Abstract
The three Akt isoforms differ in their ability to transduce oncogenic signals initiated by the Neu and PyMT oncogenes in mammary epithelia. As a result, ablation of Akt1 inhibits and ablation of Akt2 accelerates mammary tumor development by both oncogenes, while ablation of Akt3 is phenotypically almost neutral. Since the risk of breast cancer development in humans correlates with multiple late pregnancies, we embarked on a study to determine whether individual Akt isoforms also differ in their ability to transduce hormonal and growth factor signals during pregnancy, lactation and post-lactation involution. The results showed that the ablation of Akt1 delays the differentiation of the mammary epithelia during pregnancy and lactation, and that the ablation of Akt2 has the opposite effect. Finally, ablation of Akt3 results in minor defects, but its phenotype is closer to that of the wild type mice. Whereas the phenotype of the Akt1 ablation is cell autonomous, that of Akt2 is not. The ablation of Akt1 promotes apoptosis and accelerates involution, whereas the ablation of Akt2 inhibits apoptosis and delays involution. Mammary gland differentiation during pregnancy depends on the phosphorylation of Stat5a, which is induced by prolactin, a hormone that generates signals transduced via Akt. Here we show that the ablation of Akt1, but not the ablation of Akt2 or Akt3 interferes with the phosphorylation of Stat5a during late pregnancy and lactation. We conclude that the three Akt isoforms have different roles in mammary gland differentiation during pregnancy and this may reflect differences in hormonal signaling.
Keywords: Akt1, Akt2, Akt3, mammary gland, differentiation, pregnancy, lactation, involution
Introduction
The rudiment of the mammary gland in mice is laid down on embryonic day 10 (E10) and consists of an invagination of epithelial cell precursors. Following some limited growth and branching, these cells establish an elementary mammary gland structure, which grows slowly until puberty, at which point increasing levels of ovarian hormones accelerate ductal morphogenesis. During this process, estrogens induce proliferation of progenitor cells that are located in the terminal end buds (TEB), dynamic structures located in the ends of the growing mammary ducts, while progesterone promotes side branching and alveolar bud formation (Hennighausen and Robinson, 2001).
Hormonal changes during pregnancy induce alveolar morphogenesis, which depends on epithelial cell proliferation and differentiation, and is characterized by the establishment of the lobulo-alveolar architecture and lactation (Hennighausen and Robinson, 1998). The risk for breast cancer in humans is significantly higher in women who had an early menarche or multiple pregnancies later in life (Lambe et al., 1994; Newcomb et al., 1994). This suggests that the response to hormonal and growth factor signals during pregnancy primes the mammary gland to respond to oncogenic signals, perhaps because the two types of signals overlap. Understanding therefore the developmental processes operating on the mammary gland during pregnancy may provide insights into our understanding of mammary oncogenesis.
The inner surface of the ducts of the mammary gland is lined-up by luminal epithelial cells. These cells in turn are surrounded by myoepithelial cells. The luminal epithelium contains progenitor cells that proliferate and differentiate in response to physiological signals, giving rise to ductal and alveolar epithelial cell types (Hennighausen and Robinson, 1998; Hennighausen and Robinson, 2005). Estrogens, whose levels increase in the early stages of pregnancy, promote the proliferation of epithelial progenitor cells. Progesterone and prolactin, whose levels increase in the late states of pregnancy, promote side branching, the formation of lobuloalveolar structures, which coalesce and dilate by day 16.5 of pregnancy, and lactogenic differentiation. The latter proceeds in two stages. Stage 1 or alveolar differentiation, begins at mid-pregnancy and is characterized by the sustained induction of genes involved in milk protein synthesis, such as WAP and β-casein and the accumulation of intracellular lipid droplets in the alveolar cells. Stage 2 or secretory activation, occurs around parturition and is characterized by higher levels of milk protein expression, the closing of the alveolar cell tight junctions and the release of the intracellular lipid droplets into the lumen (Hennighausen and Robinson, 2005)
Mice heterozygous for the ablation of the prolactin receptor and mice homozygous for the ablation of Stat5a, a downstream target of the prolactin receptor, exhibit severe defects in mammary gland differentiation during pregnancy(Brisken et al., 1999; Cui et al., 2004; Ormandy et al., 1997). A number of secreted molecules and transcription factors regulated by them also contribute to alveolar morphogenesis and lactogenic differentiation during pregnancy (Brisken and Rajaram, 2006). Ablation of some of these molecules inhibits, while overexpression enhances differentiation. Prolactin and other secreted molecules initiate signals that are transduced via the Akt kinase, raising the question whether Akt is required for the transduction of differentiation signals induced by these molecules.
Akt is a serine-threonine protein kinase (Bellacosa et al., 1991) that is activated by a variety of signals via PI3K-dependent mechanisms (Chan et al., 1999; Franke et al., 1995; Manning and Cantley, 2007). Akt regulates a host of cellular functions including growth, proliferation, survival, cell migration and intermediary metabolism (Ahmed et al., 1997; Chan et al., 1999; Plas & Thompson, 2005; Rathmell et al., 2003; Chan et al., 1999; Franke et al., 1995; Manning & Cantley, 2007). Due to its central role in the regulation of cell function, Akt has been shown to play a critical role in a variety of human and animal cancers (Bellacosa et al., 2005; Vivanco and Sawyers, 2002). There are three Akt isoforms, Akt1, Akt2 and Akt3. The three isoforms are very similar in sequence. Despite their sequence similarity however, they are functionally distinct, as suggested by the different phenotypes of Akt1−/− (Chen et al., 2001; Cho et al., 2001b), Akt2−/− (Cho et al., 2001a; Garofalo et al., 2003), Akt3−/− (Tschopp et al., 2005), as well as Akt1−/−/Akt2−/− (Peng et al., 2003), Akt1−/−/Akt3−/− (Yang et al., 2005) and Akt2−/−/Akt3−/− mice (Dummler et al., 2006).
More relevant in the context of this report are recent studies showing that Akt1 promotes epithelial cell proliferation and inhibits epithelial cell migration and invasiveness in response to oncogenic signals, while Akt2 has the opposite effects (Irie et al., 2005; Liu et al., 2006; Maroulakou et al., 2007; Yoeli-Lerner et al., 2005). A more direct demonstration of the opposing roles of Akt1 and Akt2 in the mammary gland was presented in a recent paper by us, which showed that ablation of Akt1 inhibits, whereas ablation of Akt2 accelerates the induction of mammary tumors in MMTV LTR-PyMT and MMTV LTR-ErbB2/Neu transgenic mice. Interestingly, despite their slow growth, Neu-induced tumors arising in Akt1−/− mice were more invasive than Neu-induced tumors arising in Akt2−/− mice (Maroulakou et al., 2007). Another study addressing the role of Akt in mammary gland differentiation during pregnancy showed that the ablation of Akt1, but not Akt2 or Akt3, interferes with milk production during lactation without interfering with mammary cell proliferation and survival or differentiation (Boxer et al., 2006).
The present study was undertaken to determine the role of individual Akt isoforms in mammary gland differentiation during pregnancy and lactation, as well as in cell survival during post-lactation involution. Our data confirmed that Akt1 ablation indeed inhibits milk production. Perinatal lethality of Akt1−/− mice however, is due to a combination of a maternal milk production defect and an intrinsic defect in the newborn mice. Finally, they showed that the ablation of Akt1 dramatically delays, while the ablation of Akt2 accelerates mammary gland differentiation during pregnancy. Interestingly, the delay in differentiation correlates with defects in Stat5 phosphorylation in Akt1−/−, but not in Akt2−/− mice. It is possible that the ablation of Akt1 interferes with the differentiation of the mammary epithelia in response to not only hormonal but also oncogenic signals. If this is the case and if less differentiated cells are more invasive, these findings may explain the increased invasiveness of mammary tumors arising in MMTV LTR-ErbB2/Neu transgenic, Akt1−/− mice.
Materials and Methods
Mice
Akt1−/−, Akt2−/− and Akt3−/− mice have been described previously [36, 30, 32 and 40]. Each Akt isoform-specific knockout and the wild-type littermates were examined.
Mammary Transplantation
Mammary gland transplantation experiments were carried out with modifications of previous procedures (Kuperwasser et al., 2000). The inguinal mammary glands from 21-to 24-day-old NOD/SCID females were cleared of mammary epithelium. Single ducts from mature 8 to 10-week-old C57Bl/6/129 Akt1−/−, Akt2−/− and wild type C57Bl/6/129 donors were dissected and transplanted into the cleared fat pads. 5 weeks following mammary transplantation and upon sexual maturation, mice were mated. Mammary glands were collected on day 17.5 of pregnancy.
Western analysis
Mammary gland protein lysates were prepared by grinding the tissue in liquid nitrogen and by solubilizing it in lysis buffer, according to a protocol recommended by Santa Cruz Biotechnology. Protein (50 µg) was resolved by SDS- polyacrylamide gel electrophoresis (SDS-PAGE), and was transferred to nitrocellulose membranes (Invitrogen). Membranes were probed with the following primary antibodies: Akt1 (cell Signaling and UBI), Akt2 (Cell Signaling), Akt3 (UBI) WAP, β-casein, β-actin STAT5a, and p-STAT5a (UBI). Following probing with secondary antibodies, western blots were developed using standard procedures.
Whole mounts of the mammary gland and histology
The left Inguinal and thoracic mammary glands were dissected and whole mounts were prepared as described (Bowe et al., 2002). Tissues were dissected, fixed in formalin, embedded in paraffin, sectioned, and five micrometer serial sections were stained with hematoxylin and eosin (H&E) or used for immunohistochemical detection, or for terminal deoxynucleotidyl-transferase-mediated dUTP end labeling (TUNEL) assays, as described below.
Immunohistochemistry and TUNEL assays
To identify cells expressing the proliferation marker Ki-67, formalin-fixed, paraffin-embedded mammary gland sections were immunostained with the anti-Ki-67 rabbit monoclonal antibody SP1 (NeoMarkers, Freemont, CA). TUNEL staining for the detection of apoptotic cells was carried out also on formalin-fixed paraffin-embedded sections, using the ApopTag kit (Intergen, NY).
Quantification of epithelial cells
Epithelial nuclei were counted in mammary gland sections harvested on day 17.5 of pregnancy. Formalin-fixed and paraffin-embedded sections were stained with Hoechst 33258. Four images per mouse and three mice per genotype were analyzed.
Statistical analysis
Data were analyzed by t test, x2 or by ANOVA factorial analysis using the StatView statistical software program (SAS). Results are expressed as mean ± standard error of the mean (SEM).
Results
Differential regulation of the three Akt isoforms during pregnancy lactation and involution
To determine whether the three Akt isoforms have similar or distinct functions during pregnancy, lactation and involution we first examined their expression in the course of these processes. To this end, western blots of mammary gland cell lysates were probed with antibodies specific for Akt1, Akt2 or Akt3. Loading was measured by probing the same blots with an antibody to β-actin. The results in Figure 1 showed that Akt1 is up-regulated during the growth phase of pregnancy, whereas Akt2 is down-regulated and Akt3 remains largely unchanged. A similar trend was observed during lactation. During involution, Akt1 was initially down-regulated, but its expression increased gradually, and by day 7 it was expressed at levels similar to its levels prior to pregnancy. Akt2 expression remained initially in the same low levels as during pregnancy, but it was gradually increased, and by day 7, it had also reached approximately the same levels as prior to pregnancy. Finally, Akt3 expression remained largely unchanged, with the exception of the first day of involution, where it was slightly increased. The differential regulation of the three Akt isoforms suggests that they may indeed have distinct functions in the course of these processes.
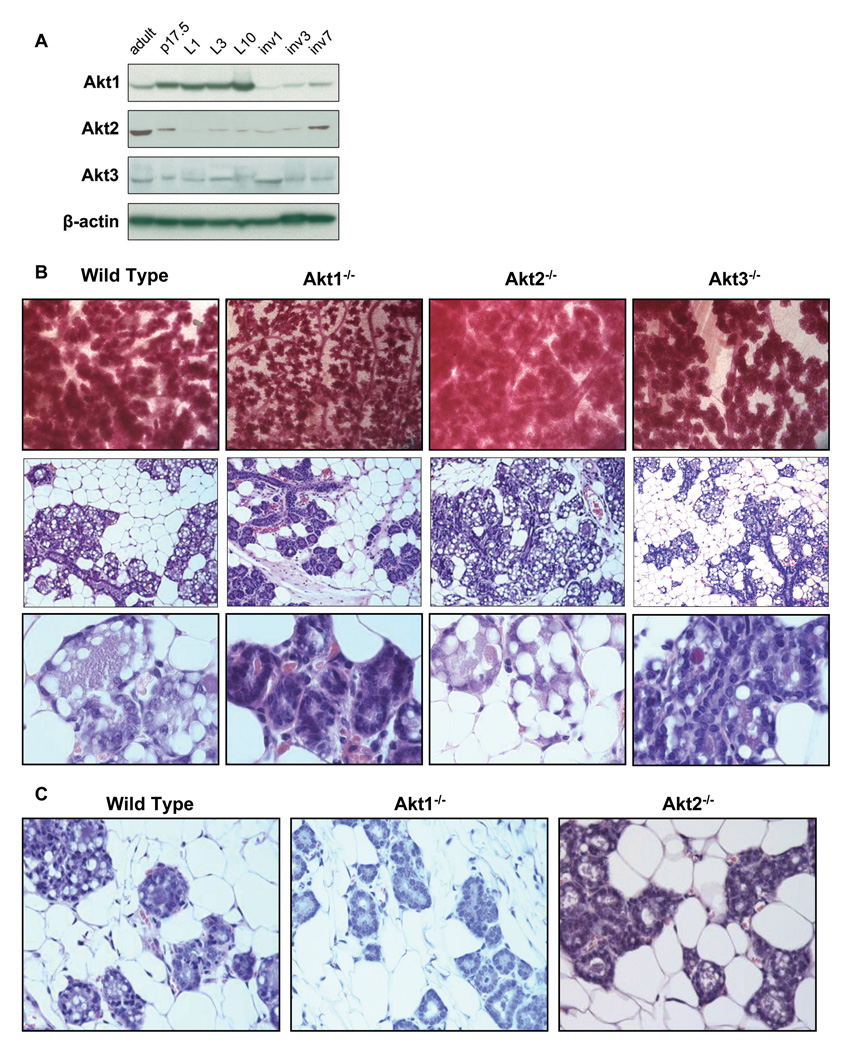
Figure 1. Akt1 ablation inhibits whereas Akt2 ablation enhances the formation of lobuloalveolar structures in the mammary gland during pregnancy. Whereas the Akt1 ablation phenotype is cell autonomous, the Akt2 ablation phenotype is not.
A. Akt isoforms are differentially expressed during pregnancy lactation and involution. Western blot analyses of mammary gland lysates harvested from wild type mice at the indicated stages of mammary gland development: p17.5 is pregnancy day 17.5; L1, L3 and L10 are postnatal (lactation) days 1,3 and 10; Inv1, Inv3 and Inv7 are involution days 1,3 and 7. Immunoblots were probed with the indicated Akt isoform-specific antibodies. β-actin was used as a loading control.
B. Upper Panel: Whole mounts of mammary glands of wild type, Akt1−/−, Akt2−/− and Akt3−/− mice harvested on day 17.5 of pregnancy. Mammary epithelia were stained with carmine red. Middle Panel: Mammary gland histology of wild type, Akt1−/−, Akt2−/−, and Akt3−/− mice at day 17.5 of pregnancy. Lower Panel: Higher magnification of mammary gland histology on day P17.5.
C. Transplantation of wild type, Akt1−/− and Akt2−/− mammary epithelia into wild type mammary fat pads shows that the phenotype of Akt1 ablation is cell autonomous, while the phenotype of Akt2 ablation is not.
Akt1 ablation inhibits, whereas Akt2 ablation enhances the formation of lobuloalveolar structures in the mammary gland during pregnancy
Whole mounts of inguinal mammary glands were prepared from wild type, Akt1−/−, Akt2−/− and Akt3−/− mice at day 17.5 of pregnancy (P17.5) (5 mice per group). A representative sample of the results (Fig. 1B, Upper panels) shows that the size of the mammary gland lobuloalveolar units were significantly smaller in Akt1−/− than in wild type mice, and that these units were smaller in both wild type and Akt1−/− than in Akt2−/− mice. Finally, their size in the mammary glands of the wild type and Akt3−/− mice was similar. These findings suggest that ablation of Akt1 inhibits, whereas ablation of Akt2 enhances the maturation of the mammary gland lobuloalveolar structures and ablation of Akt3 is phenotypically almost neutral.
Mammary gland histology at day 17.5 of pregnancy was in agreement with the whole mount analysis. Specifically, histology revealed that the developing lobuloalveolar structures gradually replace the mammary gland fat pad in wild type mice and that, whereas the replacement is impaired in Akt1−/− mice, it is more pronounced in Akt2−/− mice (Fig. 1B, Middle and Lower panels). Histology also revealed that the lobuloalveolar structures were significantly smaller in Akt1−/− than in wild type and Akt2−/− mice. More important, the alveolar epithelia of the Akt1−/− mice were completely devoid of the characteristic lipid droplets which are very prominent in the advanced stages of pregnancy in wild type, Akt2−/− and Akt3−/− mice.
The size differences of the lobuloalveolar structures were also demonstrated by DAPI staining of histological sections of the mammary gland (Supplemental Fig. 1A). The differences between Akt1−/−, Akt2−/− and wild type mice could result from differences in the number and/or the size of the epithelial cells in these structures. To address this question we first counted the number of cells in individual alveoli using again the DAPI stained sections of the mammary gland (Supplementary Fig. 1B). To measure the cell size in the same samples, tissue sections were stained with antibodies against phospho-Ezrin, Radixin and Moesin (p-ERM), which stain the cell membrane. The results (Supplementary Fig. 1C) showed that the Akt1−/− cells were indeed smaller, while the Akt2−/− cells were larger than the wild type cells. Since these cells also differ with regard to their loading with lipid droplets, the observed differences could be the result of differences in lipid loading (see also Fig. 1B Middle and Lower panels).
Whereas the inhibition of mammary gland differentiation induced by ablation of Akt1 is cell autonomous, the acceleration of differentiation induced by ablation of Akt2 is not
Akt2 is expressed primarily in the stroma (Maroulakou et al., 2007). We therefore hypothesized that the Akt2-mediated inhibition of mammary gland differentiation may be due to the effects of Akt2 on the interaction between the mammary epithelia and the stroma. To address this question, we transplanted wild type, Akt1−/− and Akt2−/− mammary epithelia into the mammary fat pads of three-week old wild type NOD/SCID mice (2 mice per genotype, 2 mammary glands per mouse). These mice became pregnant five weeks later and their mammary glands were examined histologically on day P17.5. The results (Fig. 1C) showed that the Akt1−/− epithelia retained the Akt1−/− phenotype when transplanted into the wild type fat pad suggesting that the Akt1−/− phenotype is cell-autonomous. However, the Akt2−/− epithelia did not exhibit the Akt2−/− phenotype in the same experiment, suggesting that the Akt2−/− phenotype is stroma dependent.
Ablation of Akt1 enhances the proliferation and inhibits the survival of mammary epithelia during pregnancy
The results of the preceding experiments suggested that ablation of Akt1 may inhibit, whereas ablation of Akt2 may enhance the growth, proliferation and survival of mammary epithelia in response to hormonal and growth factor signals during pregnancy. Alternatively, they suggested that the luminal epithelia of the Akt1−/− mice may fail to differentiate in response to such signals. The latter hypothesis was further supported by the finding that Akt1−/− mammary glands fail to develop the prominent lipid droplets that develop in normal mammary epithelia, as they differentiate to assume a lactogenic phenotype.
To distinguish between these alternatives, histologic sections of the mammary gland of wild type, Akt1−/−, Akt2−/− and Akt3−/− mice were analyzed at day 17.5 of pregnancy for the rate of cell proliferation, by staining for the proliferation markers Ki-67 and Cyclin D1, and for the rate of apoptosis by the TUNEL assay. These assays were carried out on four mice per group. Representative samples of the results are shown in figures 2A, 2C and supplementary figure 2.
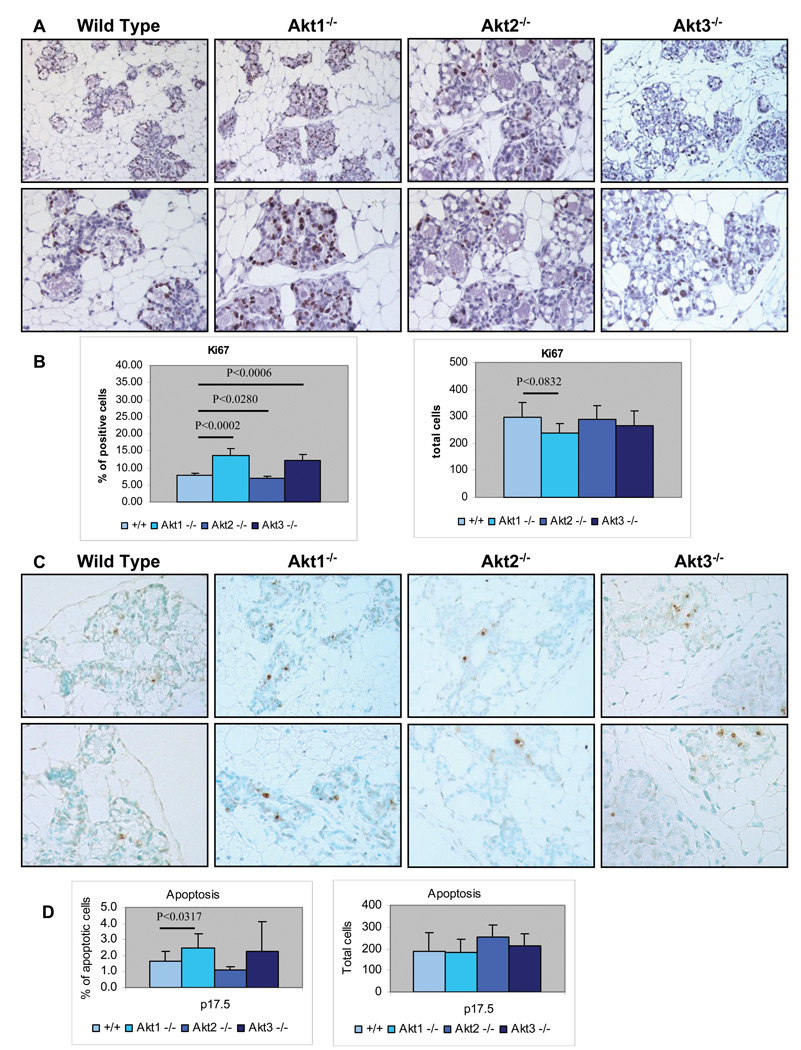
Figure 2. Ablation of Akt1 enhances the proliferation and inhibits the survival of mammary epithelia during pregnancy.
A. Tissue sections of mammary glands from wild type, Akt1−/−, Akt2−/− and Akt3−/− mice, harvested at pregnancy day 17.5 (p17.5), were stained with an antibody against the proliferation marker, Ki-67. B. Graphical representation of the data shown in figure 2A. Left Panel The bars show the mean percentage of Ki-67-positive cells +/− the SE of the mean for each genotype. The number of Ki-67-positive cells was measured in sections of mammary glands derived from four mice of each genotype. Four randomly picked fields were counted in each mouse specimen. Right Panel. The bars show the total number of cells counted for the data analysis shown in the left panel. C. Apoptotic cells in mammary gland tissue sections of wild type, Akt1−/−, Akt2−/− and Akt3−/− mice at pregnancy day 17.5 (p17.5) were detected with the TUNEL assay in a panel of slide-mounted methyl green-stained mammary tissue sections. The TUNEL assay utilizes horseradish peroxidase-conjugated anti-digoxigenin antibodies to detect digoxigenin-labeled DNA ends. D. Cumulative data of the experiment shown in figure 2C. Left Panel. The bars show the mean percentage of apoptotic cells +/− the SE of the mean for each genotype. The number of TUNEL-positive apoptotic cells was measured in sections of mammary glands derived from four mice of each genotype. Four randomly picked fields were counted in each mouse specimen. Right Panel. The bars show the total number of cells counted for the data analysis shown in the left panel.
To determine the percentage of proliferating and apoptotic cells in the mammary glands of these mice, Ki-67 and TUNEL-positive cells and the total number of epithelial cells were counted in four random fields in each specimen. Graphical presentation of the data is shown in figures 2B and 2D. Overall, these data were surprising in that they showed that ablation of Akt1 enhances the proliferation of the mammary epithelia during the growth phase of pregnancy. Since the overall number of cells did not change significantly, we hypothesized that ablation of Akt1 may raise the rate of apoptosis. The data in figure 2D confirmed the hypothesis.
The peak proliferation of the mammary epithelia occurs between days P10.5 and P14.5. The enhanced proliferation in the Akt1−/− mice at day P17.5 therefore could mean that either the proliferation was enhanced throughout pregnancy, or that the decline in proliferation which normally occurs around day P14.5 was delayed in these mice. To address this question, we stained mammary gland tissue sections harvested from wild type, Akt1−/− and Akt2−/− mice on days P10.5 and P14.5 for Ki-67. The results (Supplementary Fig. 3) showed that enhanced proliferation of the mammary epithelia in Akt1−/− mice persists throughout the proliferative stage.
The data in figure 2, combined with the data on the proliferation and survival of luminal ductal and alveolar cells during pregnancy suggest that the loss of Akt1 function may inhibit luminal cell differentiation.
Ablation of Akt1 delays luminal cell differentiation during pregnancy and lactation and impairs milk production
The preceding data suggested that Akt1 ablation might cause a developmental defect in the mammary gland during pregnancy. Such a defect may impair milk production, which in turn may be responsible for the perinatal lethality characteristic of Akt1−/− mice. To address this question, we examined the histology of the mammary gland in wild type, Akt1−/−, Akt2−/− and Akt3−/− mice in the first day of lactation (5 mice per group). A representative sample of the results (Fig. 3A) shows that the epithelial component of the mammary gland is underdeveloped in Akt1−/− overdeveloped in Akt2−/− mice relative to the wild type mice. The mammary gland of Akt3−/− mice exhibited near normal development. To determine whether the developmental defect in the mammary gland of the Akt1−/− mice represents a delay in maturation or a permanent defect that continues throughout lactation, we compared the histology of the mammary gland of wild type and Akt1−/− mice at day 17.5 of pregnancy and at lactation days 1 and 10. The results (Fig. 3B) showed that whereas at lactation day 1, the Akt1−/− mammary gland is severely underdeveloped relative to the wild type mammary gland, at lactation day 10, the two are very similar. The difference between the two at this stage is the apparent reduction of milk secretion and the higher number of adipocytes in the stroma of the mammary gland of the Akt1−/− mice. Since the lipid reserves in stromal adipocytes are mobilized within days following parturition, the higher number of adipocytes suggests altered lipid metabolism in these mice.
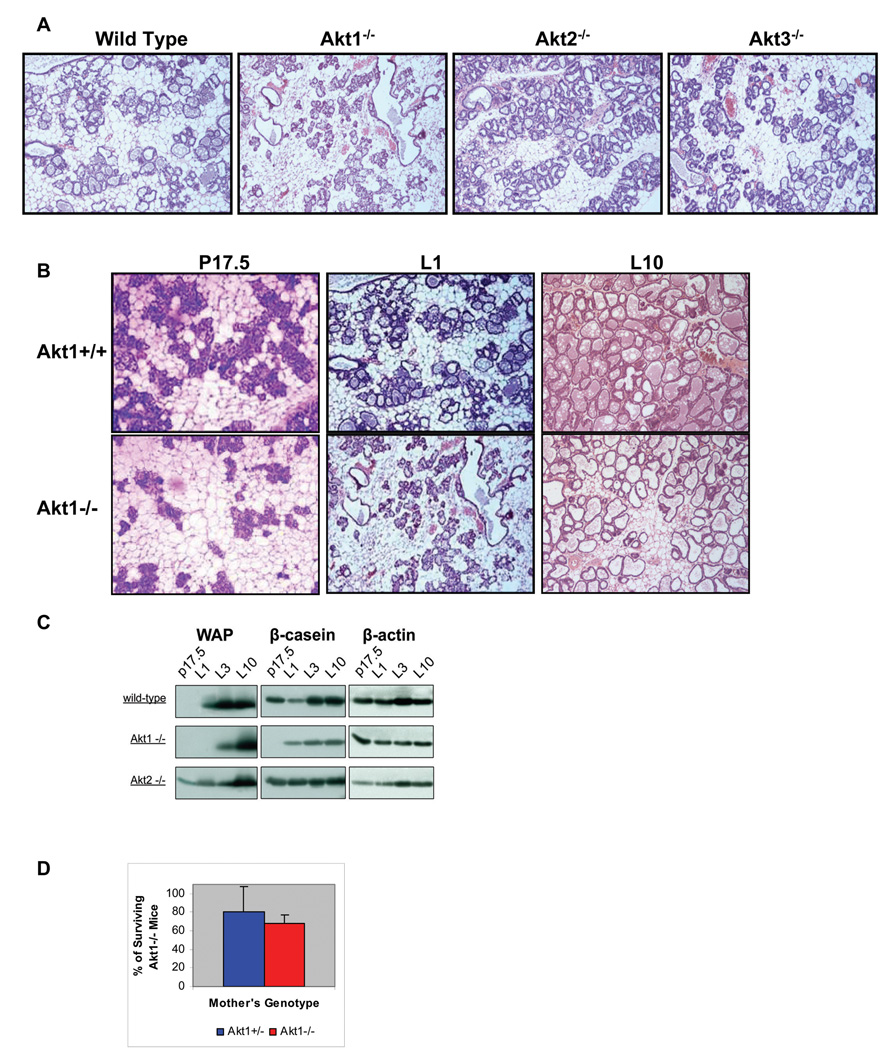
Figure 3. Ablation of Akt1 delays luminal cell differentiation during pregnancy and lactation and impairs milk production.
A. Histology of the mammary gland of wild type, Akt1−/−, Akt2−/− and Akt3−/− mice on the first day of lactation. B. Histology of the mammary gland of wild type mice (upper panel) and Akt1−/− mice (lower panel) on pregnancy day 17.5 (p17.5) and on lactation days 1 (L1) and 10 (L10). C. The expression of the milk protein WAP is delayed in the mammary gland of Akt1−/− mice and it is accelerated in the mammary gland of Akt2−/− mice. The expression of β-casein is also delayed in Akt1−/− but not in Akt2−/− mice. Milk protein expression was measured by western blotting of mammary gland lysates harvested at the indicated time points. P17.5 is pregnancy day 17.5. L1, L3 and L10 are lactation days 1, 3 and 10. These results are reproducible and were similar in three different experiments. D. The perinatal lethality of Akt1−/− mice is due to a combination of maternal milk production defects and intrinsic embryonic defects. Percentage of surviving Akt1−/− mice born to Akt1+/− (n=24) and Akt1−/− (n=15) mothers. Neonatal lethality was defined as death within the first 9 days of life. The rate of survival of the Akt1−/− offspring of Akt1+/− mothers and the rate of survival of the Akt1−/− offspring of Akt1−/− mothers were calculated as described in the results section. The statistical significance of the difference in rate of survival between mice born to Akt1+/− and Akt1−/− mothers is p<0.0547.
To address the role of Akt in milk secretion, extracts of the mammary gland of wild type, Akt1−/−, Akt2−/− and Akt3−/− mice harvested at day 17.5 of pregnancy and at lactation days 1, 3 and 10 were probed for the expression of the milk components β-casein and WAP. All mothers used in these experiments were nursing between 5 and 6 pups. The results (Fig. 3C) showed a delay in the expression of β-casein and WAP in the mammary gland of Akt1−/− mice, as expected. In contrast, the induction of WAP was accelerated in the mammary gland of Akt2−/− mice. We conclude that Akt1 and Akt2 regulate the differentiation of the mammary gland and milk production, in opposite ways.
The preceding data suggested that the perinatal lethality of Akt1−/− mice might be due to inadequate milk production. To determine whether an intrinsic embryonic defect may also contribute to the lethality, we examined the rate of survival of Akt1−/− mice born to Akt1−/− mothers, which exhibit a defect in milk production, and to Akt1+/− mothers, which produce normal amounts of milk. The Akt1−/− mothers were crossed to Akt1−/− males and the Akt1+/− mothers were crossed to Akt1+/− males. The progeny of the first type of cross should therefore be 100% Akt1−/−, while the progeny of the second type of cross should be ~25% Akt1−/−. The number of mice born to each mother and the number of mice surviving on day 9 were recorded. The rate of survival of the Akt1−/− offspring of Akt1−/− mothers was calculated by the formula, (Number of surviving Akt1−/− mice/Number of mice born) × 100, and the rate of survival of the Akt1−/− offspring of Akt1+/− mothers was calculated by the formula, (Number of surviving Akt1−/− mice/Number of expected Akt1−/− mice) × 100. The results (Fig. 3D) showed that 68% of Akt1−/− mice born to Akt1−/− mothers, and 80% of Akt1−/− mice born to Akt1+/− mothers survived. The difference, although relatively small, was statistically significant (P<0.0547), suggesting that milk production is a significant factor in the perinatal lethality of Akt1−/− mice. However, the substantial perinatal lethality of Akt1−/− mice born to Akt1+/− mothers suggests that intrinsic embryonic defects may also contribute to the high death rate of the Akt1−/− newborn mice. Milk production is impaired early because of a delay in the maturation of the mammary gland, and late because of a defect in the production of milk components. Interestingly the majority (80%) of the Akt1−/− offspring died within the first three days of lactation when the maternal mammary gland is underdeveloped.
Ablation of Akt1 interferes with the differentiation of mammary epithelia during pregnancy by interfering with the phosphorylation of Stat5a
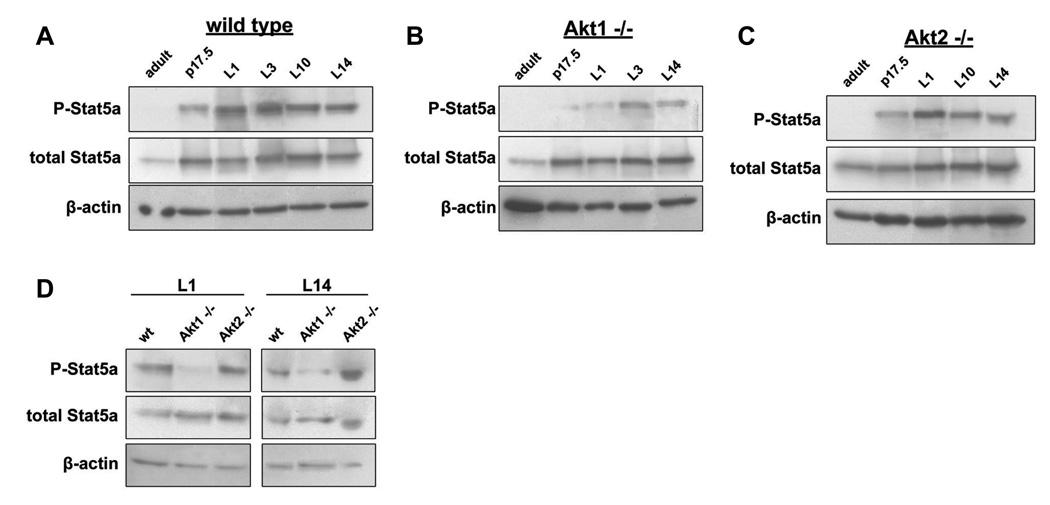
Data presented in the preceding paragraphs suggested that Akt isoforms may be differentially involved in the transduction of hormonal and growth factors signals during pregnancy. Akt isoforms may therefore differentially regulate the expression and posttranslational modification of signaling molecules involved in the regulation of lactogenic differentiation. To test this hypothesis, we examined the phosphorylation of Stat5a at tyrosine 694 on day 17.5 of pregnancy and on several lactation days, from L1 to L14. Five wild type, Akt1−/− and Akt2−/− mice were analyzed for each time point. A representative sample of the results shown in figure 4A, 4B and 4C suggests a delay in Stat5a phosphorylation in the mammary gland of Akt1−/− mice. Figure 4D directly compares the level of phosphorylation of Stat5a in wild type, Akt1 and Akt2 knockout mice on lactation days L1 and L14. The results show that the ablation of Akt1, but not Akt2 interferes with the phosphorylation of Stat5a. Since, the phosphorylation of Stat5a is downstream event of prolactin signaling, and since prolactin activates Akt, we conclude that the three Akt isoforms may function differentially in the transduction of prolactin signals in the mammary epithelia during pregnancy.
Figure 4. Ablation of Akt1 inhibits the differentiation of mammary epithelia during pregnancy by interfering with the phosphorylation of Stat5a.
A1, A2 and A3. Kinetics of Stat5a phosphorylation on tyrosine 694 in wild type, Akt1−/− and Akt2−/− mice during late pregnancy and lactation. Western blots of lysates from wild type, Akt1−/− and Akt2−/− mice mammary gland tissue, harvested at adult, pregnancy day 17.5 (p17.5), and lactation days 1, 3, 10 and 14 (L1, L3, L10 and L14) time points were probed with antibodies against total and tyrosine-phosphorylated Stat5a and β-actin (loading control). B. Stat5a phosphorylation in the mammary glands of wild type, Akt1−/− and Akt2−/− mice on lactation days 1(L1) and 14 (L14).
Ablation of Akt1 accelerates, while ablation of Akt2 delays post-lactation involution of the mammary gland
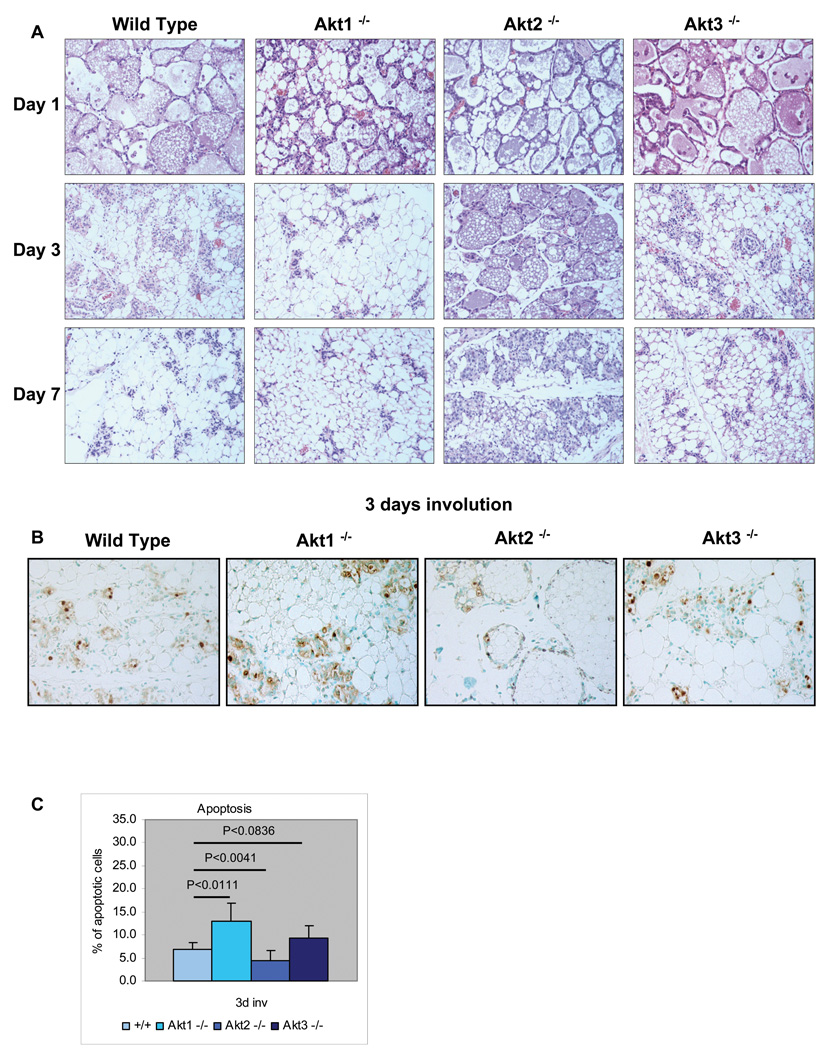
The mammary gland undergoes a process of death and remodeling after weaning. To determine whether Akt1, Akt2 and Akt3 regulate this process, we examined the histology of the mammary gland of wild type, Akt1−/−, Akt2−/− and Akt3−/− mice at days 1, 3 and 7, following weaning. The results (Fig. 5A) showed that the involution of the mammary gland in Akt1−/− mice was accelerated and in Akt2−/− mice is delayed relative to the wild type and Akt3−/− mice.
Figure 5. Ablation of Akt1 accelerates, while ablation of Akt2 delays post-lactation involution of the mammary gland.
A. Histology of the mammary gland of wild type, Akt1−/−Akt2−/−, and Akt3−/− mice on post-lactation involution days 1, day 3, and 7. B. Sections of the mammary gland of wild type, Akt1−/−, Akt2−/−, and Akt3−/− mice, harvested on the 3rd day of post-lactation involution, were stained for apoptotic cells, using the TUNEL assay. C. Cumulative data of the experiment shown in figure 5B. The bars show the mean percentage of apoptotic cells +/− the SE of the mean for each genotype. The number of apoptotic cells was measured in sections derived from four mice of each genotype. Four randomly picked fields were counted in each mouse specimen.
Involution is due primarily to apoptosis. To determine the effects of ablation of Akt1, Akt2 and Akt3 on apoptosis during involution, tissue sections of wild type, Akt1−/−, Akt2−/− and Akt3−/− mammary glands harvested on involution day 3 were analyzed for apoptosis using the TUNEL assay (4 mice per group). A representative sample of the results is shown in figure 5B. The ratio of the TUNEL-positive to the total number of epithelial cells, calculated by counting the cells in four fields in each specimen, is shown in figure 5C. The ratio shows that whereas Akt1 ablation accelerates (P<0.0111), Akt2 ablation inhibits apoptosis (P<0.0041). Akt3 ablation slightly increased apoptosis, although the difference in apoptosis between Akt3−/− and wild type mice was not statistically significant (P<0.0836). We conclude that Akt1 inhibits, while Akt2 accelerates apoptosis of the mammary epithelia during involution.
Discussion
The studies reported here addressed the role of the three Akt isoforms in the response of the mammary gland to hormonal signals during pregnancy, lactation and involution. The results revealed that the roles of the three isoforms in these physiological processes are also distinct. Of the three isoforms, Akt1 promotes the mammary gland differentiation of the mammary epithelia during pregnancy, Akt2 inhibits it and Akt3 has minimal effects. As a result, whereas ablation of Akt1 delays and ablation of Akt2 accelerates differentiation, ablation of Akt3 has no obvious effects. In addition to differentiation and lactation, mammary gland involution is also differentially regulated by the three Akt isoforms. Specifically, ablation of Akt1 accelerates apoptosis at days 1 and 3 of involution, while ablation of Akt2 delays apoptosis and Akt3 has only minor effects. Interestingly, the regeneration and remodeling of the mammary gland, which takes place at later time points (see day 7) is also delayed in the Akt2−/− mice. However, about one month from the start of the involution, the mammary glands of all mice, including the Akt1−/−, the Ak2−/− and the Akt3−/− mice, exhibit a normal architecture. Interestingly, whereas Akt1 and Akt2 play important roles in the response to hormonal signals during pregnancy lactation and involution, they are not required for mammary gland development during embryogenesis and for ductal morphogenesis during puberty. As a result, ablation of any of the three Akt isoforms has no effect on mammary gland development from embryogenesis to adulthood in virgin mice. The mice used in these experiments were of a mixed genetic background. However, we are confident that the differences we observed between Akt1, Akt2 and Akt3 knockout mice are not the result of background differences between strains. Thus, the genetic backgrounds of all knockout mice represent combinations of the same inbred strains, and the results obtained with Akt1 and Akt2 knockout mice derived from different crosses were consistent and reproducible. In addition, the whole mounts and the histologic sections of the differentiating mammary glands of our Akt1 and Akt2 knockout mice appear very similar to those reported by Boxer et al. who used knockout mice in different genetic backgrounds(Boxer et al., 2006).
Epithelial cell differentiation in the mammary gland is defined by a cascade of interdependent events that are initiated by reproductive hormones and other factors involved in cellular communication. Earlier studies discussed in the introduction have identified a host of signaling molecules that are regulated by these factors and play obligatory roles in lactogenic differentiation. Genetic disruption of any of the events induced by these hormonally regulated molecules may block the entire differentiation process. The data presented in this report place the three Akt isoforms into the network of molecules that regulate differentiation. Our findings show that the delay of differentiation observed during pregnancy and lactation in Akt1−/− mice, is phenotypically similar to the block of differentiation observed in Prlr+/−, STAT5a−/−, OPGL−/−, Elf5−/− and GATA3−/− mice. Given that the pathways regulated by some of these molecules, such as Prlr and OPGL transduce signals via Akt (Fata et al., 2000), these findings suggest that Akt is an integral part of the differentiation pathways activated by these molecules. Our findings showed that the ablation of Akt1 but not the ablation of Akt2 or Akt3 interferes with the phosphorylation of Stat5a, a mediator of prolactin signaling (Brisken and Rajaram, 2006). These findings may indicate that Akt1 and Akt2 differ in their ability to transduce hormonal signals that promote the phosphorylation of Stat5a. Given that Akt2 is expressed primarily in the stroma of the mammary gland however (Maroulakou et al., 2007), it is possible that this difference between Akt1 and Akt2 may be due to differences in the ability of hormonal signals to target Akt and Stat5a in epithelial and stromal .cells. Alternatively, Akt2 expressed in the stroma may regulate the expression of molecules that promote Stat5a phosphorylation in the epithelial cells. These are issues that will be addressed in future studies. However, we would like to point out that our studies to-date have provided strong evidence that the stroma plays an important role in mammary gland development during pregnancy. Our transplantation experiments indeed suggest that whereas the phenotype of Akt1 ablation is cell autonomous, the phenotype of the ablation of Akt2 is not.
The expression of Akt1, Akt2 and Akt3 during pregnancy, lactation and involution correlates with their physiological role in these processes. Thus, during pregnancy the expression of Akt1, which promotes cell survival, increases gradually, while the expression of Akt2, which inhibits cell proliferation and survival, decreases and the expression of Akt3 remains stable. During lactation Akt1 is the predominantly expressed form. In the first day of involution, Akt1 reaches its lowest levels of expression, while Akt2 expression remains very low, as during lactation. The expression of both isoforms increases gradually during involution and reaches near pre-pregnancy levels by day 7. These findings suggest that the three Akt isoforms are functionally distinct and that their expression changes in the course of physiological processes such as pregnancy lactation and involution according to patterns that allow the proper isoforms to be expressed when needed. Alternatively, the functional differences between isoforms may be the result of differences in their pattern of expression.
The findings presented in this report are consistent with the results of earlier studies addressing the effects of constitutively active MMTV LTR-MyrAkt transgenes, as well as the effects of ablation or mammary gland overexpression of the PIP3 phosphatase PTEN. These earlier studies had indeed shown that expression of MyrAkt does not disturb mammary gland development during puberty and does not induce mammary adenocarcinomas. However lipid droplets appeared precociously in the mammary epithelia of these mice during pregnancy and they became significantly larger and persistent throughout lactation. Consistent with our data and with the well established anti-apoptotic activity of Akt1, mammary gland involution was also delayed in these mice (Schwertfeger et al., 2003; Schwertfeger et al., 2001) Mammary-specific loss of PTEN function, which is associated with high Akt activity, leads to precocious lobuloalveolar development and delayed involution with severely reduced apoptosis (Li et al., 2002). Additionally, overexpression of PTEN in mammary epithelia decreases cell proliferation, increases apoptosis, and impairs lactogenic differentiation of the mammary epithelia (Dupont et al., 2002).
While this work was in progress, a paper was published showing that Akt1 has a distinct role in the functional maturation of the mammary gland during lactation(Boxer et al., 2006). This paper suggested that the perinatal death in Akt1 knockout mice is due to a defect in milk production. Moreover, it suggested that the defect in milk production results from defects in the metabolism of the mammary epithelia and not from defects in mammary cell proliferation, apoptosis and differentiation. Our work, presented here, confirmed that the mammary gland of Akt1−/− mice exhibits a defect in milk production, even at late points in lactation, when the development of the Akt1−/− mammary gland is comparable to that of wild type mice. However our data also showed that Akt1 ablation gives rise to a significant delay in lactogenic differentiation, during late pregnancy and lactation. The characteristic defect in milk production in these mice is due in the underdevelopment of the mammary gland in the early stages of lactation (this report) and to metabolic defects that interfere with the synthesis of milk components by the fully developed mammary gland at the late stages of lactation(Boxer et al., 2006). Since 80% of the Akt1−/− mice that die perinatally, die within the first three days of life, our data also suggest that the early defect may be responsible for the majority of perinatal deaths.
Although a maternal defect in milk production appears to play a major role in the perinatal lethality of Akt1−/− mice, intrinsic defects of the newborn Akt1−/− mice contribute to the lethality. This was suggested by the data in figure 3D, which showed that Akt1−/− mice born to Akt1−/+ mothers, who do not exhibit defects in lactogenesis, survive better than Akt1−/− mice born to Akt1−/− mothers. However, these mice also exhibit high perinatal lethality. We have not investigated the direct cause of this lethality. However, the fact that the Akt1−/−/Akt2−/− mice die perinatally with severe musculoskeletal and adipose tissue defects (Peng et al., 2003) suggests that similar defects may also occur in the Akt1−/− mice. The incomplete penetrance of these defects may allow the majority of the mice to survive.
Earlier studies on the role of the three Akt isoforms in oncogenesis in both mice and humans had shown that ablation of Akt1, but not Akt2 or Akt3 may specifically increase tumor cell migration and invasiveness(Irie et al., 2005; Liu et al., 2006; Maroulakou et al., 2007; Yoeli-Lerner et al., 2005). The increased invasiveness could potentially be explained by the signaling defects associated with the ablation or knockdown of Akt1, which may be causally responsible for the phenotype. Alternatively, the ablation or knockdown of Akt1 may promote EMT and the change in differentiation status of the tumor cells may be associated with increased invasiveness. The data in this report show that Akt1, but not Akt2 or Akt3, is required for the response of the mammary gland to physiological differentiation signals. If the differentiation of mammary epithelia in response to oncogenic signals also depends on Akt1, the ablation of the kinase may enhance invasiveness by promoting the selection of undifferentiated tumor cells.
In conclusion, the three different Akt isoforms expressed in the mammary gland respond differently to both the physiological signals associated with pregnancy, lactation and involution and to the oncogenic signals induced by PyMT and Neu. A likely point where the Akt1 and Akt2 roles in the response to physiological and oncogenic signals may overlap is the differentiation and the invasiveness of the mammary epithelia. The data in this report identify a physiological setting where the three Akt isoforms have functionally distinct roles. These data therefore define the path for future studies addressing the molecular mechanisms responsible for the functional differences between them.
Supplementary Material
Acknowledgments
We thank Kris Bolen for technical assistance. Support was provided by National Institutes of Health grant R01 CA57436 (PNT) and the Simeon J. Fortin Foundation at the Bank of America (IGM).
References
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254(5029):274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Bowe DB, Kenney NJ, Adereth Y, Maroulakou IG. Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene. 2002;21(2):291–298. doi: 10.1038/sj.onc.1205025. [DOI] [PubMed] [Google Scholar]
- Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, Belka GK, Cho H, Rathmell JC, Thompson CB, Birnbaum MJ, Chodosh LA. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 2006;4(6):475–490. doi: 10.1016/j.cmet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, Kelly PA, Ormandy CJ. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210(1):96–106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- Brisken C, Rajaram RD. Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia. 2006;11(3–4):239–248. doi: 10.1007/s10911-006-9026-0. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15(17):2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001b;276(42):38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24(18):8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26(21):8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110(6):815–825. doi: 10.1172/JCI13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103(1):41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81(5):727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112(2):197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12(4):449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171(6):1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperwasser C, Hurlbut GD, Kittrell FS, Dickinson ES, Laucirica R, Medina D, Naber SP, Jerry DJ. Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. A model for Li-Fraumeni syndrome. Am J Pathol. 2000;157(6):2151–2159. doi: 10.1016/S0002-9440(10)64853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129(17):4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, Bissell MJ. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci U S A. 2006;103(11):4134–4139. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, Horie K, Gaitanaris GA, Fidanza V, Ludwig T, Wiest DL, Gounari F, Tsichlis PN. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178(9):5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67(1):167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Newcomb PA, Storer BE, Longnecker MP, Mittendorf R, Greenberg ER, Clapp RW, Burke KP, Willett WC, MacMahon B. Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med. 1994;330(2):81–87. doi: 10.1056/NEJM199401133300201. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Binart N, Kelly PA. Mammary gland development in prolactin receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2(4):355–364. doi: 10.1023/a:1026395229025. [DOI] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res. 2003;44(6):1100–1112. doi: 10.1194/jlr.M300045-JLR200. [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol. 2001;15(6):867–881. doi: 10.1210/mend.15.6.0663. [DOI] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132(13):2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25(23):10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20(4):539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.