Abstract
Background
A large proportion of HIV-infected subjects on antiretroviral medication develop insulin resistance, especially in the context of fat redistribution. This study investigates the interrelationships among fat distribution, hepatic lipid content, and insulin resistance in HIV-infected men.
Design and methods
We performed a cross-sectional analysis of baseline data from twenty-three HIV-infected participants in 3 prospective clinical studies. Magnetic resonance spectroscopy was applied to quantify hepatic lipid concentrations. Magnetic resonance imaging was used to quantify whole body adipose tissue compartments, i.e., subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) volumes as well as inter-muscular adipose tissue (IMAT) subcompartment, and omental-mesenteric adipose tissue (OMAT) and retroperitoneal adipose tissue (RPAT) subcompartments of VAT. Homeostasis model for assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin concentrations.
Results
Hepatic lipid content correlated significantly with total VAT (r=0.62, p=0.0014) but not with SAT (r=0.053, p=0.81). In univariate analysis, hepatic lipid content was associated with the OMAT (r=0.67, p=0.0004) and RPAT (r=0.53, p=0.009) subcompartments; HOMA-IR correlated with both VAT and hepatic lipid contents (r=0.61, p=0.057 and 0.68, p=0.0012, respectively). In stepwise linear regression models, hepatic lipid had the strongest associations with OMAT and with HOMA-IR.
Conclusion
Hepatic lipid content is associated with VAT volume, especially the omental-mesenteric subcompartment, in HIV-infected men. Hepatic lipid content is associated with insulin resistance in HIV-infected men. Hepatic lipid content might mediate the relationship between VAT and insulin resistance among treated, HIV-infected men.
Keywords: liver fat, visceral adipose tissue, subcutaneous adipose tissue, inter-muscular adipose tissue, omental-mesenteric adipose tissue, retroperitoneal adipose tissue, HOMA, insulin resistance, HIV
Introduction
Highly active antiretroviral therapy (HAART) dramatically reduces overall and AIDS-specific mortality (1). Metabolic-related health risks are now emerging as health concerns in the long-term care of HIV-infected people (2), so that risk stratification has clinical significance. A substantial proportion of HAART-treated, HIV-infected patients develop fat redistribution, which includes subcutaneous lipoatrophy and/or visceral adipose tissue (VAT) accumulation or preservation (3, 4). Both lipoatrophy and central fat accumulation have been associated with insulin resistance in HIV-infected subjects (5). We previously showed that VAT is not homogenous regarding its relationship to whole body insulin sensitivity, with the omental-mesenteric adipose tissue (OMAT) sub-compartment having a stronger association with insulin resistance than does the anatomically-distinct retroperitoneal adipose tissue (RPAT) subcompartment (6).
Since insulin is multifunctional, insulin resistance may manifest in several ways. The specific nature of insulin resistance also is organ-specific. The liver plays a special role in insulin resistance as it is the organ most responsible for endogenous glucose production and release into the circulation. Insulin normally suppresses hepatic glucose production and promotes hepatic glycogen and lipid synthesis. Hepatic insulin resistance leads to hepatic glucose over-production, which is a feature of untreated diabetes mellitus. Insulin resistance precedes the development of overt diabetes mellitus in many human and animal models (7-9), and is associated with elevated hepatic lipid contents in multiple disease models in non-HIV infected (10) and HIV-infected patients (11-13). Given the associations between central fat deposition and insulin resistance, and between hepatic lipid content and insulin resistance, it is reasonable to study a potential relationship between VAT and hepatic lipid content. However, Sutinen et al were unable to demonstrate a significant relationship between hepatic lipid content and VAT (12) in HIV-infected men, though a study by Hadigan and colleagues did demonstrate such a relationship (13). The discordant results might be related to the sampling bias, related to a relatively narrow range of VAT in Sutinen's study of lipodystrophic patients. In this study, we investigate the hypothesis that there is a correlation between VAT and hepatic lipid by examining a sample covering a wide range of VAT contents.
Recently, Gallagher et al reported that inter-muscular adipose tissue (IMAT) also might influence cardiovascular risk (14, 15). We reported an association between IMAT and insulin resistance in HIV-infected women (16). The associations between IMAT and hepatic lipid, and between IMAT and insulin resistance have not previously been reported in HIV infected men.
Patients and methods
Design
This was a cross-sectional data analysis of baseline data from prospective studies performed in our General Clinical Research Centers.
Subjects
Data from twenty-three HIV-infected men enrolled in three clinical trials performed at St. Luke's-Roosevelt Hospital Center and New York Presbyterian Hospital were used. Thirteen subjects were enrolled in a clinical trial investigating the effect of growth hormone and/or rosiglitazone in subjects with both VAT accumulation and insulin resistance, who were stable on antiviral regimens (NCT00130286). Nine subjects were enrolled in a study examining the effect of switching from the nucleoside reverse transcriptase inhibitor zidovudine (Retrovir® – Glaxo Smith Kline) to the nucleotide reverse transcriptase inhibitor tenofovir (Viread®, Gilead Sciences) on mitochondrial function, in virologically-suppressed subjects on established antiretroviral treatment longer than one year. (NCT00389194). One subject was enrolled in a trial investigating the effect of diet and exercise and/or rosiglitazone on insulin sensitivity in HIV-infected subjects with hyperinsulinemia. All subjects signed informed consent forms for their measurements, which were approved by the Institutional Review Boards at each institution.
Measurements
Whole body Magnetic Resonance Imaging (MRI)
Scans were performed using a 1.5 T system (Signa LX version 10; GE Medical Systems, Milwaukee). A previously validated research protocol was applied to all subjects (3). Subjects lay supine with arms extended above their heads and were scanned in two segments with a common landmark at the L4-L5 inter-vertebral space. The protocol dictated a spin-echo sequence with a 200ms repetition time and a 14ms echo time and acquired about 40 axial T1-weighted images with 10-mm thickness with 40-mm intervals from fingers to toes. The MRI images were subsequently analyzed using research image analysis software (SliceOmatic, version 4.0; Tomovision Inc, Montreal) by a single analyst (QH). Whole body adipose tissue was first segmented into visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) compartments. The abdominal portion of the former compartment was further segmented into omental-mesenteric adipose tissue (OMAT) and retroperitoneal adipose tissue (RPAT) subcompartments following a previously published protocol (6). The SAT compartment was further segmented into inter-muscular adipose tissue (IMAT) and IMAT-free SAT following a previous protocol (14-16). Adipose tissue volumes were calculated according to the following formula: V=Σ(T+I) × Si, where T and I are slice thickness and inter-slice interval respectively, and Si is the area of the tissue of interest on an individual image slice.
Localized proton magnetic resonance spectroscopy (MRS)
Was applied on the same system as MRI to measure hepatic lipids in a single voxel (2 × 2 × 1.9 cm3) within the right lobe of the liver. This methodology has been extensively validated by others (17,18). Subjects lay prone on the MRI table and were requested to breathe shallowly. A whole body coil was used for radio frequency excitation and a 5-inch round general-purpose surface coil as receiver of the spectroscopic signal. T1-weighted MRI scouts were used for localization of the voxel of interest (VOI) to avoid major ductal and vascular structures, and subcutaneous fat tissue. A single-voxel point resolved spectroscopy (PRESS) technique (GE PROBE program) with 3 second repetition time (TR) = 3 seconds and 35 milli-second echo time (TE) was applied. One hundred twenty-eight acquisitions with 8 number of excitation (NEX) were recorded in order to obtain a sufficient signal-noise ratio over a measuring time of 6 minutes. Automatic shimming of the VOI was performed. The proton signals from water (H2O at 4.7 ppm) and the methylene group of lipids (-CH2- at 1.3ppm) were quantified using jMRUI analytic software (http://www.mrui.uab.es/mrui/). Hepatic lipid content was calculated by the ratio of Integration (methylene proton) over Integration (water proton), which represent a proton concentration ratio in two chemical group among the measurement sample.
Fasting glucose and insulin
Subjects fasted overnight before blood sampling. Insulin assays were performed via radioimmunassay (Human insulin RIA kit, Linco Research, Inc. St. Charles, MO) in the New York Obesity Research Center, St. Luke's-Roosevelt Hospital Center. To conform to standardization, two subjects, whose insulin level was measured outside the research center, was not included in the analysis for HOMA. However, including those two subjects in analysis, there was no apparent difference in the relationship we examined. Fasting glucose was measured either on a VITROS Fusion 5.1 IS system (Ortho-Clinical Diagnostics, Raritan, NJ) in the clinical lab of St. Luke's-Roosevelt Hospital Center or by a Synchron LX 20 Clinical Chemistry analyzer (Beckman Coulter Inc. Brea, CA) at the New York-Presbyterian Hospital (Cornell campus). HOMA-IR index was calculated as (glucose (mg/dl) × insulin (μIU/ml)/405).
Data analysis
Pearson's correlation analysis was performed to determine correlations between individual adipose tissue depots and hepatic lipid and between individual adipose tissue depots and HOMA-IR. A stepwise selection linear regression model was performed to analyze relationships among HOMA-IR, hepatic lipid and adipose tissue depots. Data were analyzed using SAS statistical package (Version 8, SAS Institute, Cary, NC). A p-value < 0.05 was considered to be statistically significant.
Results
All subjects studied were non-diabetic males, 40-58 years of age, and their characteristics are presented in Table 1. All subjects were taking HAART, defined as dual nucleosides plus an protease inhibitor (13 subjects) or a non-nucleoside reverse transcriptase inhibitor (8 subjects), or 3 NRTIs (2 subjects). The mean VAT in the study group was 5.6±2.4L (mean±SD, median 5.96L) and ranged from 1.5L to 10.6L. Subcompartments of VAT, i.e. OMAT and RPAT, were 3.4±1.6L (range 0.74-6.84L) and 1.8±0.7L (range 0.7-3.2L), respectively. There was a strong linear correlation between OMAT and RPAT compartments (r=0.85, p<0.0001).
Table 1.
Characteristics of the participants
| Age (years, mean±SD) | 49±6 |
| Body Mass index (kg/m2, mean±SD) | 28.1±2.9 |
| Race: % Caucascian: Latin/Hispanic: African American | 48:47:5 |
| CD4 count (cells/ml, median) | 510 |
| HIV viral load (% undetectable) | 74 |
| Current antiretroviral therapy (%) | |
| HAART | 100% |
| Protease inhibitor | 57 |
| Nucleoside reverse transcriptase inhibitor | 100 |
| Non-nucleoside reverse transcriptase inhibitor | 35 |
| Hepatitis C co-infection (%) | 22 |
Total body SAT was 20.8±10.6L. There was no linear association between SAT and VAT (r=0.32, p=0.13). The IMAT compartment was a small depot of 0.6±0.3L. There was a trend of association between total body adipose tissue (sum of SAT and VAT) and IMAT (r=0.40, p=0.08). There was no correlation between IMAT and the IMAT-free SAT (r=0.25, p=0.30) or between IMAT and VAT (r=0.28, p=0.30) in linear correlation models.
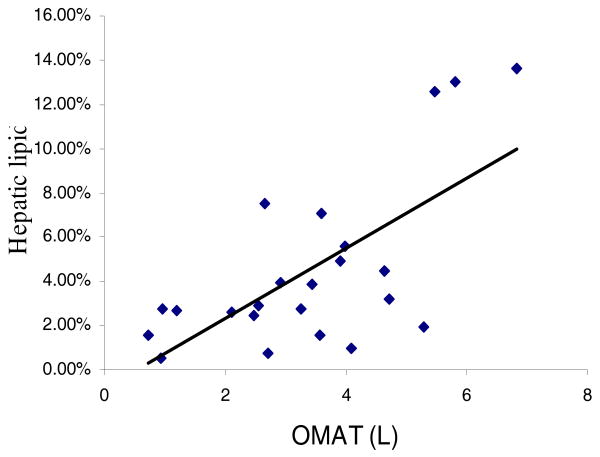
Mean hepatic lipid content was 4.5±3.9% with a range from 0.5 to 13.6%. Hepatic lipid content correlated with total VAT (r=0.62, p=0.0014). Further segmentation of VAT into OMAT and RPAT showed that hepatic lipid strongly related to both OMAT (Figure 1, r=0.67, p=0.0004) and RPAT (r=0.53, p=0.009). Hepatic lipid content was not associated with whole body SAT (r=0.05, p=0.81) or its IMAT subcompartment (r=0.01, p=0.95). In a stepwise multiple regression model with hepatic lipid as the dependent variable and subcompartments of VAT (OMAT and RPAT) and SAT (IMAT and IMAT-free SAT) as independent variables, only OMAT had a significant relationship with hepatic lipid. Inclusion of class of antiretroviral medication and hepatitis C status as variables in the model didn't alter the final output.
Figure 1.
A simple correlation between omental-mesenteric adipose tissue volume and hepatic lipid concentration
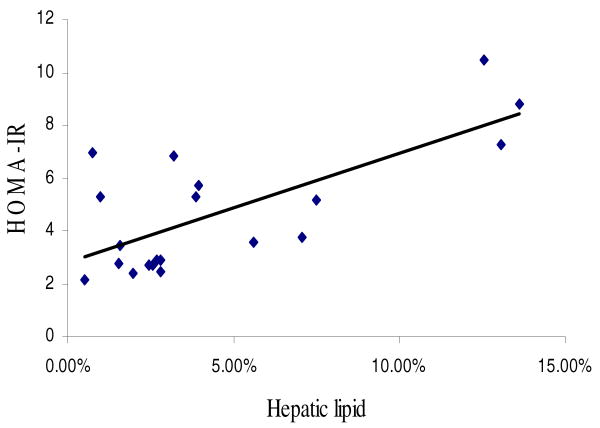
Mean fasting glucose concentration was 92±14 mg/dl (range 66-125 mg/dl, n=19) and fasting insulin was 22.3±10.6 μIU/ml (range 9.5-42 μIU/ml, n=20). The mean calculated HOMA-IR was 4.8±2.4 (range 2.2-10.4, n=19). HOMA-IR was related to total VAT (r=0.61, p=0.0057, n=19) and OMAT (r=0.66, p=0.0019), but only obtained a trend with RPAT (r=0.44, p=0.054). HOMA-IR was not related to whole body SAT (r=0.30, p=0.21) nor with IMAT (r=0.27, p=0.26). HOMA-IR also correlated strongly with hepatic lipid content (Figure 2, r=0.68, p=0.0012). In a multiple regression model with HOMA-IR as the dependent variable and hepatic lipid, OMAT and RPAT as independent variables, only hepatic lipid was a significant, independent predictor.
Figure 2.
A simple correlation between HOMA-IR index and hepatic lipid concentration
Discussion
This study included subjects with a wide range of VAT over a correspondingly wide range of adiposity. We report that both hepatic lipid content and HOMA-IR correlated with VAT in HIV-infected men. More specifically, the omental-mesenteric, rather than the retroperitoneal adipose tissue subcompartment of VAT, was the major contributor to these associations. Despite the strong association between VAT and HOMA-IR on univariate analysis, multivariate analysis showed that hepatic lipid content is a stronger independent predictor of insulin resistance.
Regarding visible adipose tissue depots on MRI, the lack of association between SAT and VAT in this sample corroborates the results of the FRAM study, which demonstrated dissociation, on cross sectional analysis, between limb lipoatrophy and visceral obesity within a large HIV cohort (4).
Regarding hepatic lipid content, our study corroborates the report of Hadigan and colleagues that there is a linear association between hepatic lipid and VAT contents (13). Our study advances their finding by further defining the subcompartment of VAT, i.e., OMAT, that contributes to such correlation. Our study also supports Sutinen and colleagues, who reported that HIV-infected subjects with excess VAT have higher hepatic lipid contents than those without excess VAT (12). However, that study failed to show a statistically significant correlation between VAT and hepatic lipid content in lipodystrophy patients. We believe that this resulted from sampling bias related to the relatively narrow range of VAT in their study group. An advantage of the current study is the wide range (1.5 to 10.6 L) of VAT sampled.
While the current study demonstrated a strong correlation between RPAT and hepatic lipid and strong co-linerarity between OMAT and RPAT in univariate analysis, the results of the multivariate analysis suggest that the strongest association is between OMAT and hepatic lipid content. This result echoes our previous study, which showed that OMAT, but not RPAT, was associated with whole body insulin resistance, measured as area under the curve of insulin concentrations during an oral glucose tolerance test (6). In this regard, it is important to remember that omental-mesenteric blood drains to the liver via the portal circulation, while retroperitoneal blood drains to the systemic circulation via the inferior vena cava. Thus, the release of mediators affecting insulin sensitivity from the RPAT compartment will exert systemic, rather than specifically hepatic effects. Increasing evidence indicates that free fatty acid might be the likely mediator connecting excessive adiposity to insulin resistance. Jensen et al reported that free fatty acid (FFA) delivery to the liver from splanchnic sources increased proportionally to abdominal visceral adipose tissue (19), while the study by Lam et al demonstrated that increased FFA supply induced hepatic insulin resistance (20). In addition, Tripathy et al showed the hepatic insulin resistance was a major determinant of HOMA-IR (21).
All of this evidence supports the notion that increased hepatic FFA flux in abdominal adiposity prmotes hepatic insulin resistance. The results of Szczepaniak's validation study suggested that increased FFA flux leads to liver lipid accumulation (17). The result of this study, showing that hepatic lipid content was only associated with OMAT in a multivariate analysis further supports the notion that VAT may exert its effect via increased portal flux of free fat acid flux to the liver.
While central obesity is regarded as a risk factor for cardiovascular diseases, the biological mechanism is not clear. Some researchers suggest that hepatic lipid directly affects hepatic insulin signaling to promote insulin resistance (22), and the current study adds support to this notion by showing that hepatic lipid predicted HOMA-IR better than did VAT.
IMAT is an adipose tissue depot located between muscle groups and between muscle bundles, associated with perimysium and epimysium of muscles. While there is no report yet of a relationship between IMAT and muscle insulin sensitivity, as has been demonstrated with intramyocellular lipid (23, 24), we previously reported an association between IMAT and insulin resistance in both HIV-infected and uninfected women (16). In contrast, the current study found IMAT was not a significant predictor of hepatic lipid level, and its association with insulin sensitivity index was not significant. Though no association was found in this study between hepatic lipid content and IMAT, the small volume of the latter depot is a possible reason (16). Therefore, study with wider ranges of SAT and IMAT are warranted. Further investigation is needed to look at a potential association between hepatic lipid and intramyocellular fat (25), a compartment that was not analyzed in this study.
Our demonstration that hepatic lipid is the only significant factor for insulin resistance on multivariate analysis may have clinical importance. While the gold-standard measure of insulin sensitivity is the euglycemic clamp technique, it is a cumbersome tool used only for research purposes. There is a close correlation between the HOMA-IR index and insulin sensitivity determined by the clamp technique (26). While the role of hepatic lipid in the pathogenesis of hepatic insulin resistance remains to be settled, it may serve as a surrogate for hepatic insulin sensitivity or even as a marker of cardiovascular risk. Several studies also have shown associations between increased hepatic insulin sensitivity by dietary, exercise or medications and reduced hepatic lipid in both animal and human models (27-32). Given the ability to measure hepatic lipid content non-invasively, this technique could serve as an index to monitor the effects of interventions on hepatic insulin sensitivity, either experimental or clinically. While these studies were limited to HIV-infected subjects, there is no reason to believe that the relationships demonstrated are limited to that group, though confirmatory studies should be performed.
The major limitation of the current study is its cross-sectional nature and small sample size. The cross-sectional nature limits the ability to determine the causality between fat accumulation and hepatic lipid deposition. In addition, the measurements of OMAT and RPAT represent estimates, as the tissue planes separating them are not always distinct on MRI scans.
In summary, hepatic lipid content in HIV-infected subjects is related to VAT and to insulin resistance. Hepatic lipid might serve as the linkage mediator between VAT and insulin resistance in the liver.
Acknowledgments
Financial support: This study was supported by grants from Serono Laboratories Inc., Gilead Sciences and the NIH (R01-DK065515, M01-RR0047 and M01-RR00645).
We thank all study participants. We are indebted to our outstanding study coordinators, Janet Sheikhan, RN and Kirsis Ham, FNP. We are grateful for the generous support from Serono Laboratories Inc., Gilead Sciences and the National Institutes of Health (R01-DK065515, M01 RR0047 and M01RR00645).
References
- 1.Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study AID. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 3.Engelson ES, Kotler DP, Tan Y, et al. Fat distribution in HIV-infected patients reporting truncal enlargement quantified by whole-body magnetic resonance imaging. Am J Clin Nutr. 1999;69:1162–9. doi: 10.1093/ajcn/69.6.1162. [DOI] [PubMed] [Google Scholar]
- 4.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosmiski LA, Kuritzkes DR, Lichtenstein KA, et al. Fat distribution and metabolic changes are strongly correlated and energy expenditure is increased in the HIV lipodystrophy syndrome. AIDS. 2001;15:1993–2000. doi: 10.1097/00002030-200110190-00012. [DOI] [PubMed] [Google Scholar]
- 6.He Q, Engelson ES, Albu JB, et al. Preferential loss of omental-mesenteric fat during growth hormone therapy of HIV-associated lipodystrophy. J Appl Physiol. 2003;94:2051–7. doi: 10.1152/japplphysiol.00845.2002. [DOI] [PubMed] [Google Scholar]
- 7.Bodkin NL, Metzger BL, Hansen BC. Hepatic glucose production and insulin sensitivity preceding diabetes in monkeys. Am J Physiol. 1989;56:E676–81. doi: 10.1152/ajpendo.1989.256.5.E676. [DOI] [PubMed] [Google Scholar]
- 8.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Zethelius B, Hales CN, Lithell HO, Berne C. Insulin resistance, impaired early insulin response, and insulin propeptides as predictors of the development of type 2 diabetes: a population-based, 7-year follow-up study in 70-year-old men. Diabetes Care. 2004;27:1433–8. doi: 10.2337/diacare.27.6.1433. [DOI] [PubMed] [Google Scholar]
- 10.Seppälä Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Torres A, Domingo P, Pujol J, et al. Liver triglyceride content in HIV-1-infected patients on combination antiretroviral therapy studied with 1H-MR spectroscopy. Antivir Ther. 2007;12:195–203. [PubMed] [Google Scholar]
- 12.Sutinen J, HÄkkinen AM, Westerbacka J, et al. Increased fat accumulation in the liver in HIV-infected patients with antiretroviral therapy-associated lipodystrophy. AIDS. 2002;16:2183–93. doi: 10.1097/00002030-200211080-00011. [DOI] [PubMed] [Google Scholar]
- 13.Hadigan C, Liebau J, Andersen R, et al. Magnetic Resonance Spectroscopy of Hepatic Lipid Content and Associated Risk Factors in HIV Infection. J Acquir Immune Defic Syndr. 2007;46:312–17. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–10. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes. 2007;31:1400–5. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albu JB, Kenya S, He Q, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr. 2007;86:100–6. doi: 10.1093/ajcn/86.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–89. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 18.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam TK, van de Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab. 2003;284:E281–90. doi: 10.1152/ajpendo.00332.2002. [DOI] [PubMed] [Google Scholar]
- 21.Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004;27(9):2204–10. doi: 10.2337/diacare.27.9.2204. [DOI] [PubMed] [Google Scholar]
- 22.Lam TK, Carpentier A, Lewis GF, et al. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003;284:E863–73. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- 23.Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–9. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 24.Kuhlmann J, Neumann-Haefelin C, Belz U, et al. Correlation between insulin resistance and intramyocellular lipid levels in rats. Magn Reson Med. 2005;53:1275–82. doi: 10.1002/mrm.20501. [DOI] [PubMed] [Google Scholar]
- 25.Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;(1):122–7. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 27.Charbonneau A, Couturier K, Gauthier MS, Lavoie JM. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Int J Sports Med. 2005;26:432–41. doi: 10.1055/s-2004-821225. [DOI] [PubMed] [Google Scholar]
- 28.Tiikkainen M, Häkkinen AM, Korsheninnikova E, et al. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–76. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 29.Juurinen L, Tiikkainen M, Häkkinen AM, et al. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E829–35. doi: 10.1152/ajpendo.00133.2006. [DOI] [PubMed] [Google Scholar]
- 30.Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, andhyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes Diabetes 200251797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lessard SJ, Rivas DA, Chen ZP, et al. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes. 2007;56:1856–64. doi: 10.2337/db06-1065. [DOI] [PubMed] [Google Scholar]