Abstract
Rationale
The synthetic sphingosine analog FTY720 is undergoing clinical trials as an immunomodulatory compound, acting primarily via sphingosine 1-phosphate receptor activation. Sphingolipid and cholesterol homeostasis are closely connected but whether FTY720 affects atherogenesis in humans is not known.
Objective
We examined the effects of FTY720 on the processing of scavenged lipoprotein cholesterol in human primary monocyte-derived macrophages.
Methods and Results
FTY720 did not affect cholesterol uptake but inhibited its delivery to the endoplasmic reticulum, reducing cellular free cholesterol cytotoxicity. This was accompanied by increased levels of Niemann-Pick C1 (NPC1) and ATP-binding cassette transporter A1 (ABCA1) proteins and increased efflux of endosomal cholesterol to apolipoprotein A-I. These effects were not dependent on sphingosine 1-phosphate receptor activation. Instead, FTY720 stimulated the production of 27-hydroxycholesterol, an endogenous ligand of the liver X receptor (LXR), leading to LXR–induced upregulation of ABCA1. Fluorescently labeled FTY720 was targeted to late endosomes, and the FTY720-induced upregulation of ABCA1 was NPC1-dependent, but the endosomal exit of FTY720 itself was not.
Conclusions
We conclude that FTY720 decreases cholesterol toxicity in primary human macrophages by reducing the delivery of scavenged lipoprotein cholesterol to the endoplasmic reticulum and facilitating its release to physiological extracellular acceptors. Furthermore, FTY720 stimulates 27-hydroxycholesterol production, providing an explanation for the atheroprotective effects and identifying a novel mechanism by which FTY720 modulates signaling.
Keywords: sphingolipid, atherogenesis, foam cell, cholesterol transport
Introduction
The synthetic sphingosine analog 2-amino-[2-(4-n-octylphenyl)ethyl]-1,3-propanediol hydrochloride (FTY720) is undergoing clinical trials for the treatment of immunological disorders.1 It is a prodrug phosphorylated by type 2 sphingosine kinase to form FTY720-phosphate (FTY720-P).2 FTY720-P is an analog of sphingosine 1-phosphate (S1P) that mediates its immunomodulatory effects primarily through G-protein coupled S1P receptors.3, 4 The S1P1 receptor regulates the egress of lymphocytes from secondary lymphoid organs. By inducing the internalization and degradation of the S1P1 receptor, FTY720-P reduces circulating lymphocyte counts, and is therefore considered a promising therapy for immune disorders, particularly multiple sclerosis.5 Sphingosine serves not only as a bioactive signaling lipid, but also constitutes the backbone of all sphingolipids that are structural components of eukaryotic cell membranes. Sphingolipids are thought to associate with cholesterol to form membrane rafts that are involved in multiple cellular functions, including membrane trafficking and signalling.6, 7
Atherosclerotic lesions are characterized by the accumulation of cholesterol in the arterial intima. The lesions form when modified low-density lipoproteins (LDL) are trapped in the intima, and elicit an inflammatory response, attracting inflammatory cell types - in particular monocyte-derived macrophages - to scavenge the particles. Because this leads to excessive cholesterol uptake, macrophages are transformed into lipid-laden foam cells.8 Such cells are found already in early atheromas, called fatty streaks. In advanced lesions, foam cells become overloaded with free cholesterol and undergo cell death, forming the necrotic core of the lesion that becomes prone to rupture.9
On a Western diet, fatty streaks are present already from childhood, and thus probably also in individuals receiving FTY720 therapy. Considering the long-term need of immunomodulatory therapies in many diseases, it is important to assess if FTY720 affects atherogenesis. To our knowledge, this has not been investigated in humans. The findings are in part contradictory in animals. Two studies reported a protective effect of FTY720 in murine atherosclerosis, using LDL-receptor or apoE deficient mice on a high-fat diet.10, 11 The effect was suggested to result from decreased recruitment of inflammatory cells into the lesions because of reduced S1P receptor activation. However, in apoE-/- mice on a normal diet, no changes in atherosclerotic lesions were observed but the animals developed hypercholesterolemia.12
Here, we investigated whether FTY720 affects cholesterol processing in human primary monocyte-derived macrophages deriving from healthy individuals. The results provide evidence that FTY720 protects macrophages from cholesterol toxicity and identify a new mechanism by which this compound affects lipid signaling.
Methods
Materials
Macrophage serum-free medium was from Gibco and recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) from Invitrogen. Other cell culture media were from Sigma-Aldrich. The anti-NPC-1 antibody was from Novus Biologicals, anti-LAMP-1 (H4A3) from Developmental Studies Hybridoma Bank, anti-ABCA1 from Abcam, anti-CHOP (B-3) from Santa Cruz Biotechnology and secondary antibodies from Invitrogen. LDL was provided by Dr. Matti Jauhiainen (National Public Health Institute, Helsinki, Finland) and lipid-free apoA-I by Dr. Peter Lerch (Swiss Red Cross, Bern, Switzerland). DiI-acLDL was from Invitrogen, [3H]oleic acid, [3H]cholesterol oleate, [14C]cholesterol oleate and [14C]oleoyl coenzymeA from Amersham. BODIPY-FTY720 was synthesized as described.13
Cell culture and viability
Human monocytes from healthy control subjects (in total, from 35 individuals) were isolated from buffy coat cells (Finnish Red Cross Blood Transfusion Service, Helsinki, Finland) as described.14 Monocytes were allowed to adhere to the flask for 1 h in RPMI medium, washed once with PBS and differentiated into macrophages for one week in macrophage serum-free medium + 10 ng/mL GM-CSF + 100 U/mL penicillin and 100 μg/mL streptomycin. CHO cells were cultured in a 1:1 solution of Ham's F12 and D-MEM supplemented with 5% FBS + 100 U/mL penicillin and 100 μg/mL streptomycin. FTY720 was added to cells from DMSO and control samples received DMSO only. DMSO concentration did not exceed 0.1%. Cell viability was measured using the CellTiter 96®Aqueous assay (Promega).
Sterol determinations
Cells were washed three times with PBS and harvested in 1% NP-40 in PBS + protease inhibitors (chymostatin, leupeptin, antipain and Pepstatin A at 25 μg/mL). Protein concentrations were determined using the BioRad protein assay kit. Cholesterol and cholesteryl ester concentrations were determined using the Amplex Red cholesterol assay kit (Invitrogen) from amounts corresponding to 10 μg of protein. Radiolabeling was carried out by incubating acLDL (1.5 mg/ml) with 1:10 volume of [3H]cholesterol in DMSO (40 μCi [3H]cholesterol/mg acLDL). The solution was incubated for 2 h at 40°C and dialyzed against 150 mmol/L NaCl + 1 mmol/L EDTA, pH 7.4. Oxysterol determinations were performed as described.15
Cholesterol esterification
Cells were labeled with [3H]-oleic acid in serum-free medium containing 2% fatty acid free BSA for 6 h, washed three times with PBS and scraped into a Kimax tube in 2% NaCl. Samples of cell suspensions were taken for protein determinations and [3H]oleate incorporation into cholesteryl esters was measured as described.16
ACAT activity
Cells were washed with ice-cold PBS, scraped and the pellet was resuspended in Buffer A (20 mmol/L Tris-HCl, 1 mmol/L EDTA; pH 7.7). The suspension was passed through a 25 gauge needle 10 times for homogenization. Aliquots of the homogenate were used for protein determination. A 40 μl aliquot was mixed with 10 μl Buffer A + 2 mg/ml fatty acid free BSA + 250 μg/ml cholesterol. The solution was incubated at 37°C for 2 min and the reaction started by adding 50 μl Buffer A + 2 mg/mL fatty acid free BSA + [14C]oleoyl CoA. The solution was vortexed briefly, incubated for 10 min at 37°C and the reaction stopped by adding methanol (1 mL) and CHCl3 (1 mL). [3H]Cholesteryl oleate was added as an internal standard and cholesteryl esters measured as described.16
Fluorescence microscopy
Cells were in CO2-independent medium at 37°C during microscopy. For wide-field microscopy, an Olympus IX71 microscope with a UAPO 40×/NA1.35 oil objective was used. TILL Photonics imago QE camera and TILL Vision v4.01 software were used for image acquisition. Confocal microscopy was performed using a Leica DM RXA2/TCS SP2 microscope with a HCX APO 63×/NA 0.9 water objective. Leica Confocal Scanning software was used for image acquisition.
Electron microscopy
Macrophages on coverslips were fixed, osmificated and embedded, and the cells sectioned horizontally. Multilamellar bodies/cell profile were counted. For quantification of mitochondrial-LE contacts, every fourth cell was photographed and the number of mitochondria making contact with LE versus the total number of mitochondria/cell profile were counted. Late endosomes/lysosomes were identified by morphological criteria as described.17 Contact was defined as a proximity of ≤ 50 nm between membranes.
Western blotting
Proteins were separated by SDS-PAGE (15 μg/lane; 6-15% gels depending on protein size), transferred to nitrocellulose membrane and incubated with primary antibodies at 4°C overnight and secondary antibodies for 45 min at room temperature. After extensive washing with 0.1% Tween in TBS, the blots were developed using the Enhanced Chemiluminiscense kit (Amersham).
Statistics
Results are expressed as mean ± SEM from a minimum of three independent experiments. Statistical analysis was made using Student's t test for paired observations. When three or more means were tested, one way ANOVA was performed followed by Dunnett's test for multiple comparisons against a single control. Statistical significance (p<0.05) is denoted with *.
Results
FTY720 affects cholesterol deposition in human monocyte-derived macrophages and protects them from death induced by cholesterol overload
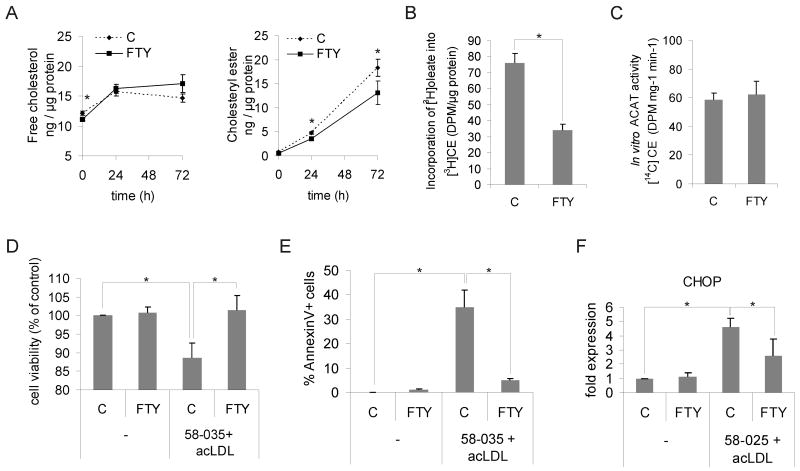
Foam cell formation in human macrophages was induced by incubating them with 50 μg/mL acetylated LDL (acLDL) for 1-3 days. This induces a several-fold increase in cellular cholesteryl esters while free (unesterified) cholesterol increases only moderately (Fig. 1A). Macrophages treated with 1 μmol/L FTY720 displayed a significant reduction in the amount of cholesteryl esters and a tendency towards increased free cholesterol upon acLDL loading as compared to control (vehicle only) cells (Fig. 1A). At 3 days of acLDL loading, there was less total cholesterol in FTY720-treated cells (30.2 ± 2.7 ng/μg protein) than in control cells (33.0 ± 2.3 ng/μg protein), (n = 12 individuals, p < 0.05).
Figure 1. FTY720 alters cholesterol homeostasis and improves survival of human macrophage foam cells.
A. Macrophages were pre-treated for 24 h ± 1 μmol/L FTY720 and foam cell formation induced by addition of 50 μg/mL acLDL for 24 h or 72 h in the continued presence or absence of FTY720. Cellular free cholesterol and cholesteryl esters were determined from at least 12 independent donors per condition (C = control, FTY = FTY720). B. Cholesterol esterification was measured as [3H]oleic acid incorporation into cholesteryl esters (CE). Cells were treated ± 1 μmol/L FTY720 for 24 h, followed by a 6-h incorporation with [3H]oleic acid in the presence of 50 μg/mL acLDL. Measurements are from three independent donors. C. Determination of ACAT activity from cell lysates. Macrophages were treated for 24 h ± 1 μmol/L FTY720 prior to cell lysis and in vitro ACAT activity measured in the continued presence or absence of 1 μmol/L FTY720. Results are from 6 independent donors. D. Macrophages were treated for 24 h ± 1 μmol/L FTY720 followed by loading with 50 μg/mL acLDL in the presence of the ACAT inhibitor PKF 58-035 (10 μg/mL) for 16 h. Cell viability was then measured from three donors in triplicate. E. Macrophages were treated ± 1μmol/L FTY720 for 24 h and loaded with 50 μg/mL acLDL in the presence of PKF 58-035 for 8 h. Externalized phosphatidylserine was stained with FITC-conjugated Annexin V, and the fraction of positive cells was counted. A minimum of 180 cells from three separate fields were quantified. F. Macrophages were treated for 24 h ± 1 μmol/L FTY720, and then incubated for 48 h with 100 μg/mL acLDL and PKF 58-035. Cellular CHOP levels were quantified by Western blotting from three independent donors.
These data indicate that FTY720 does not affect the extent of cholesterol uptake from modified LDL but suggest a stimulatory effect on cholesterol removal and an inhibitory effect on cholesterol esterification. In support of the latter, we found that FTY720 led to a reduction in the amount of [3H]oleic acid incorporated into cholesteryl esters in acLDL-loaded macrophages (Fig. 1B). Importantly, the activity of acyl-CoA cholesteryl acyl transferase (ACAT) in macrophage lysates was not affected (Fig. 1C), suggesting that FTY720 impairs the delivery of cholesterol to ACAT in the endoplasmic reticulum (ER), but not the enzyme activity per se.
FTY720 may induce cell death at high concentrations.18 We found that 1 μmol/L FTY720 did not impair macrophage viability (Fig. 1D). On the contrary, when macrophages were challenged with free cholesterol overloading as in 19, FTY720-treated cells were more resistant to death (Fig. 1D and 1E) and showed decreased induction of the unfolded protein response mediator CHOP (Fig. 1F). Together, these results indicate that FTY720 protects macrophages from free cholesterol-induced toxicity and suggest that this protection results from inhibition of cholesterol transport to the ER.
FTY720 reduces the incorporation of acLDL-derived cholesterol into cholesteryl esters and stimulates its release to apolipoprotein A-I
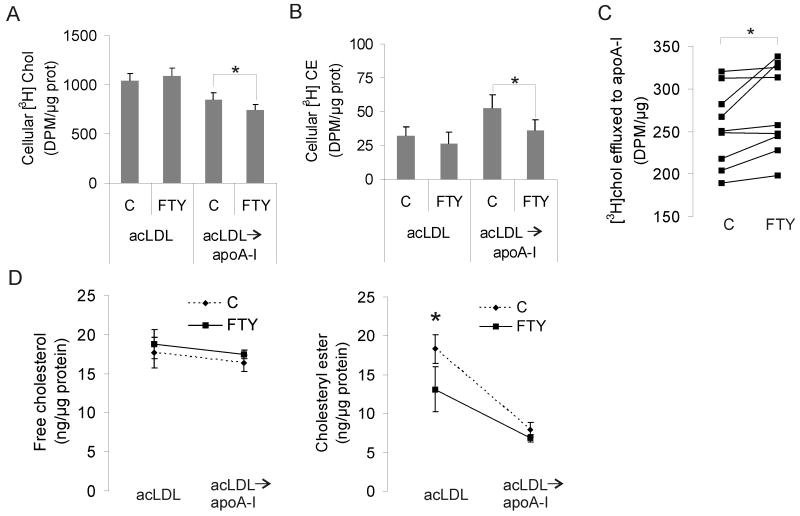
To specifically address how FTY720 affects cholesterol that enters macrophages via acLDL, we incubated the cells for 4 h with [3H]cholesterol-labeled acLDL, followed by a 3-h chase in the presence of the extracellular cholesterol acceptor apolipoprotein A-I (apoA-I). FTY720-treated cells took up similar amounts of [3H]cholesterol as control cells (Fig. 2A), in agreement with the results with unlabeled cholesterol. However, [3H]cholesterol was more avidly released from FTY720-treated cells to apoA-I (Fig. 2A, C). Moreover, the esterification of [3H]cholesterol was reduced in FTY720 treated cells (Fig. 2B), in accordance with the earlier results (Fig. 1).
Figure 2. FTY720 induces re-routing of acLDL derived cholesterol.
Macrophages were pre-treated for 24 h ± 1 μmol/L FTY720. Cells were then pulse labeled with 50 μg/mL [3H]cholesterol-labeled acLDL for 4 h, followed by a 3-h chase in the presence of 10 μg/mL apoA-I. Lipids were extracted from cells immediately after the pulse, or from cells and medium after the chase. The amounts of cellular free cholesterol (Chol) (A), cellular cholesteryl esters (CE) (B) and cholesterol effluxed to apoA-I (C) were measured by scintillation counting. A and B show pooled data from 9 donors, C shows the results from individual donors separately. D. Macrophages were treated for 24 h ± 1 μmol/L FTY720 and loaded with 50 μg/mL acLDL for 72 h, followed by cholesterol efflux to 10 μg/mL apoA-I for 72 h ± 1 μmol/L FTY720. Cellular free cholesterol and cholesteryl esters were determined before and after cholesterol efflux from at least 6 donors per experimental condition.
To study the effect of FTY720 on the removal of the foam cell cholesterol mass to apoA-I, we incubated macrophages with acLDL for 3 days as in Fig. 1, followed by a 3-day incubation with apoA-I. We found that after incubation with apoA-I, control and FTY720-treated cells reached similar levels of both free and esterified cholesterol (Fig. 2D). Taken together, these data show that FTY720 induced a shift in the handling of acLDL derived cholesterol from esterification toward efflux from cells.
Effects of FTY720 on late endosomes and on proteins involved in cholesterol exit from late endosomes
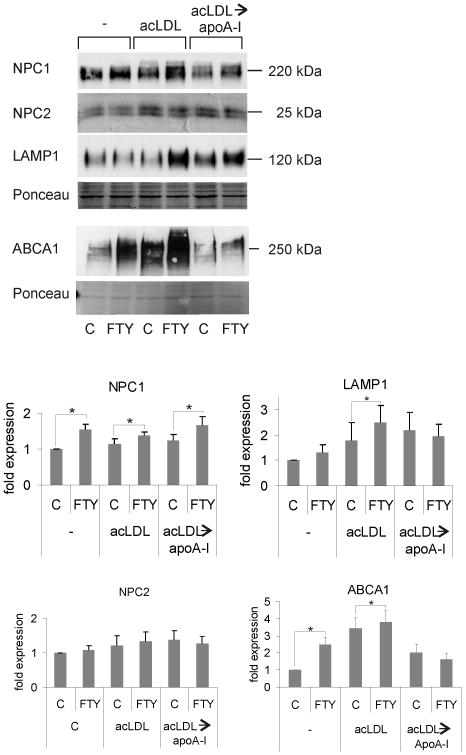
We found that FTY720 induced a significant increase in the amounts of Niemann-Pick C1 protein (NPC1) and ATP-binding cassette transporter A1 (ABCA1) (Fig. 3), two large multitransmembrane proteins involved in the exit of cholesterol from late endosomal compartments (LE) and in its efflux to apoA-I, respectively.20, 21 Notably, the levels of the soluble sterol-binding Niemann-Pick C2 protein (NPC2) were not affected by FTY720 (Fig. 3) although NPC1 and NPC2 are considered to function in the same pathway.22
Figure 3. Upregulation of proteins involved in endosomal cholesterol trafficking in FTY720 treated cells.
Representative Western blots and quantification of NPC1, NPC2, LAMP1 and ABCA1 levels from macrophages. Cells were treated for 24 h ± 1 μmol/L FTY720. Then, foam cell formation was induced by addition of 50 μg/mL acLDL for 24 h. At this stage, not loaded (-) and acLDL-loaded (acLDL) cells were harvested. A parallel set of acLDL loaded macrophages were allowed to undergo cholesterol efflux to 10 μg/mL apoA-I for 24 h (acLDL→apoA-I) before harvesting for analysis. Bars show the relative quantities of the proteins from at least 3 independent donors.
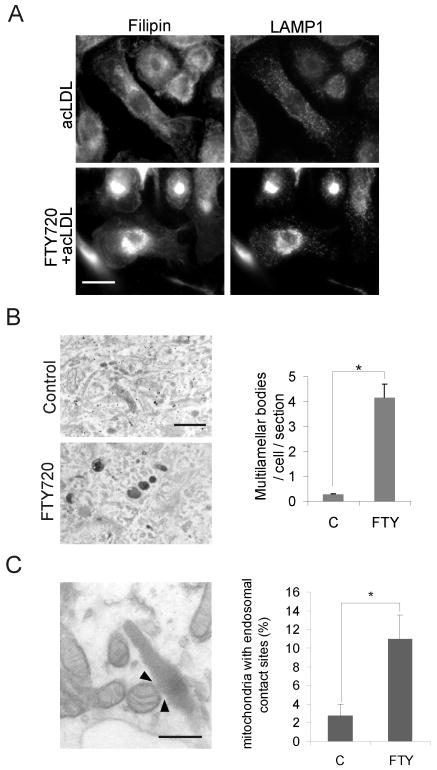
To further address the endosomal effects of FTY720, we investigated the ultrastructure of the LE. We found that FTY720 induced a proliferation of LE, as judged by the increased immunoreactivity against lysosomal acidic membrane protein 1 (LAMP1) in acLDL-loaded cells (Fig 4A) and an increased number of multilamellar bodies as assessed by electron microscopy (Fig. 4B). The LE of FTY720-treated cells were also strongly filipin positive (Fig. 4A), indicating that these compartments contained ample free cholesterol. Unexpectedly, electron microscopy also revealed that the LE were often in close proximity to mitochondria. Indeed, quantification revealed an increased number of contacts between mitochondria and LE in FTY720 treated cells (Fig. 4C).
Figure 4. Late endosomal organelles are affected by FTY720.
A. Macrophages were treated ± 1 μmol/L FTY720 for 48 h and 50 μg/mL acLDL for the last 24 h of incubation. Cells were fixed and stained with filipin and anti-LAMP-1 antibodies. B. Electron microscopic images of cells treated ± 1 μmol/L FTY720 for 24 h, followed by a 2-h pulse with 50 μg/mL DiI-acLDL and a 4-h chase. The number of multilamellar bodies was quantified from 44 control cells and 48 FTY720 treated cells. Scale bar, 500 nm. C. Macrophages were treated for 24 h ± 1 μmol/L FTY720 prior to fixing and processing for electron microscopy. The percentage of mitochondria with endosomal contacts (example indicated by arrowheads) was quantified from 21 and 19 randomly chosen visual fields from control and FTY720 treated cells, respectively. Scale bar, 250 nm.
Cellular itineraries of fluorescently labeled FTY720
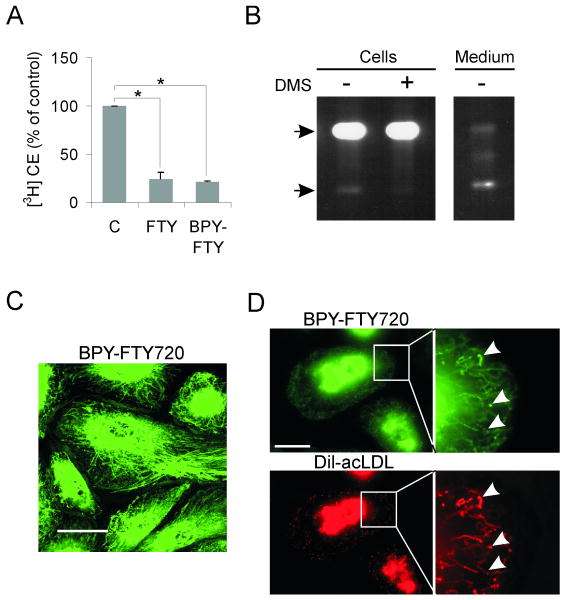
We next studied the localization of FTY720 by using a fluorescently labeled FTY720. BODIPY-FTY720 reduced cholesterol esterification similarly as the nonfluorescent compound (Fig. 5A). Most of the cellular BODIPY-FTY720 was non-phosphorylated, whereas the phosphorylated form predominated in the medium (Fig. 5B). This is in line with the low concentrations of S1P in the cell and high concentrations in the extracellular milieu.3 In living macrophages, BODIPY-FTY720 was localized in perinuclearly concentrated tubular structures (Fig. 5C). These structures became more punctuate upon acLDL loading and colocalized with endocytosed DiI-labeled acLDL (Fig. 5D), indicating that they represent LE involved in the processing of modified lipoproteins.
Figure 5. Cellular targeting and effects of BODIPY-FTY720.
A. Cholesterol esterification in macrophages was measured by [3H]oleic acid incorporation into cholesteryl esters. The cells were pre-treated with ± 1 μmol/L FTY720 or 1 μM BODIPY-FTY720 for 24 h, followed by a 6-h incubation with [3H]oleic acid in the presence of 50 μg/mL acLDL ± FTY720 or BODIPY-FTY720. Measurements are from three individual donors. B. Macrophages were incubated for 24 h with 1 μmol/L BODIPY-FTY720 ± 5 μmol/L DMS. Lipids from the cell pellet and from 1/8 of the medium were analyzed by TLC. The upper arrow indicates the position of BODIPY-FTY720 and the lower arrow that of BODIPY-FTY720-P. C. Macrophages were labeled with μmol/L BODIPY-FTY720 for 16 h and imaged live by confocal microscopy. A confocal section from the basal part of the cells is shown. Scale bar, 10 μm. D. Macrophages were labeled with 0.5 μmol/L BODIPY-FTY720 for 24 h followed by a 2-h pulse and a 2-h chase with DiI-acLDL. Wide field microscopy images show BODIPY-FTY720 and DiI-acLDL distribution in macrophages. The insets are composite time lapse sequences showing the movement of BODIPY-FTY720 and DiI-acLDL during 10 s (higher magnification of the area indicated). Images were acquired at a rate of 5 Hz, arrowheads indicate structures with high colocalization of BODIPY-FTY720 and DiI-acLDL. Scale bar, 10 μm.
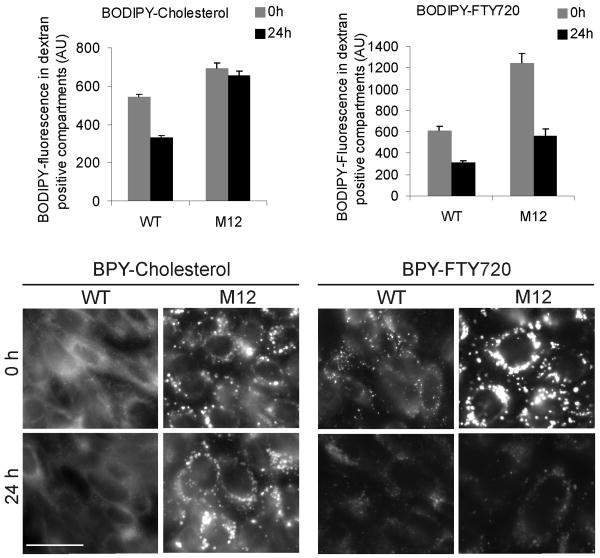
Given the upregulation of NPC1 (but not NPC2) in FTY720-treated cells (Fig. 3) and the recent suggestion that NPC1 may function as a sphingosine transporter 23, we considered that NPC1 might transport FTY720 out of the LE. Therefore, we compared the ability of wild-type and NPC1-deficient CHO cells (M12 cells) to transport BODIPY-FTY720. We have shown that BODIPY-cholesterol accumulates in dextran-positive LE in M12 cells.24 Using this method, we found that while BODIPY-cholesterol accumulated in the LE of M12 cells, BODIPY-FTY720 did not (Fig. 6). This indicates that NPC1 function is not required for the exit of FTY720 from LE.
Figure 6. BODIPY-FTY720 trafficking in NPC1-deficient cells.
Wild-type and NPC1-deficient (M12) CHO cells were labeled with 1 mg/mL rhodamine-dextran for 24 h followed by incubation with 1 μmol/L BODIPY-cholesterol or 1 μmol/L BODIPY-FTY720 for 24 h and chasing for 0 h or 24 h. Living cells were imaged and BODIPY intensity in dextran-positive organelles was quantified directly after loading (0 h) and following the 24 h chase. Quantification from eight independent measurements and representative fluorescence micrographs are shown. Scale bar, 10 μm.
Mechanisms involved in the FTY720-induced alterations of cholesterol transport
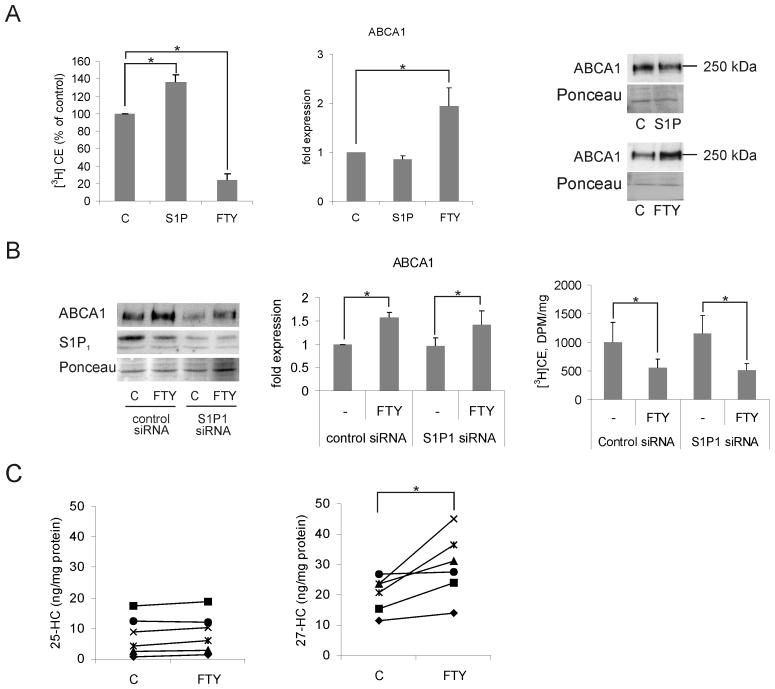
To investigate whether phosphorylation of FTY720 was necessary for the observed alterations in cellular cholesterol handling, we treated macrophages with S1P, the natural equivalent of FTY720-P. This did not reduce cholesterol esterification or increase the cellular levels of ABCA1 (Fig. 7A). Furthermore, FTY720 was able to inhibit cholesterol esterification in the presence of the sphingosine kinase inhibitor N,N-dimethylsphingosine (DMS) (not shown). Finally, knockdown of the S1P1 receptor did not affect the ability of FTY720 to reduce cholesterol esterification or increase ABCA1 expression (Fig. 7B). These data suggest that generation of FTY720-P and modulation of S1P receptors are not necessary for the observed effects on macrophage cholesterol balance.
Figure 7. FTY720 stimulates 27-hydroxycholesterol production and upregulates ABCA1 independently of S1P receptor activation.
A. Macrophages were pre-treated ± 1 μmol/L S1P for 24 h, followed by a 6-h incubation with [3H]oleic acid in the presence of 50 μg/mL acLDL ± S1P. Cholesterol esterification was measured by [3H]oleic acid incorporation into cholesteryl esters (CE) from six individual donors (left panel). Cells were treated for 24 h ± 1 μmol/L S1P or FTY720 and ABCA1 expression analyzed by Western blotting. Results are from three individual donors (middle and right panels). B. Macrophages electroporated with control siRNA or S1P1 siRNA were treated ± 1μmol/L FTY720 for 24 h. Cell lysates were subjected to Western blotting using S1P1 and ABCA1 antibodies. S1P1 siRNA reduced S1P1 protein levels by ∼70% (left panel). ABCA1 levels were quantified from three individuals (middle panel). Cholesterol esterification was measured from a parallel set of siRNA and FTY720 treated macrophages (right panel). C. Quantification of 25- and 27-hydroxycholesterol (HC) content in macrophages treated for 24 h ± 1 μmol/L FTY720. Results are from six individual donors.
ABCA1 expression is transcriptionally regulated by liver X receptor (LXR)/retinoid X-receptor (RXR) heterodimers that are activated by oxysterols. We found that FTY720 increased the ABCA1 mRNA levels (36±6%, p<0.05, quadruplicate measurements from three donors). We therefore measured oxysterol levels in macrophages upon FTY720 treatment. FTY720 induced a significant increase in cellular 27-hydroxysterol, an endogenous LXR ligand, while the levels of another oxysterol, 25-hydroxysterol, remained unaltered (Fig. 7C). This indicates that FTY720 exerts a potent but selective effect on oxysterol generation.
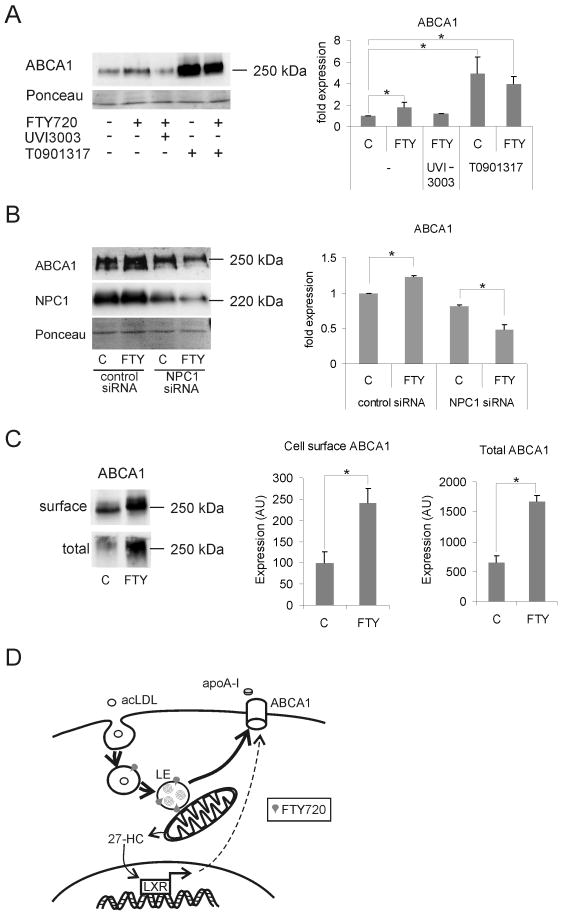
Next, we treated macrophages either with FTY720 alone or in combination with the retinoid X receptor (RXR) antagonist UVI3003.25 The FTY720-induced increase in ABCA1 was abrogated by UVI3003, indicating that ABCA1 upregulation upon FTY720 administration indeed occurs via nuclear hormone receptor activation (Fig. 8A). When compared with a synthetic LXR ligand, T0901317, FTY720 was a weaker LXR activator, as assessed by ABCA1 upregulation. Importantly, FTY720 did not further enhance the stimulatory effect of T0901317 on ABCA1 expression (Fig. 8A), suggesting that they act in the same pathway. These findings provide evidence that FTY720 stimulates 27-hydroxycholesterol formation, thereby enhancing LXR/RXR-dependent transcriptional activation and ABCA1 expression.
Figure 8. Mechanism for FTY720 induced upregulation of ABCA1.
A. Macrophages were treated for 24 h ± 1 μmol/L FTY720 together with 10 μmol/L T0901317 or 5 μmol/L UVI3003 as indicated. Representative Western blot and quantification of ABCA1 levels from three donors in duplicate. B. Control siRNA or NPC1 siRNA treated macrophages were treated ± 1 μmol/L FTY720 for 24 h. The cellular levels of NPC1 and ABCA1 were analyzed by Western blotting and ABCA1 was quantified from three individual donors in duplicate. C. Macrophages were treated for 24 h ± 1 μmol/L FTY720. Surface proteins were biotinylated, pulled down with streptavidin agarose, and surface ABCA1 analyzed by Western blotting. Ont-tenth of cell lysate was removed before pull-down of the biotinylated proteins to reflect the total cellular amount of ABCA1. The level of ABCA1 on the surface was 15-20% of total ABCA1 in both control and FTY720 treated cells. Macrophages from a total of five donors were analyzed. D. Proposed model of FTY720 action in macrophage atheroprotection. 27-HC, 27-hydroxycholesterol. The thick arrows indicate the intracellular routing of cholesterol.
NPC1 expression is known to be important for the generation of 27-hydroxycholesterol in macrophages.26, 27 We therefore depleted NPC1 in human primary macrophages using siRNAs. In NPC1-silenced cells, the stimulation of ABCA1 expression upon FTY720 treatment was not observed (Fig. 8B). This suggests that NPC1 plays a critical role in the process by which FTY720 stimulates 27-hydroxycholesterol production and ABCA1 upregulation. The targeting of ABCA1 to the plasma membrane is important for its function.20 FTY720 significantly increased ABCA1 levels in the plasma membrane as assessed by surface biotinylation (Fig 8C).
Discussion
We show here that FTY720 reduces cholesterol deposition in human macrophage foam cells and uncover a mechanism by which FTY720 regulates sterol balance. The concentration of FTY720 producing these effects was similar to that found in target tissues during FTY720 treatment.28 Moreover, the results were surprisingly consistent in the cells of the over 40 blood donors investigated. Although the in vivo situation is clearly much more complex, the prediction from these in vitro findings would be that FTY720 does not increase but would rather decrease the lipid burden in pre-existing human atheromatous lesions.
The FTY720 induced cholesterol reduction resulted from a significant re-routing of intracellular cholesterol. This re-routing appeared distinct from that induced by other amphiphils known to modulate the membrane activity of cholesterol (see Supplemental Table I). We found that upon FTY720 treatment, endocytosed lipoprotein cholesterol was shunted less avidly to the ER for esterification and more efficiently to the extracellular acceptor apoA-I. This was observed by following the itinerary of radiolabeled cholesterol introduced in acLDL. It was also reflected in the cellular cholesterol mass, with an increase in the free vs. esterified cholesterol pool and an absolute cholesterol reduction upon acLDL loading in FTY720-treated cells.
Despite the relative increase in free cholesterol, FTY720 protected macrophages from free cholesterol induced cytotoxicity. This is likely to result from the FTY720-induced reduction of cholesterol transport to the ER, as reduced cholesterol delivery to the ER has been shown to protect macrophages from apoptosis.29, 30 Filipin staining demonstrated that most of the free cholesterol was localized in the LE, and from there it apparently became transferred to apoA-I. ABCA1 plays a key role in the removal of endosomal cholesterol to apoA-I.20, 31 The increased ABCA1 levels upon FTY720 treatment thus fit well with the enhanced cholesterol efflux to apoA-I (and a parallel decreased delivery of cholesterol to the ER). Of note, FTY720 increased ABCA1 levels and decreased the cholesterol content of macrophages also in the absence of acLDL, indicating that the process does not necessitate an excess of cholesterol.
The immunomodulatory effects of FTY720 are considered to largely derive from the modulation of S1P receptors via FTY720-P.3, 4 However, we found that S1P failed to reduce cholesterol esterification and increase ABCA1 levels. In addition, cholesterol esterification was reduced under conditions where FTY720 phosphorylation was inhibited and knockdown of the receptor S1P1 did not affect the FTY720-induced reduction of cholesterol esterification or ABCA1 regulation. These results argued for an alternative mechanism of action for FTY720. As increased ABCA1 levels appeared to represent an important mediator in the FTY720-induced sterol changes, and ABCA1 is upregulated by oxysterols via LXR, we investigated whether FTY720 directly regulates oxysterol generation. We found that FTY720 induced a significant increase in 27-hydroxycholesterol production and ABCA1 transcriptional activation. To our knowledge, these results provide the first demonstration that FTY720 affects oxysterol generation and signalling.
27-hydroxycholesterol is the most abundant circulating oxysterol and is produced by sterol-27 hydroxylase (CYP27) in several tissues. This enzyme is localized in the inner mitochondrial membrane where the cholesterol content is very low. The rate-limiting step for 27-hydroxycholesterol synthesis is considered to be the delivery of cholesterol into mitochondria.32 With this in mind, it is tempting to speculate that the increased LE-mitochondrial contacts observed upon FTY720 treatment may assist in the movement of cholesterol from the LE into mitochondria.
Moreover, NPC1 activity is known to be important for the generation of 27-hydroxycholesterol in macrophages 26, 27 and our data implicate a functional role for NPC1, as the FTY720-induced ABCA1 upregulation was absent in NPC1 silenced cells. Taken the sphingolipid structure of FTY720 and the LE targeting of the fluorescently labeled compound, it seems plausible that FTY720 exhibits membrane effects promoting an increase in NPC1 levels. This in turn might lead to increased delivery of cholesterol for mitochondrial 27-hydroxycholesterol production – possibly via increased LE-mitochondrial contacts – resulting in elevated LXR activation and ABCA1 expression (see Fig 8D).
In conclusion, this study shows that FTY720 confers atheroprotective effects in human macrophage foam cells by inhibiting the delivery of cholesterol to the ER and promoting its removal to physiological acceptors. These effects are not mediated by sphingosine 1-phosphate receptor activation but rather by FTY720-induced production of 27-hydroxycholesterol, consequent LXR activation and ABCA1 upregulation. The findings indicate 27-hydroxycholesterol generation as a novel mechanism of action for FTY720. This encourages the assessment of circulating 27-hydroxycholesterol levels in human patients receiving FTY720 treatment and evaluation of the potential role of oxysterol(s) in the therapeutic actions of FTY720.
Novelty and significance.
What is known?
The sphingolipid FTY720 is in clinical trials for treating immunological disorders and acts via sphingosine-1-phosphate receptors.
Cellular sphingolipid and cholesterol metabolism are closely connected.
Whether FTY720 affects cholesterol deposition in human cells or tissues is not known.
What new information does this article contribute?
FTY720 stimulates cholesterol removal and decreases cholesterol toxicity in human macrophage foam cells.
These effects are not mediated via sphingosine-1-phosphate receptors.
Instead, we identify a novel mechanism of action for FTY720: it stimulates 27-hydroxycholesterol production, and leads to a liver X receptor dependent upregulation of the cholesterol efflux protein ABCA1.
Summary
FTY720 is a synthetic sphingolipid currently in clinical trials as an immunological drug, e.g. for multiple sclerosis. It acts by regulating sphingosine-1-phosphate receptors in lymphocytes. On a Western diet, atherosclerotic lesions are present from childhood; therefore, they are likely to be present also in individuals receiving FTY720 therapy. Sphingolipids are known to modify cholesterol metabolism. Therefore, we wondered if FTY720 might affect atherosclerotic lesion development. Since this process is not easy to monitor in living humans, we studied monocyte-derived macrophages that represent a key cell type in atherosclerotic lesions. We found that FTY720 treated macrophages got rid of cholesterol better than control cells and showed increased resistance to cholesterol cytotoxicity. These effects were not mediated by sphingosine-1-phosphate receptors. Instead, we found a new mechanism that can explain the atheroprotective effects: FTY720 stimulated the production of an oxygenated cholesterol derivative, 27-hydroxycholesterol, which acts as a transcriptional activator, increasing the expression of proteins involved in cholesterol removal. These data are the first to document the effects of FTY720 on human lipid-laden macrophages (foam cells). The study identifies a novel mechanism of action for FTY720 and provides a rationale for investigating whether FTY720 increases 27-hydroxycholesterol formation and exhibits atheroprotective effects in patients receiving FTY720 therapy.
Supplementary Material
Acknowledgments
Sources of Funding: This study was financially supported by the Academy of Finland (grant numbers 210969 and 123261), European Union Framework Programme 7 (LipidomicNet, grant number 202272), NIH Grant HL-083187, The Finnish Foundation for Cardiovascular Research and The Perklén Foundation. The Wihuri Research Institute is maintained by The Jenny and Antti Wihuri Foundation.
Non-standard abbreviations and acronyms
- ABCA1
ATP-binding cassette transporter A1
- ACAT
acyl-CoA cholesterol acyltransferase
- acLDL
acetylated low-density lipoprotein
- apoA-I
apolipoprotein A-I
- CHOP
C/EBP-homologous protein
- DMS
N,N-dimethylsphingosine
- ER
endoplasmic reticulum
- FTY720
2-amino-[2-(4-n-octylphenyl)ethyl]-1,3-propanediol hydrochloride
- LE
late endosomal compartments
- LAMP1
lysosomal acidic membrane protein 1
- NPC1
Niemann-Pick C1 protein
- S1P
sphingosine 1-phosphate
Footnotes
Disclosures: None.
References
- 1.Mansoor M, Melendez AJ. Recent trials for FTY720 (fingolimod): a new generation of immunomodulators structurally similar to sphingosine. Rev Recent Clin Trials. 2008;3:62–69. doi: 10.2174/157488708783330486. [DOI] [PubMed] [Google Scholar]
- 2.Kihara A, Igarashi Y. Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim Biophys Acta. 2008;1781:496–502. doi: 10.1016/j.bbalip.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 5.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 8.Vainio S, Ikonen E. Macrophage cholesterol transport: a critical player in foam cell formation. Ann Med. 2003;35:146–155. doi: 10.1080/07853890310008198. [DOI] [PubMed] [Google Scholar]
- 9.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 10.Keul P, Tolle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 11.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 12.Klingenberg R, Nofer JR, Rudling M, Bea F, Blessing E, Preusch M, Grone HJ, Katus HA, Hansson GK, Dengler TJ. Sphingosine-1-phosphate analogue FTY720 causes lymphocyte redistribution and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2392–2399. doi: 10.1161/ATVBAHA.107.149476. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Bittman R. Synthesis and spectral properties of cholesterol- and FTY720-containing boron dipyrromethene dyes. J Org Chem. 2007;72:8376–8382. doi: 10.1021/jo701475q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 15.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 16.Holtta-Vuori M, Tanhuanpaa K, Mobius W, Somerharju P, Ikonen E. Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol Biol Cell. 2002;13:3107–3122. doi: 10.1091/mbc.E02-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsch S, Habermann A, Jager S, Muller B, Krijnse-Locker J, Krausslich HG. Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular-vesicular membranes in ESCRT function. Traffic. 2006;7:1551–1566. doi: 10.1111/j.1600-0854.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 18.Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK, Muthusamy N, Byrd JC, Cheng AL, Chen CS. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008;68:1204–1212. doi: 10.1158/0008-5472.CAN-07-2621. [DOI] [PubMed] [Google Scholar]
- 19.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Wang N, Tall AR. A PEST deletion mutant of ABCA1 shows impaired internalization and defective cholesterol efflux from late endosomes. J Biol Chem. 2005;280:29277–29281. doi: 10.1074/jbc.M505566200. [DOI] [PubMed] [Google Scholar]
- 21.Wojtanik KM, Liscum L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J Biol Chem. 2003;278:14850–14856. doi: 10.1074/jbc.M300488200. [DOI] [PubMed] [Google Scholar]
- 22.Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc Natl Acad Sci U S A. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 24.Holtta-Vuori M, Uronen RL, Repakova J, Salonen E, Vattulainen I, Panula P, Li Z, Bittman R, Ikonen E. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic. 2008;9:1839–1849. doi: 10.1111/j.1600-0854.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 25.Nahoum V, Perez E, Germain P, Rodriguez-Barrios F, Manzo F, Kammerer S, Lemaire G, Hirsch O, Royer CA, Gronemeyer H, de Lera AR, Bourguet W. Modulators of the structural dynamics of the retinoid X receptor to reveal receptor function. Proc Natl Acad Sci U S A. 2007;104:17323–17328. doi: 10.1073/pnas.0705356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frolov A, Zielinski SE, Crowley JR, Dudley-Rucker N, Schaffer JE, Ory DS. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J Biol Chem. 2003;278:25517–25525. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JR, Coleman T, Langmade SJ, Scherrer DE, Lane L, Lanier MH, Feng C, Sands MS, Schaffer JE, Semenkovich CF, Ory DS. Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking. J Clin Invest. 2008;118:2281–2290. doi: 10.1172/JCI32561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 29.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neufeld EB, Stonik JA, Demosky SJ, Jr, Knapper CL, Combs CA, Cooney A, Comly M, Dwyer N, Blanchette-Mackie J, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The ABCA1 transporter modulates late endocytic trafficking: insights from the correction of the genetic defect in Tangier disease. J Biol Chem. 2004;279:15571–15578. doi: 10.1074/jbc.M314160200. [DOI] [PubMed] [Google Scholar]
- 32.Pandak WM, Ren S, Marques D, Hall E, Redford K, Mallonee D, Bohdan P, Heuman D, Gil G, Hylemon P. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J Biol Chem. 2002;277:48158–48164. doi: 10.1074/jbc.M205244200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.