Summary
Tissue expression microarrays, employed to determine the players and mechanisms leading to prostate cancer development, have consistently demonstrated that myosin VI, a unique actin-based motor, is upregulated in medium-grade human prostate cancers (Dunn et al., 2006). Thus to understand the role of myosin VI in prostate cancer development, we have characterised its intracellular localisation and function in the prostate cancer cell line LNCaP. Using light and electron microscopy we identified myosin VI on Rab5-positive early endosomes as well as on recycling endosomes and the trans-Golgi network. Intracellular targeting appears to involve two myosin VI interacting proteins, GIPC and LMTK2, both of which can be co-immunoprecipitated with myosin VI from LNCaP cells. The absence of Dab2, a tumour suppressor and myosin VI binding partner, inhibits recruitment of myosin VI to endocytic structures at the plasma membrane in LNCaP cells, but interestingly has no effect on endocytosis. SiRNA-mediated down regulation of myosin VI expression results in a significant reduction in prostate-specific antigen (PSA) and vascular endothelial growth factor (VEGF) secretion in LNCaP cells. Our results suggest that in prostate cancer cells myosin VI regulates protein secretion, but the over expression of myosin VI has no major impact on clathrin-mediated endocytosis.
Introduction
Although prostate cancer is the most frequent malignancy detected in men in Western Countries, there is a general lack of information regarding the molecular mechanisms involved in the progression of this cancer. To identify the cellular processes and proteins that are involved in the development of prostate cancers, tissue expression microarrays have been used to monitor the changes in gene expression during malignant transformation. Microarray data from 174 human epithelial tumours highlighted myosin VI as one of the genes most frequently over-expressed in prostate and breast cancer tissues (Su et al., 2001). Furthermore the highest level of myosin VI expression was detected in medium-grade androgen-dependent prostate cancers, whereas androgen-independent more advanced and aggressive cancers show less dramatic over-expression (Dunn et al., 2006). Although these initial observations are intriguing, very little is known about the roles that myosin VI plays in human cancers.
In the myosin superfamily myosin VI has unique cellular properties and functions, because it moves towards the minus end of actin filaments in the opposite direction to all other myosins (Wells et al., 1999). Myosin VI contains a N-terminal motor domain that binds actin and ATP, a short neck region with bound calmodulin, and a C-terminal tail, the cargo binding domain (Hasson & Mooseker, 1994). The tail region is alternatively spliced containing either a large or a small insert generating at least 4 different myosin VI splice isoforms with different tissue specific expression patterns and possible functions (Buss et al., 2001; Kellerman & Miller, 1992). Myosin VI is ubiquitously expressed in multicellular organisms. In mammalian cells myosin VI is recruited to membrane ruffles, to cell-cell contact sites, into the perinuclear region at/around the Golgi complex and onto clathrin-coated and uncoated endocytic structures (Buss et al., 1998) (Aschenbrenner et al., 2003) (Buss et al., 2001; Maddugoda et al., 2007). Functional studies have demonstrated a role for myosin VI in the secretory pathway, for the delivery of cargo to the plasma membrane (Au et al., 2007; Warner et al., 2003), in clathrin-mediated endocytosis (Buss et al., 2001) and in the transport of endocytosed cargo to the recycling compartment (Chibalina et al., 2007). Loss of myosin VI reduces the motility of ovarian and prostate cancer cells (Yoshida et al., 2004) (Dunn et al., 2006) and in Drosophila melanogaster ovaries it inhibits border cell migration (Geisbrecht & Montell, 2002). These diverse roles of myosin VI are mediated by interactions with different binding partners; e.g. recruitment of myosin VI to the Golgi complex is mediated by binding to optineurin (Sahlender et al., 2005), whereas targeting of myosin VI to clathrin-coated structures at the plasma membrane requires binding to Disabled-2 (Dab2) (Morris et al., 2002). Dab2 is a tumour suppressor gene that is dramatically down-regulated in breast, prostate and ovarian cancers and in a number of cancer cell lines (Schwahn & Medina, 1998) (Fazili et al., 1999) (Tseng et al., 1998). On early endosomes and in the recycling pathway myosin VI interacts with the glucose transporter binding protein GLUT1CBP (GIPC) (Bunn et al., 1999) and the Ser/Thr transmembrane protein kinase LMTK2 (Chibalina et al., 2007). A genome-wide association study recently identified LMTK2 as one of the three novel common alleles associated with prostate cancer (Eeles et al., 2008).
Since myosin VI is a multifunctional motor protein involved in different cellular pathways, it may promote prostate carcinogenesis by a number of distinct mechanisms/pathways. In prostate cancer LNCaP cells we observed that myosin VI is present on early endosomes, on recycling endosomes and to a lesser extent on the trans-Golgi network. No myosin VI was associated with clathrin-coated structures at the plasma membrane despite the expression of the large insert myosin VI isoform that normally targets to this location. GIPC and LMTK2 co-immunoprecipitate with myosin VI from LNCaP cells and reflect myosin VI’s cellular localisation in early endosomes and the perinuclear recycling compartment. When myosin VI expression was knocked down, secretion of prostate-specific antigen (PSA), a serine protease of the kallikrein family (Balk et al., 2003), and the vascular endothelial growth factor (VEGF), a growth factor important for tumor angiogenesis (Ellis & Hicklin, 2008), were significantly reduced. Thus myosin VI over-expression may enhance prostate tumor growth and metastasis by increasing the secretion of growth factors and potential extracellular matrix proteases.
Results
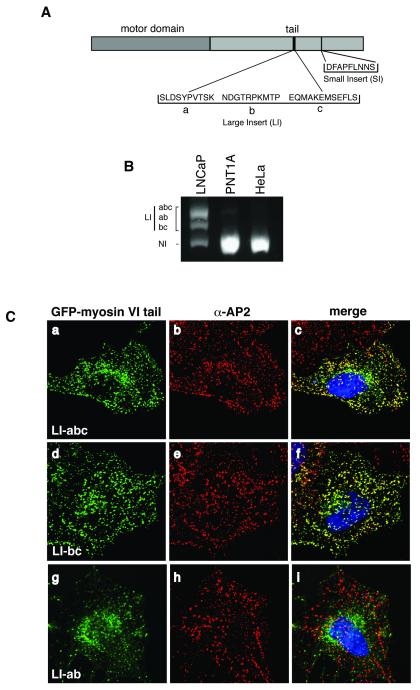
In mammalian cells splice variants of myosin VI are expressed that contain either no insert (NI), or a small insert (SI, 9aa) or a large insert (LI, 22 or 32aa) or both large and small inserts in the C-terminal tail domain (Buss et al., 2001; Kellerman & Miller, 1992). The large insert encoded by three different exons of roughly equal size (a, b and c) can be rearranged to form different versions (Dance et al., 2004) (see figure 1A). Myosin VI isoforms are expressed in a tissue specific manner; the large insert isoform (abc or bc) is predominantly expressed in polarised epithelial cells, specifically targeting to clathrin coated structures at the plasma membrane, whereas the NI isoform is mostly present in uncoated endocytic structures and in the perinuclear region at/around the Golgi complex (Buss et al., 2001). Using RT-PCR we observed that LNCaP cells express the myosin VI NI isoform and three splice variants of the LI, the long abc, the bc and the ab, whereas the non-malignant prostate cell line PNT1A and HeLa cells express only the myosin VI NI isoform (figure 1B). Since the myosin VI LI-abc and bc isoforms target to clathrin coated structures at the plasma membrane, we expressed GFP-myosin VI tail constructs of all three LNCaP LI isoforms (abc, bc and ab) in HeLa cells (figure 1 C). Whereas the myosin VI tail LI-abc or bc isoforms colocalise with AP-2 on clathrin coated structures at the plasma membrane as expected, (figure 1 C, a-c and d-f), the ab isoform is concentrated in the perinuclear region and not at the plasma membrane (figure 1 C, g-i). Since this short version of the LI, missing exon c, is no longer able to target myosin VI to endocytic structures, exon c must play a role in targeting/recruiting myosin VI to clathrin-coated structures at the plasma membrane.
Fig. 1. Four different myosin VI splice isoforms are expressed in LNCaP cells.
(A) Schematic diagram of myosin VI splice isoforms. The tail domain contains two splice insertions: a large insert with maximal three exons (a, b and c) and a small insert with a single exon. (B) myosin VI isoforms expressed in LNCaP, PNT1A or HeLa cells were identified by RT-PCR using primers flanking the large insert and run on a 1.5% agarose gel. Whereas HeLa cells and PNT1A cells express only the no insert (NI) isoform of myosin VI, LNCaP cells express four different isoforms of myosin VI, the NI form and three splice variants of the large insert (LI) abc, ab and bc. (C) The LI-ab splice variant does not target to CCS at the plasma membrane. To analyse the targeting of the three splice variants of the large insert to clathrin coated structures, the GFP-tagged myosin VI tails containing either the LI-abc (a), the LI-bc (d) or the LI-ab (g) were expressed in HeLa cells and processed for immunofluorescence microscopy using anti-GFP and anti-AP2 antibodies. As shown in the merged images the tail LI-abc (a-c) and LI-bc (d-f) isoforms are recruited to clathrin coated structures at the plasma membrane whereas the tail LI-ab (g-i) isoform is present on perinuclear vesicles and shows very little colocalisation with AP2 in CCS.
The subcellular localisation of myosin VI in LNCaP cells
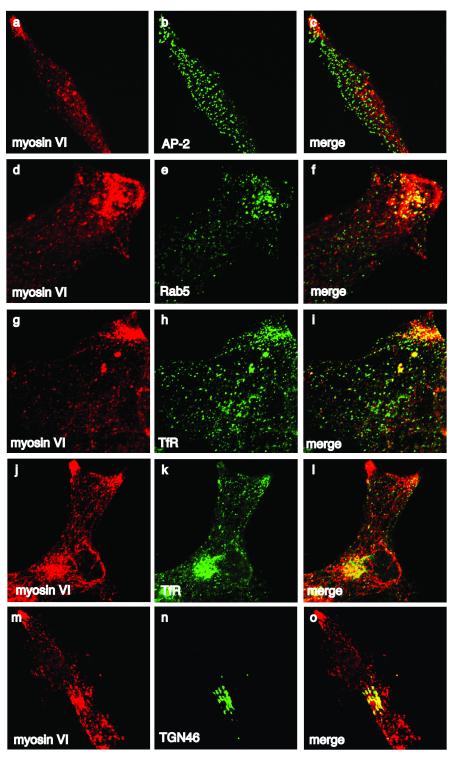
In LNCaP cells over-expression of endogeneous myosin VI causes it to accumulate in the cytosol and since not bound to cargo or target membranes, it could be removed by pre-permeabilising the cells with saponin prior to fixation (Morriswood et al., 2007). Under these conditions myosin VI was observed in a vesicular staining pattern throughout the cell, with high concentrations in cell extensions and in the perinuclear area (figure 2). To characterise the intracellular distribution of myosin VI we performed double labelling experiments with antibodies to marker proteins for the different subcellular compartments. Although LNCaP cells express myosin VI isoforms containing the LI-abc and bc inserts, no endogenous myosin VI was detected in AP-2 positive clathrin-coated structures at the plasma membrane (figure 2, a-c) due to the lack of Dab2 (see figure 4). The myosin VI in cell extensions in the cell periphery colocalises with Rab5 (figure 2, d-f) and the transferrin receptor (TfR) (figure 2, g-l), indicating that in LNCaP cells myosin VI is recruited to early endosomes and is also concentrated in the perinuclear region, where it colocalises with TGN46, a marker of the trans Golgi network (figure 2, m-o), and with TfR, which is present in the endocytic recycling compartment (figure 2, j-l). A similar juxtanuclear concentration of myosin VI was recently observed in surgical prostate cancer tissues (Wei et al., 2008). In the non-malignant prostate cell line PNT1A myosin VI colocalises with TfR and Rab5-positive early endocytic compartment in the cell periphery, however, less myosin VI is present in the perinuclear region at/around the Golgi complex. PTN1A cells do not express the myosin VI LI isoform and therefore although Dab2 is present, very little colocalisation with AP2 in clathrin-coated structures at the plasma membrane was detected (supplementary figure 1).
Fig. 2. Intracellular localisation of myosin VI in LNCaP cells.
The endogenous myosin VI was detected with a polyclonal antibody to myosin VI (a, d, g, j, and m) and double labelled with a monoclonal antibody to AP2 (b), Rab5 (e), TfR (h and k) and TGN46 (n) in immunofluorescence. The merged images are shown in (c, f, i, l and o). Myosin VI is present in peripheral Rab5 and TfR positive endosomes and overlapps with the localisation of TfR and TGN46 in the perinuclear region. No myosin VI is colocalising with AP2 in clathrin-coated structures at the plasma membrane.
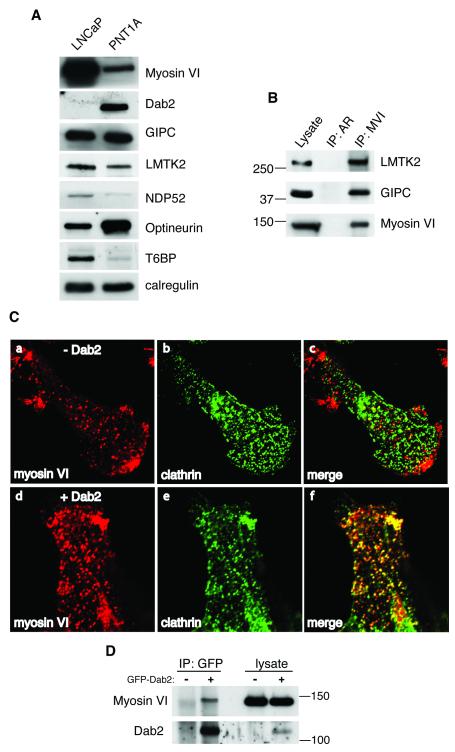
Fig. 4. Myosin VI can be co-immunoprecipitated from LNCaP cells together with GIPC and LMTK2.
(A) The expression of various myosin VI binding proteins was compared between normal prostate cells (PNT1A) and LNCaP cells by Western blotting. Calreticulin was used as a loading control. The expression of Dab2 is completely lost in LNCaP cells, whereas the expression of optineurin is downregulated. (B) Co-immunoprecipitation of myosin VI with LMTK2 or GIPC from LNCaP cells. Myosin VI was immunoprecipitated using polyclonal antibodies against the myosin VI tail. A control immunoprecipitation was performed with polyclonal antibodies to the androgen receptor (AR). The lysate lane contains about 5 % (GIPC and LMTK2) or 1 % (myosin VI) of the input used for each immunoprecipitation. The immunoprecipitated protein complexes were blotted with antibodies against LMTK2, GIPC or myosin VI. A quarter of the immunoprecipitated myosin VI is shown on the blot. (C) Expression of Dab2 in LNCaP cells rescues recruitment of myosin VI to clathrin coated structures at the plasma membrane. Untransfected LNCaP cells (a) (−Dab2) or LNCaP cells transfected with full-length Dab2 (d) (+Dab2) were processed for immunofluorescence microscopy and labelled with anti myosin VI (a and d) and anti clathrin (b and e) antibodies. In cells transfected with the Dab2 construct a dramatic colocalisation between endogenous myosin VI (d) and clathrin (e) can be observed in the merged image (f). (D) Endogenous myosin VI coimmunoprecipitates with GFP-Dab2 from LNCaP cells. GFP-Dab2 was transiently expressed in LNCaP cells and immunoprecipitated using polyclonal GFP antibodies. The immunoprecipitated complexes were blotted with antibodies against myosin VI or Dab2.
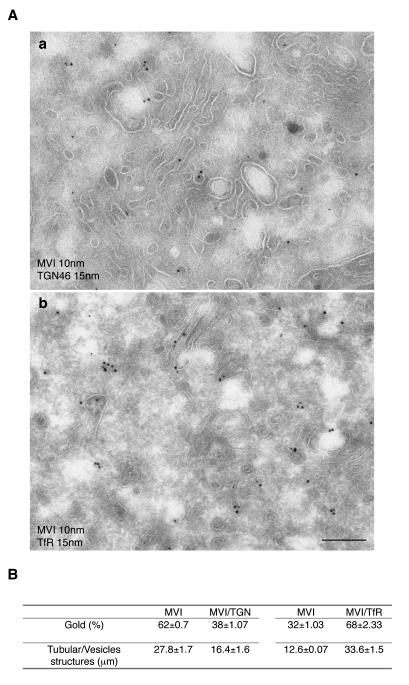
The precise localisation of myosin VI in the perinuclear region was investigated at the ultrastructural level. Cryosections of LNCaP cells double labelled with antibodies to myosin VI and TfR or TGN46 demonstrate that myosin VI is associated with vesicular and tubular membranes at the Golgi complex, showing partial colocalisation with TGN 46 (a) and TfR (b) (figure 3 A). A morphometric analysis was performed to quantify the colocalisation of myosin VI with TfR or with TGN46 and determine whether myosin VI is recruited to the perinuclear endocytic recycling compartment (TfR) or to the trans-Golgi network (TGN46). In each double labelling experiment 100 myosin VI associated gold particles present on a vesicle or tubule were analysed for colocalisation with TfR or TGN46 on the same membrane structure. The same length of membrane of about 45 μm was scored in each experiment. Figure 3 B shows that 68 out of 100 myosin VI molecules are on a compartment that also contains TfR, whereas only 38 molecules out of 100 myosin VI molecules share a membrane with TGN46, indicating that most of the myosin VI in the perinuclear region is present in the TfR-positive endocytic recycling compartment and less is associated with the trans-Golgi network.
Fig. 3. Myosin VI concentrates in perinuclear recycling endosomes in LNCaP cells.
To analyse the relative distribution of myosin VI between the recycling endosome and the TGN, cryosections of LNCaP cells were double labelled with antibodies against endogenous myosin VI (10 nm gold) and TGN46 (15 nm gold) or with antibodies against myosin VI (10 nm gold) and TfR (15 nm gold). Myosin VI and TGN46 (a) or myosin VI and TfR (b) colocalise on tubular vesicular membranes near a stack of membranes, the Golgi complex. Bar: 300 nm. (B) The relative amounts of myosin VI associated with either the recycling endosome or the TGN compartment were quantified on pictures of the Golgi area at 64K magnification. In both experiments 100 myosin molecules were assessed for colocalisation with either TGN46 or TfR on the same length of membrane (about 45 μm each). To measure the length of the membrane the number of intersections with the Photoshop 1cm grid lines were counted (Rabouille et al JCB 1995). Whereas 68 out of 100 myosin VI molecules can be found on a membrane compartment also containing the TfR, only 38 out of 100 myosin VI molecules share a membrane with TGN46.
Binding partners of myosin VI in LNCaP cells
Since a number of binding partners have been described previously that link myosin VI to specific functions in the endocytic and secretory pathway (Buss & Kendrick-Jones, 2008), we compared their expression levels in PNT1A and LNCaP cells by Western blotting (figure 4 A). Myosin VI is highly expressed in LNCaP cells, whereas most binding partners show very little change in expression between normal and malignant prostate cells, Dab2 expression however is completely absent in LNCaP cells. Dab2 is a tumour suppressor gene that is dramatically down regulated in breast, prostate and ovarian cancers (Schwahn & Medina, 1998) (Fazili et al., 1999) (Tseng et al., 1998). Its absence in LNCaP cells may explain why myosin VI is missing from clathrin-coated structures at the plasma membrane although the myosin VI LI-abc and bc isoforms are expressed (Morris et al., 2002; Spudich et al., 2007). To test this suggestion, we re-expressed Dab2 in LNCaP cells and monitored the changes in myosin VI localisation (figure 4 C). In untransfected cells myosin VI shows very little overlap with clathrin (figure 4 C, a-c), whereas in cells expressing Dab2 endogenous myosin VI is now present in clathrin-coated structures at the plasma membrane (figure 4 C, d-f). The expressed Dab2 binds to endogenous myosin VI in LNCaP cells and both proteins can be coimmunoprecipitated (figure 4 D). Thus in LNCaP cells absence of Dab2 blocks myosin VI association with endocytic structures at the plasma membrane and allows this myosin VI pool to be available to function in other subcellular compartments.
Of all the binding partners expressed in LNCaP cells, only GIPC and LMTK2 bind to and coimmunoprecipitate with myosin VI (figure 4 B). This suggests that in LNCaP cells GIPC is involved in recruiting myosin VI to the peripheral Rab5-positive early endosomes and LMTK2 may link myosin VI to the perinuclear recycling compartment (see figures 2 and 3). Although myosin VI is also present at the trans-Golgi network in LNCaP cells (figure 3) and we previously showed that optineurin links myosin VI to the Golgi complex, no optineurin coimmunoprecipitates with myosin VI from LNCaP cells (data not shown).
Clathrin-mediated endocytosis is not compromised in LNCaP cells
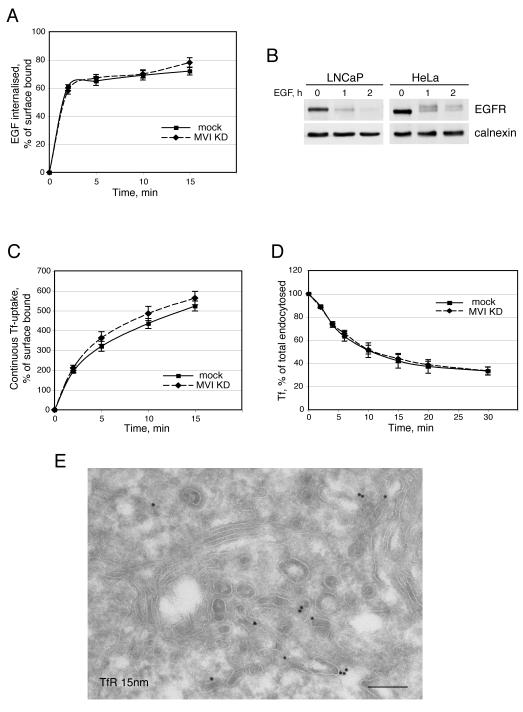
In LNCaP cells, since Dab2 is absent, the myosin VI LI-abc and LI-bc isoforms are not targeted to clathrin-coated structures at the plasma membrane. To determine whether this causes defects in cellular uptake of cell surface receptors, we analysed TfR and EGFR trafficking in LNCaP cells. First we assessed clathrin-dependent and ligand-induced EGFR uptake and sorting from endosomes to lysosomes by measuring EGFR endocytosis and EGFR degradation. No dramatic difference in the rate of EGFR uptake was observed in LNCaP compared to myosin VI KD LNCaP cells (figure 5 A). Furthermore, LNCaP cells showed no delay in EGFR degradation by Western blotting compared to HeLa cells, indicating that endocytosis and sorting of the EGFR from endosomes to late endosomes/lysosomes is intact (figure 5B). We next analysed the uptake and recycling of the TfR. Measuring continuous endocytosis of Tf-Alexa647 by FACS analysis we observed a very similar rate of Tf-Alexa647 uptake in LNCaP cells compared to myosin VI KD LNCaP cells, suggesting that over expression of myosin VI does not lead to a defect in clathrin mediated endocytosis (figure 5 C). To assess the endocytic recycling pathway, the cells were allowed to internalize TfAlexa-647 for 30 min at 37°C, before a chase with unlabelled Tf for varying times. The amount of Tf-Alexa647 remaining in the cell was quantified by FACS analysis. Figure 5 D indicates that the rate of Tf recycling is very similar in LNCaP and HeLa cells and there is no significant increase or delay in Tf recycling in LNCaP cells. Interestingly in the EM the internalized TfR is localised in vesicular/tubular structures in the perinuclar region close to the Golgi stack (figure 5 E). Thus the absence of Dab2 and myosin VI from CCS at the plasma membrane has no apparent effect on the rate of TfR uptake in LNCaP cells.
Fig. 5. Endocytosis and trafficking of TfR and EGFR in LNCaP cells.
(A) To assess EGFR endocytosis, the internalisation of fluorescently labelled EGF was measured. LNCaP and myosin VI KD LNCaP cells were allowed to bind to Alexa-647-EGF for 1 h at 4°C and then were shifted to 37°C for the indicated length of time, after washing to remove unbound EGF. The amount of endocytosed EGF was determined by FACS analysis. (B) To measure ligand induced EGFR degradation in LNCaP cells and HeLa cells, the cells were serum starved over night, incubated in medium with 100 μg/ml cycloheximide for 2 h before stimulation with 100 ng/ml EGF for 0, 1 or 2 h. The cell lysates were blotted with antibodies to EGFR or calnexin as a loading control. (C) To measure the amount of internalised transferrin, LNCaP and myosin VI KD LNCaP cells were incubated for 30 min on ice in medium with 50 μg/ml Tf-Alexa647 before warming up to 37°C for different times in the continous presence of Tf-Alexa647. The amount of endocytosed transferrin was determined by FACS analysis. (D) To assess the recycling pathway LNCaP and myosin VI KD LNCaP cells were allowed to internalise Tf-Alexa647 for 30 min at 37°C. After washing the cells were incubated at 37°C for various times in the presence of 100 μg/ml unlabelled transferrin. The amount of Tf-Alexa647 remaining in the cell was measured by FACS analysis. (E) Immuno-electron microscopy showing the steady state distribution of the TfR in the recycling compartment in the perinuclear region of the cell. Bar: 225 nm.
We also investigated, whether myosin VI is involved in androgen receptor translocation into the nucleus, since myosin VI is specifically over expressed in androgen dependent prostate cancer (Dunn et al., 2006). However, no defects in androgen receptor recruitment into the nucleus were observed in myosin VI KD cells or in cells expressing the dominant negative myosin VI tail domain (supplementary figure 2).
Myosin VI plays a role in PSA and VEGF secretion in LNCaP cells
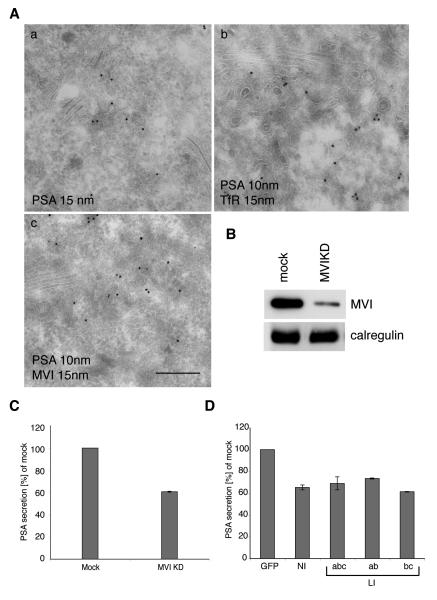
In cells derived from the myosin VI knock out mouse (Snell’s waltzer), or siRNA knock down cells or cells over-expressing dominant negative myosin VI tail mutants we showed that myosin VI is required for the steady state organisation of the Golgi complex, for post-Golgi membrane traffic and for sorting of newly synthesized transmembrane proteins via the recycling endosome to the basolateral domain in polarised epithelial cells (Au, 2007; Chibalina et al., 2007; Warner et al., 2003). Since in LNCaP cells myosin VI is present on tubular/vesicular structures that appear to be part of the trans Golgi network or the perinuclear recycling endosome, we tested whether myosin VI functions in the secretion of the prostate-specific antigen (PSA), a serine protease and member of the kallikrein protein family that is secreted by normal and malignant prostatic epithelial cells and is widely used as a serum prostate cancer biomarker (Balk et al., 2003). In LNCaP cells PSA is present in tubular/vesiclular structures in the perinuclear region (Fig. 6 A,a), where it colocalises with TfR (Fig. 6 A, b) and myosin VI (fig. 6 A, c). The localisation of myosin VI and PSA in TfR-positive recycling endosomes (figure 3 and figure 6) suggests that myosin VI may regulate the delivery of PSA to the plasma membrane possibly at the level of the recycling endosome. To test this hypothesis we measured PSA secretion in LNCaP cells transfected with myosin VI siRNA to knock down myosin VI expression. We achieved a dramatic reduction in myosin VI expression in LNCaP cells and observed a 40% reduction in PSA secretion. Similar results were obtained by over-expressing the different dominant negative myosin VI tail isoforms in LNCaP cells, where there was also a 30 – 40 % reduction in PSA secretion (Fig. 6 D). These results indicate that all four myosin VI isoforms may play a role in protein secretion in LNCaP cells.
Fig. 6. Myosin VI modulates PSA secretion in LNCaP cells.
(A) Immuno-EM showing the localisation of PSA in the perinuclear region in LNCaP cells. Cryosections of LNCaP cells were either labelled with antibodies against endogenous PSA (a) (15 nm gold), with antibodies against PSA (10 nm gold) and the TfR (15 nm gold) (b) or with antibodies to PSA (10 nm gold) and to myosin VI (15 nm gold) (c). PSA colocalises with TfR (b) and myosin VI (c) on tubular/vesicular membranes in the perinuclear region close to the Golgi complex. Bar: 340 nm. (B) To reduce expression of myosin VI in LNCaP cells, the cells were transfected twice with siRNA targeting myosin VI or with non-specific control siRNA (mock). Two days after the second transfection myosin VI depletion was assessed by immunoblotting with antibodies to myosin VI and to calregulin as a loading control. (C) The medium of mock transfected and myosin VI siRNA knock down cells was collected and the amount of secreted PSA was measured using a commercially available ELISA kit. (D) To assess the role of different myosin VI isoforms for PSA secretion, LNCaP cells were transfected with control pEGFP vector or with the different dominant negative tail isoforms of myosin VI: GFP-tail NI, GFP-tail LI-abc, GFP-tail LI-ab or GFP-tail LI-bc. Transfected cells expressing GFP-tagged tails were isolated by FACS sorting and grown for 48 h before the PSA concentration in the medium was determined by ELISA.
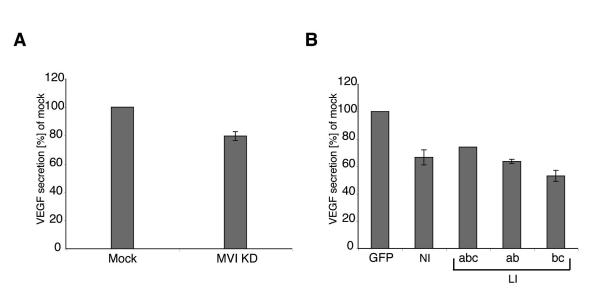
Finally we determined whether loss of myosin VI only affects PSA secretion or whether it also affects secretion of the vascular endothelial growth factor (VEGF), which is an important factor released by many tumours that stimulates vascularisation and tumour angiogenesis (Ellis & Hicklin, 2008). Loss of functional myosin VI leads to a reduction in VEGF secretion ranging from 20 % in siRNA KD cells to a maximal 40% in LNCaP cells over-expressing the dominant negative myosin VI tails (Fig. 7 A and B). In summary our results indicate that the over-expressed myosin VI in LNCaP cells enhances exocytosis of PSA and VEGF.
Fig. 7. Inhibition of myosin VI function reduces VEGF secretion in LNCaP cells.
(A) The expression of myosin VI in LNCaP cells was reduced by siRNAs targeting myosin VI. Mock transfections were performed with non-specific control siRNAs. Two days after the second transfection VEGF secretion was measured using ELISA. (B) To measure the role of different myosin VI isoforms for VEGF secretion, LNCaP cells were transfected with control pEGFP vector or with the different dominant negative tail isoforms of myosin VI: GFP-tail NI, GFP-tail LI-abc, GFP-tail LI-ab or GFP-tail LI-bc. Transfected cells expressing GFP-tagged tails were isolated by FACS sorting and grown for 48 h before the VEGF concentration in the medium was determined by ELISA.
We next analysed whether any factors secreted into conditioned medium by LNCaP cells compared to LNCaP myosin VI KD cells change the viability of prostate cells (supplementary figure 3 A). We observed no significant change in the viability of the prostate cell line PNT1A when grown in conditioned medium harvested from LNCaP myosin VI KD cells compared to medium from LNCaP cells (supplementary figure 3A). Therefore, the total amount of proteins that is secreted into protein-free conditioned cell culture medium, is not affected by the expression levels of myosin VI in mock transfected or myosin VI KD LNCaP cells (supplementary figure 3B), clearly indicating that myosin VI selectively enhances exocytosis of only a limited number of secreted factors.
Discussion
In LNCaP cells, where myosin VI is dramatically over-expressed, we observed that it is involved in secretion of the prostate specific marker PSA and the endothelial growth factor VEGF. Down regulation of myosin VI expression in LNCaP cells by siRNA mediated knock-down, reduces secretion of PSA and VEGF by about 40 % indicating that myosin VI over-expression in prostate but also probably in other human cancer tissues (Dunn et al., 2006; Yoshida et al., 2004) may be linked to elevated protein secretion. These cancer tissues are derived from epithelial cells, which are specialised secretory cells that line ducts and cavities in tissues. At present it is unclear why myosin VI is specifically over-expressed in these secretory adenocarcinomas and how exactly up-regulation of secretion generates a malignant tissue.
PSA is the most widely used biomarker for prostate cancer detection and there is now increasing evidence that it has a pathophysiological role in prostate cancer biogenesis (Whitbread et al., 2006). It has been demonstrated that secreted PSA can increase cellular migration in two ways by either promoting epithelial-mesenchymal cell transition (Lawrence et al., 2007; Veveris-Lowe et al., 2005) or by cleaving extracellular matrix proteins (Borgono & Diamandis, 2004; Clements et al., 2004), which could propagate the invasiveness of prostate carcinoma cells. It is, therefore, conceivable that the up-regulation of PSA secretion, caused by the over-expression of myosin VI, may support prostate cancer growth. Similarly VEGF is a key angiogenic factor that plays several roles in the complex process of blood vessel formation required for tumour growth (Hicklin & Ellis, 2005). VEGF mediates numerous changes within the tumour including the recruitment and cell proliferation of endothelial precursor cells by binding to its own cognate tyrosine kinase receptor on the surface of endothelial cells (Ellis & Hicklin, 2008). Therefore an increase in VEGF secretion in cells over-expressing myosin VI could be a significant factor promoting tumour growth.
Although myosin VI could be involved in endocytosis, exocytosis and/or cell migration in LNCaP cells we clearly demonstrate that myosin VI has an important function in protein secretion. In NRK cells we observed that myosin VI’s role in secretion was mediated by interaction with optineurin, which links it to the Golgi complex (Sahlender et al., 2005). In LNCaP cells, however, very little optineurin binds to and can be co-immunoprecipitated with myosin VI and in these cells far less myosin VI is associated with the trans-Golgi network and more is present at the perinuclear recycling endosome. In polarised epithelial cells in addition to the Golgi complex the recycling endosome represents an important sorting station in the biosynthetic secretory pathway for the delivery of proteins to the plasma membrane (Ang et al., 2004). We recently showed that myosin VI is present at the recycling endosome in a polarised cell line derived from canine kidney (MDCK cells) (Au, 2007) and since LNCaP cells are derived from prostate epithelial cells they may have maintained this specialised delivery route via the recycling endosome to the plasma membrane.
Our observation that out of the different myosin VI binding partners that are expressed in LNCaP cells, only GIPC and LMTK2 co-immunoprecipitate with myosin VI is extremely important in the light of the recent observation that LMTK2 is a new candidate susceptibility gene for prostate cancer (Eeles et al., 2008). We previously demonstrated that myosin VI and LMTK2 function in the Rab11-positive recycling endosome in HeLa cells (Chibalina et al., 2007) and it is likely that both proteins have similar functions in LNCaP cells. LMTK2 appears to act as a transmembrane protein to recruit myosin VI to the surface of endosomes. The evidence that in LNCaP cells myosin VI interacts with LMTK2, and both proteins play a role at the recycling endosome and are linked to prostate cancer progression highlights the importance of the secretory pathway via the recycling endosome for prostate cancer pathology.
The targeting of myosin VI to CCS at the plasma membrane requires myosin VI binding to Dab2 (Spudich et al., 2007) (Morris et al., 2002). However in many prostate, breast and ovarian cancer cell lines that up-regulate myosin VI expression, the expression of the tumour suppressor protein Dab2 has been lost. At present we do not know whether in these cell lines loss of Dab2 and up-regulation of myosin are linked. The absence of Dab2 from LNCaP cells ensures that the myosin VI isoforms LI-abc and LI-bc, which are normally recruited by Dab2 to CCS, are available for other intracellular function, such as protein secretion. Indeed our results show (see figure 6 and 7) that over- expression of not only the dominant negative NI tail but also the LI-abc, LI-bc and LI-ab tail isoforms are able to inhibit secretion of PSA and VEGF from LNCaP cells, suggesting that all the different myosin VI isoforms play equal roles in protein secretion. Interestingly in LNCaP cells the absence of myosin VI in CCS does not affect clathrin-dependent endocytosis of the TfR or the EGFR, indicating that in these prostate cancer cells, which have lost apical-basolateral polarity, myosin VI is no longer required for clathrin mediated endocytosis from the apical domain (Ameen & Apodaca, 2007). The transition from apical-basolateral to planar polarity is required for the increase in cell motility that underlies the invasive dissemination of cancer cells. In prostate and ovarian cancer cells knock down of myosin VI has been shown to reduce cell migration and dissemination (Yoshida et al., 2004). Thus although the molecular mechanism of myosin VI function in cancer cell migration remains to be established, increased protein secretion is very likely to play a role in enhancing cell motility.
Materials and methods
Antibodies, cell lines and plasmids
Antibodies used: GFP (Molecular Probes), AP2 (AP6, ABR), Rab5 (BD Transduction Laboratories), TfR (Zymed), TGN46 (Serotec), Dab2, androgen receptor, calregulin, calnexin, EGFR (all from Santa Cruz), anti-GIPC (Proteus Biosciences), clathrin (Abcam), PSA (Sigma), myosin VI tail (Buss et al., 1998), T6BP and NDP52 (Morriswood et al., 2007), optineurin (Sahlender et al., 2005) and LMTK2 (Chibalina et al., 2007). LNCaP, HeLa and PNT1A cells from the European Collection of Cell Cultures. Dab2-GFP (Morris et al) and GFP-myosin VI tails with (LI, exones abc) and without (NI) the large insert were described previously (Spudich et al, 2007). Myosin VI tail-LI constructs containing ab and bc exones were amplified by PCR from LNCaP cDNA and cloned into pEGFPC3 (Clontech). Expression of myosin VI isoforms in HeLa cells, normal prostate (PNT1A) and prostate cancer (LNCaP) cells was assessed by RT-PCR (Buss et al., 2001) (Au, 2007).
Cell culture and transfection
LNCaP cells grown in RPMI 1640 medium containing 10% fetal calf serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 10 mM Hepes and 1 mM sodium pyruvate were transfected using Lipofectamine 2000 (Invitrogen). Cells expressing GFP-tagged proteins were isolated by FACS sorting.
Knock down of myosin VI by siRNA
For efficient siRNA knock down of myosin VI in LNCaP cells, Lipofectamine 2000 (Invitrogen) and ON-TARGET plus Smart pool siRNAs (Dharmacon) were used. The cells were transfected twice with siRNA on day 1 and 3 and on day 5 the efficiency of protein depletion assessed by immunoblotting.
Immuofluorescence microscopy
LNCaP and HeLa cells were grown on coverslips, transfected and after 24h processed for immunofluorescence (Buss et al., 2001). To remove cytosolic proteins, LNCaP and HeLa cells were first permeabilized with 0.05% saponin in cytosol buffer (Morris & Cooper, 2001) before fixation with 4% formaldehyde. Cells were visualized and photographed using a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging Inc.)
Immuno-EM and morphometric analysis
LNCaP and PNT1A cells in growing conditions were fixed in a mixture of 2% paraformaldheyde and 0.2% glutaraldehyde or 2% paraformaldehyde and 1% acrolein in PBS buffer and processed for ultra thin cryosectioning (Puri et al., 2005)
Immunoblotting and immunoprecipitation
LNCaP cells were lysed in 50 mM Tris pH 7.5, 100 mM NaCl, 20 nM NaF, 20 mM Na4P2O7, 5 mM MgCl2, 5 mM ATP, 1 mM activated Na-o-vanadate, 1% Igepal CA-630 and protease inhibitor cocktail (Roche). Myosin VI was immunoprecipitated from the cleared lysate (15 min at 13,000xg) using polyclonal tail antibodies (Buss et al., 1998) (Chibalina et al., 2007) and the immunoprecipitated complexes analysed by SDS-PAGE and immunoblotting. For co-immunoprecipitation of myosin VI and GFP-Dab2, LNCaP cells were transfected with GFP-Dab2 construct and 24 hours later FACS-sorted into GFP-positive and GFP-negative populations. GFP-Dab2 was immunoprecipitated using GFP antibody; immunoprecipitation from GFP-negative cells was performed as control.
FACS-based endocytosis assays
Endocytic uptake and recycling assays in HeLa and LNCaP cells were as described (Peden et al., 2004) (Chibalina et al., 2007). For transferrin uptake, the cells were trypsinized and incubated in medium with 50 μg/ml Alexa-647-Tf for 30 min at 4°C. After shifting the temperature to 37°C for set times, transferrin internalisation was stopped by placing the samples on ice for 10 min. For recycling assay, cells were incubated with Alexa-647-Tf for 30 min at 37°C, washed and incubated at 37°C with 100 μg/ml unlabelled tranferrin for various times before fixing in 4% PFA.
For EGF internalisation, the cells were serum starved for 18h, detached with non-enzymatic cell dissociation solution and incubated in serum free medium containing 0.5 μg/ml Alexa-647-EGF for 1h, then washed in serum-free medium to remove unbound EGF. After shifting the temperature to 37°C for set times, internalisation was stopped by placing cells on ice. Cell surface bound EGF was stripped with 0.5 M acetic acid/0.5 M NaCl and the cells fixed with 4% PFA. Cell associated Alexa-647-Tf and Alexa-647-EGF were determined using FACSCalibur flow cytometer (BD Biosciences).
EGFR degradation
LNCaP cells and HeLa cells were serum starved overnight, incubated in medium with 100 μg/ml cycloheximide for 2 h before stimulation with 100 ng/ml EGF for 0, 1 or 2 h. The cell lysates were blotted with antibodies to EGFR.
PSA/VEGF secretion assay
LNCaP cells were transfected with myosin VI siRNA or dominant negative myosin VI tails and after 72 h the amounts of PSA and VEGF in medium measured by ELISA according to manufacturer’s instructions (MP Biomedicals and R&D Systems). The cell number was quantified using Cell Titer-Blue® dye (Promega) and EnVision HTS microplate Reader using a 405 filter.
Supplementary Material
Supplementary figure 1. Intracellular localisation of myosin VI in PNT1A cells. (A) To visualise the localisation of the endogenous no insert isoform of myosin VI in normal prostate cells in immunofluorescence, PTN1A cells were double labelled with a polyclonal antibody to myosin VI (a, d and g) and with a monoclonal antibody to AP2 (b), Rab5 (e) and TfR (h). The merged images are shown in (c, f and i). Myosin VI colocalises with Rab5 and TfR in peripheral endosomes. However, very little of the endogenous no insert-myosin VI colocalises with AP2 in clathrin-coated structures at the plasma membrane.
(B) Cryosections of PNT1A cells were double labelled with antibodies against endogenous myosin VI (10 nm gold) and TfR (15 nm gold) or with antibodies against myosin VI (10 nm gold) and TGN46 (15 nm gold). Myosin VI and TfR (a) or myosin VI and TGN46 (c) colocalise on tubular vesicular membranes close to the Golgi complex. In addition in PTN1A cells myosin VI and TfR are also present on tubular vesicular structures close to the plasma membrane, which are most likely peripheral early endosomes (b). Bar: 300 nm.
Supplementary figure 2. Myosin VI is not required for androgen receptor translocation into the nucleus Myosin VI is specifically over expressed in androgen-dependent prostate cancers and prostate cancer cell lines, which express the androgen receptor (AR). To further analyse the link between myosin VI and the AR, we tested whether myosin VI is involved in AR translocation into the nucleus, since the actin cytoskeleton has been shown to facilitate AR shuttling between cytosol and nucleus. To delineate the function of myosin VI in AR translocation, we either over expressed myosin VI or depleted the functional pool of myosin VI in HeLa cells transfected with GFP-tagged AR. (A) HeLa cells either mock treated or depleted of myosin VI by siRNA were transfected with GFP-AR. 16 h after transfection the cells were starved for 2 h in serum-free medium with 0.1 % BSA, before stimulated with 50 nM testosterone (Sigma) (+T) for 1 h at 37 °C to induce nuclear import. Control cells were left unstimulated (−T). After washing the cells were processed for immunofluorescence with anti-GFP antibodies and labelled with DAPI to visualise nuclei. In mock transfected, unstimulated cells, the GFP-tagged AR displayed a uniform distribution in the cytosol and the nucleus, whereas upon testosterone stimulation the AR was completely imported into the nucleus. In myosin VI KD cells no delay in transport of AR receptor was observed compared to mock transfected cells. (B) (C) NI-myosin VI tail (B) or His-tagged full-length NI-myosin VI were expressed together with the GFP-tagged AR in HeLa cells, which were either stimulated with testosterone (+T) or left unstimulated (−T) and processed for immunoflourescence and labelled with anti-myosin VI antibodies (B) or with anti-His antibodies (C) and anti-GFP antibodies as well as stained with DAPI to visualise the nuclei. Overall no significant difference in GFP-AR translocation into the nucleus could be observed between mock treated and myosin VI siRNA KD cells (A) or cells over expressing the dominant negative myosin VI tail (B). Since myosin VI expression is dramatically upregulated in AR-positive LNCaP cells, we decided to simulate this situation by overexpressing the full-length myosin VI in HeLa cells transfected with the GFP-AR. However, upregulation of myosin VI expression in these HeLa cells did not inhibit the translocation of the AR from the cytosol into the nucleus (C). These results indicate that although in our HeLa cell model we can clearly observe a testosterone-dependent import of the AR into the nucleus, myosin VI does not seem to be involved in this process and therefore is unlikely to play a role in nuclear import of the AR and in androgen-dependent activation of transcription in prostate cancer cells. The construct for expressing GFP-AR was kindly provided by Dr Ian Mills (Cambridge Research Institute, Cambridge, UK).
Supplementary figure 3. Characterisation of LNCaP total protein secretion (A) To further characterise the proteins secreted by LNCaP cells into conditioned medium, normal prostate PNT1A cells were cultured for 3 days in the medium harvested from control or myosin VI KD LNCaP cells. On day 1, 2 and 3 viable cells were stained with Cell Titer-Blue® dye (Promega) and counted using an EnVision HTS microplate reader with a 405 filter. The cell viability graph indicates no significant change in the viability of PTN1A cells when grown in conditioned medium harvested from LNCaP myosin VI KD cells compared to mock treated LNCaP cells.
(B) To compare the total amount of proteins secreted by mock transfected LNCaP or LNCaP myosin VI KD cells, the cells were cultured for 72 h in chemically defined medium (CD CHO, Gibco) (Martin et al., 2004). To analyse proteins in the medium, the supernatant was cleared from cell debris by centrifugation and the protein concentration was determined using a standard BCA proteins assay (Pierce). Proteins in the supernatant were precipitated by TCA and the same amount of conditioned medium relative to cell number was loaded in each lane; a: conditioned medium only, b: conditioned medium harvested from control LNCaP cells (44 μg total protein), c: conditioned medium from myosin VI KD LNCaP cells (42 μg total protein). The SDS-PAGE shows no major difference in the total amount of protein present in the medium of control or myosin VI KD LNCaP cells.
Acknowledgements
We thank A. Peden for help with transferrin uptake assays and J. P. Luzio for helpful discussions. This work was funded by a Cancer Research UK project grant (C.P.), a Wellcome Trust Senior Fellowship (to F.B.), a Wellcome Trust PhD studentship (A.J.K.) and was supported by the Medical Research Council. CIMR is in receipt of a strategic award from the Wellcome Trust.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- Ameen N, Apodaca G. Traffic. 2007;8:998–1006. doi: 10.1111/j.1600-0854.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. J Cell Biol. 2004;167:531–43. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner L, Lee T, Hasson T. Mol Biol Cell. 2003;14:2728–43. doi: 10.1091/mbc.E02-11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. J Cell Biol. 2007;177:103–14. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SP, Ko YJ, Bubley GJ. J Clin Oncol. 2003;21:383–91. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- Borgono CA, Diamandis EP. Nat Rev Cancer. 2004;4:876–90. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- Bunn RC, Jensen MA, Reed BC. Mol Biol Cell. 1999;10:819–32. doi: 10.1091/mbc.10.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J. Embo J. 2001;20:3676–84. doi: 10.1093/emboj/20.14.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Kendrick-Jones J. Biochem Biophys Res Commun. 2008;369:165–75. doi: 10.1016/j.bbrc.2007.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Kendrick-Jones J, Lionne C, Knight AE, Cote GP, Paul Luzio J. J Cell Biol. 1998;143:1535–45. doi: 10.1083/jcb.143.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalina MV, Seaman MN, Miller CC, Kendrick-Jones J, Buss F. J Cell Sci. 2007;120:4278–88. doi: 10.1242/jcs.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JA, Willemsen NM, Myers SA, Dong Y. Crit Rev Clin Lab Sci. 2004;41:265–312. doi: 10.1080/10408360490471931. [DOI] [PubMed] [Google Scholar]
- Dance AL, Miller M, Seragaki S, Aryal P, White B, Aschenbrenner L, Hasson T. Traffic. 2004;5:798–813. doi: 10.1111/j.1600-0854.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- Dunn TA, Chen S, Faith DA, Hicks JL, Platz EA, Chen Y, Ewing CM, Sauvageot J, Isaacs WB, De Marzo AM, Luo J. Am J Pathol. 2006;169:1843–54. doi: 10.2353/ajpath.2006.060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF. Nat Genet. 2008;40:316–21. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX. Oncogene. 1999;18:3104–13. doi: 10.1038/sj.onc.1202649. [DOI] [PubMed] [Google Scholar]
- Geisbrecht ER, Montell DJ. Nat Cell Biol. 2002;4:616–20. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- Hasson T, Mooseker MS. J Cell Biol. 1994;127:425–40. doi: 10.1083/jcb.127.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin DJ, Ellis LM. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Kellerman KA, Miller KG. J Cell Biol. 1992;119:823–34. doi: 10.1083/jcb.119.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MG, Veveris-Lowe TL, Whitbread AK, Nicol DL, Clements JA. Cells Tissues Organs. 2007;185:111–5. doi: 10.1159/000101311. [DOI] [PubMed] [Google Scholar]
- Maddugoda MP, Crampton MS, Shewan AM, Yap AS. J Cell Biol. 2007;178:529–40. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DB, Gifford DR, Wright ME, Keller A, Yi E, Goodlett DR, Aebersold R, Nelson PS. Cancer Res. 2004;64:347–55. doi: 10.1158/0008-5472.can-03-2062. [DOI] [PubMed] [Google Scholar]
- Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, Buss F. Traffic. 2002;3:331–41. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Cooper JA. Traffic. 2001;2:111–23. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- Morriswood B, Ryzhakov G, Puri C, Arden SD, Roberts R, Dendrou C, Kendrick-Jones J, Buss F. J Cell Sci. 2007;120:2574–85. doi: 10.1242/jcs.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden AA, Schonteich E, Chun J, Junutula JR, Scheller RH, Prekeris R. Mol Biol Cell. 2004;15:3530–41. doi: 10.1091/mbc.E03-12-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F, Luzzi P, Di Fiore PP, Tacchetti C. Mol Biol Cell. 2005;16:2704–18. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F. J Cell Biol. 2005;169:285–95. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwahn DJ, Medina D. Oncogene. 1998;17:1173–8. doi: 10.1038/sj.onc.1202038. [DOI] [PubMed] [Google Scholar]
- Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J. Nat Cell Biol. 2007;9:176–83. doi: 10.1038/ncb1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H, Schultz PG, Powell SM, Moskaluk CA, Frierson HF, Jr., Hampton GM. Cancer Res. 2001;61:7388–93. [PubMed] [Google Scholar]
- Tseng CP, Ely BD, Li Y, Pong RC, Hsieh JT. Endocrinology. 1998;139:3542–53. doi: 10.1210/endo.139.8.6159. [DOI] [PubMed] [Google Scholar]
- Veveris-Lowe TL, Lawrence MG, Collard RL, Bui L, Herington AC, Nicol DL, Clements JA. Endocr Relat Cancer. 2005;12:631–43. doi: 10.1677/erc.1.00958. [DOI] [PubMed] [Google Scholar]
- Warner CL, Stewart A, Luzio JP, Steel KP, Libby RT, Kendrick-Jones J, Buss F. Embo J. 2003;22:569–79. doi: 10.1093/emboj/cdg055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Dunn TA, Isaacs WB, De Marzo AM, Luo J. Prostate. 2008;68:1387–95. doi: 10.1002/pros.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Nature. 1999;401:505–8. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Whitbread AK, Veveris-Lowe TL, Lawrence MG, Nicol DL, Clements JA. Biol Chem. 2006;387:707–14. doi: 10.1515/BC.2006.089. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Cheng W, Hung J, Montell D, Geisbrecht E, Rosen D, Liu J, Naora H. Proc Natl Acad Sci U S A. 2004;101:8144–9. doi: 10.1073/pnas.0400400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Intracellular localisation of myosin VI in PNT1A cells. (A) To visualise the localisation of the endogenous no insert isoform of myosin VI in normal prostate cells in immunofluorescence, PTN1A cells were double labelled with a polyclonal antibody to myosin VI (a, d and g) and with a monoclonal antibody to AP2 (b), Rab5 (e) and TfR (h). The merged images are shown in (c, f and i). Myosin VI colocalises with Rab5 and TfR in peripheral endosomes. However, very little of the endogenous no insert-myosin VI colocalises with AP2 in clathrin-coated structures at the plasma membrane.
(B) Cryosections of PNT1A cells were double labelled with antibodies against endogenous myosin VI (10 nm gold) and TfR (15 nm gold) or with antibodies against myosin VI (10 nm gold) and TGN46 (15 nm gold). Myosin VI and TfR (a) or myosin VI and TGN46 (c) colocalise on tubular vesicular membranes close to the Golgi complex. In addition in PTN1A cells myosin VI and TfR are also present on tubular vesicular structures close to the plasma membrane, which are most likely peripheral early endosomes (b). Bar: 300 nm.
Supplementary figure 2. Myosin VI is not required for androgen receptor translocation into the nucleus Myosin VI is specifically over expressed in androgen-dependent prostate cancers and prostate cancer cell lines, which express the androgen receptor (AR). To further analyse the link between myosin VI and the AR, we tested whether myosin VI is involved in AR translocation into the nucleus, since the actin cytoskeleton has been shown to facilitate AR shuttling between cytosol and nucleus. To delineate the function of myosin VI in AR translocation, we either over expressed myosin VI or depleted the functional pool of myosin VI in HeLa cells transfected with GFP-tagged AR. (A) HeLa cells either mock treated or depleted of myosin VI by siRNA were transfected with GFP-AR. 16 h after transfection the cells were starved for 2 h in serum-free medium with 0.1 % BSA, before stimulated with 50 nM testosterone (Sigma) (+T) for 1 h at 37 °C to induce nuclear import. Control cells were left unstimulated (−T). After washing the cells were processed for immunofluorescence with anti-GFP antibodies and labelled with DAPI to visualise nuclei. In mock transfected, unstimulated cells, the GFP-tagged AR displayed a uniform distribution in the cytosol and the nucleus, whereas upon testosterone stimulation the AR was completely imported into the nucleus. In myosin VI KD cells no delay in transport of AR receptor was observed compared to mock transfected cells. (B) (C) NI-myosin VI tail (B) or His-tagged full-length NI-myosin VI were expressed together with the GFP-tagged AR in HeLa cells, which were either stimulated with testosterone (+T) or left unstimulated (−T) and processed for immunoflourescence and labelled with anti-myosin VI antibodies (B) or with anti-His antibodies (C) and anti-GFP antibodies as well as stained with DAPI to visualise the nuclei. Overall no significant difference in GFP-AR translocation into the nucleus could be observed between mock treated and myosin VI siRNA KD cells (A) or cells over expressing the dominant negative myosin VI tail (B). Since myosin VI expression is dramatically upregulated in AR-positive LNCaP cells, we decided to simulate this situation by overexpressing the full-length myosin VI in HeLa cells transfected with the GFP-AR. However, upregulation of myosin VI expression in these HeLa cells did not inhibit the translocation of the AR from the cytosol into the nucleus (C). These results indicate that although in our HeLa cell model we can clearly observe a testosterone-dependent import of the AR into the nucleus, myosin VI does not seem to be involved in this process and therefore is unlikely to play a role in nuclear import of the AR and in androgen-dependent activation of transcription in prostate cancer cells. The construct for expressing GFP-AR was kindly provided by Dr Ian Mills (Cambridge Research Institute, Cambridge, UK).
Supplementary figure 3. Characterisation of LNCaP total protein secretion (A) To further characterise the proteins secreted by LNCaP cells into conditioned medium, normal prostate PNT1A cells were cultured for 3 days in the medium harvested from control or myosin VI KD LNCaP cells. On day 1, 2 and 3 viable cells were stained with Cell Titer-Blue® dye (Promega) and counted using an EnVision HTS microplate reader with a 405 filter. The cell viability graph indicates no significant change in the viability of PTN1A cells when grown in conditioned medium harvested from LNCaP myosin VI KD cells compared to mock treated LNCaP cells.
(B) To compare the total amount of proteins secreted by mock transfected LNCaP or LNCaP myosin VI KD cells, the cells were cultured for 72 h in chemically defined medium (CD CHO, Gibco) (Martin et al., 2004). To analyse proteins in the medium, the supernatant was cleared from cell debris by centrifugation and the protein concentration was determined using a standard BCA proteins assay (Pierce). Proteins in the supernatant were precipitated by TCA and the same amount of conditioned medium relative to cell number was loaded in each lane; a: conditioned medium only, b: conditioned medium harvested from control LNCaP cells (44 μg total protein), c: conditioned medium from myosin VI KD LNCaP cells (42 μg total protein). The SDS-PAGE shows no major difference in the total amount of protein present in the medium of control or myosin VI KD LNCaP cells.