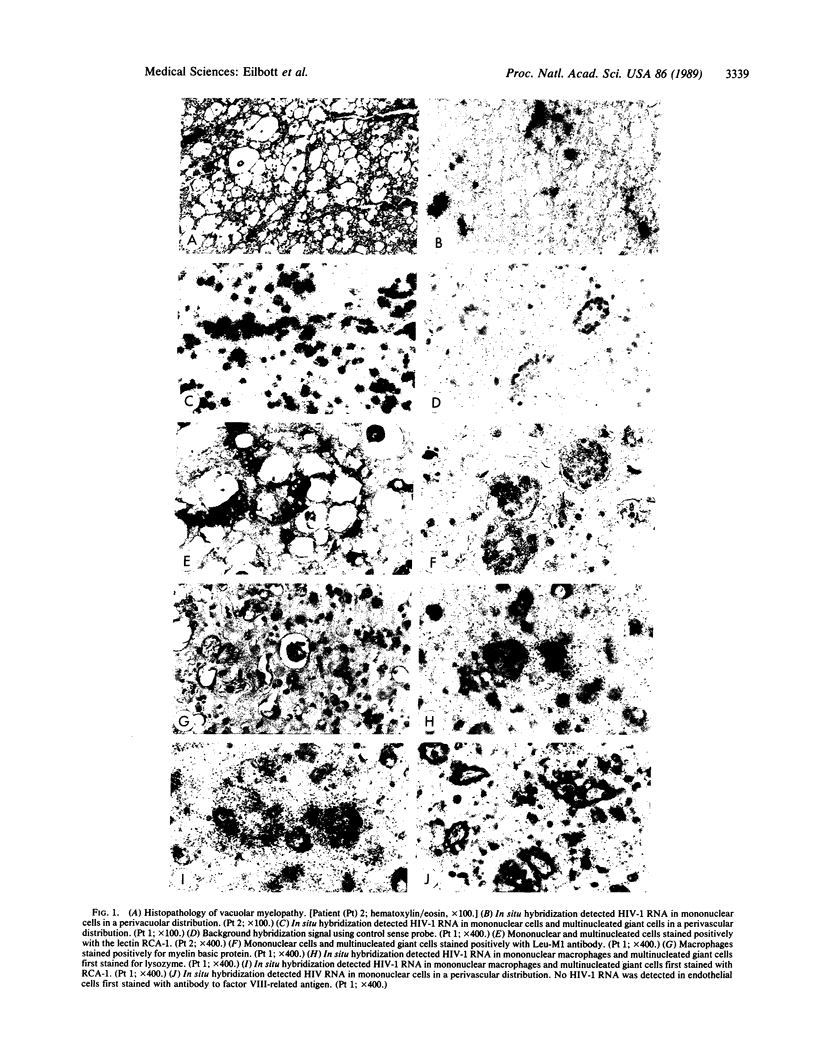
Spinal cord disease is common in patients infected with human immunodeficiency virus type 1 (HIV-1), and a characteristic vacuolar myelopathy is present at autopsy in approximately one-fourth of acquired immunodeficiency syndrome patients. Pathologic examination of the spinal cord shows vacuolation of white matter and infiltration by macrophages, a process distinct from HIV-1 encephalopathy. To determine the presence and localization of HIV-1 RNA in the spinal cords of acquired immunodeficiency syndrome patients with vacuolar myelopathy, we used the technique of combined in situ hybridization and immunohistochemical staining on the same slide. Spinal cord tissue sections were stained with markers for macrophages, endothelial cells, oligodendroglia, astrocytes, and myelin and then hybridized in situ with HIV-1-specific RNA probes. Combined in situ hybridization and immunohistochemical staining on three spinal cords showed HIV-1 expression in mononuclear and multinucleated macrophages localized mainly to areas of myelopathy in spinal cord white matter. Immunohistochemical staining and electron microscopy showed myelin within macrophages and electron microscopy revealed HIV-1 budding from macrophages. These data suggest a role for HIV-1-infected macrophages locally in the pathogenesis of vacuolar myelopathy and add to the body of evidence that these cells play a role systemically in the development of HIV-1-related disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Flavell D. J., Jones D. B., Wright D. H. Identification of tissue histiocytes on paraffin sections by a new monoclonal antibody. J Histochem Cytochem. 1987 Nov;35(11):1217–1226. doi: 10.1177/35.11.3309045. [DOI] [PubMed] [Google Scholar]
- Gabuzda D. H., Ho D. D., de la Monte S. M., Hirsch M. S., Rota T. R., Sobel R. A. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986 Sep;20(3):289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E., Clements J. E. A double labeling technique for performing immunocytochemistry and in situ hybridization in virus infected cell cultures and tissues. J Virol Methods. 1985 Jun;11(2):93–103. doi: 10.1016/0166-0934(85)90033-3. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Sinkovics J. G., Gyorkey P. Retrovirus resembling HTLV in macrophages of patients with AIDS. Lancet. 1985 Jan 12;1(8420):106–106. doi: 10.1016/s0140-6736(85)91995-6. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Schooley R. T., Kaplan J. C., Allan J. D., Groopman J. E., Resnick L., Felsenstein D., Andrews C. A., Hirsch M. S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- Itoyama Y., Sternberger N. H., Kies M. W., Cohen S. R., Richardson E. P., Jr, Webster H. Immunocytochemical method to identify myelin basic protein in oligodendroglia and myelin sheaths of the human nervous system. Ann Neurol. 1980 Feb;7(2):157–166. doi: 10.1002/ana.410070211. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Mannoji H., Yeger H., Becker L. E. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71(3-4):341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Maples J. A. A method for the covalent attachment of cells to glass slides for use in immunohistochemical assays. Am J Clin Pathol. 1985 Mar;83(3):356–363. doi: 10.1093/ajcp/83.3.356. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975 Feb;28(2):124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein J. M., Jannotta F. Human immunodeficiency virus and papovavirus infections in acquired immunodeficiency syndrome: an ultrastructural study of three cases. Hum Pathol. 1988 Mar;19(3):350–361. doi: 10.1016/s0046-8177(88)80531-8. [DOI] [PubMed] [Google Scholar]
- Peluso R., Haase A., Stowring L., Edwards M., Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985 Nov;147(1):231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Navia B. A., Cho E. S., Jordan B. D., George D. C., Price R. W. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985 Apr 4;312(14):874–879. doi: 10.1056/NEJM198504043121402. [DOI] [PubMed] [Google Scholar]
- Sehested M., Hou-Jensen K. Factor VII related antigen as an endothelial cell marker in benign and malignant diseases. Virchows Arch A Pathol Anat Histol. 1981;391(2):217–225. doi: 10.1007/BF00437598. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Cho E. S., Epstein L. G. Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol. 1985 Aug;16(8):760–760. doi: 10.1016/s0046-8177(85)80245-8. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Smith D. A., Lantos P. L. Immunocytochemistry of cerebellar astrocytomas: with a special note on Rosenthal fibres. Acta Neuropathol. 1985;66(2):155–159. doi: 10.1007/BF00688691. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Simpson D. M., Nielsen S., Gold J. W., Metroka C. E., Posner J. B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983 Oct;14(4):403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Eskin T. A., Benn S., Angerer R. C., Angerer L. M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986 Nov 7;256(17):2360–2364. [PubMed] [Google Scholar]
- Tschachler E., Groh V., Popovic M., Mann D. L., Konrad K., Safai B., Eron L., diMarzo Veronese F., Wolff K., Stingl G. Epidermal Langerhans cells--a target for HTLV-III/LAV infection. J Invest Dermatol. 1987 Feb;88(2):233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- Vazeux R., Brousse N., Jarry A., Henin D., Marche C., Vedrenne C., Mikol J., Wolff M., Michon C., Rozenbaum W. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987 Mar;126(3):403–410. [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]