Abstract
Objective
To compare the effectiveness of corticosteroid injections with physiotherapy for the treatment of painful stiff shoulder.
Design
Randomised trial.
Setting
40 general practices.
Subjects
109 patients consulting general practitioners for shoulder pain were enrolled in the trial.
Interventions
Patients were randomly allocated to 6 weeks of treatment either with corticosteroid injections (53) or physiotherapy (56).
Main outcome measures
Outcome assessments were carried out 3, 7, 13, 26, and 52 weeks after randomisation; some of the assessments were done by an observer blind to treatment allocation. Primary outcome measures were the success of treatment as measured by scores on scales measuring improvement in the main complaint and pain, and improvement in scores on a scale measuring shoulder disability.
Results
At 7 weeks 40 (77%) out of 52 patients treated with injections were considered to be treatment successes compared with 26 (46%) out of 56 treated with physiotherapy (difference between groups 31%, 95% confidence interval 14% to 48%). The difference in improvement favoured those treated with corticosteroids in nearly all outcome measures; these differences were statistically significant. At 26 and 52 weeks differences between the groups were comparatively small. Adverse reactions were generally mild. However, among women receiving treatment with corticosteroids adverse reactions were more troublesome: facial flushing was reported by 9 women and irregular menstrual bleeding by 6, 2 of whom were postmenopausal.
Conclusions
The beneficial effects of corticosteroid injections administered by general practitioners for treatment of painful stiff shoulder are superior to those of physiotherapy. The differences between the intervention groups were mainly the result of the comparatively faster relief of symptoms that occurred in patients treated with injections. Adverse reactions were generally mild but doctors should be aware of the potential side effects of injections of triamcinolone, particularly in women.
Key messages
There is little evidence supporting the effectiveness of either corticosteroid injections or physiotherapy in painful stiff shoulder
Few studies of the effectiveness of treatments for shoulder pain have been done in a primary care setting even though most patients with shoulder pain are treated there
This randomised trial shows that patients treated with corticosteroid injections are significantly more likely to improve on measures of pain and disability than patients treated with physiotherapy
The differences between those who received injections and those treated with physiotherapy result mainly from comparatively fast relief of symptoms that occurs after injections
Doctors and patients should be aware of mild, but sometimes troublesome, adverse reactions to corticosteroids that may occur
Introduction
Shoulder pain is a common complaint in primary care; estimates of the annual incidence in general practice vary from 6.6 to 25 cases per 1000 patients.1–3 Shoulder conditions that are characterised by a painful restriction of the passive range of motion, particularly of lateral rotation and abduction, are usually referred to as painful stiff shoulder or capsular syndrome.3,4 Despite the fact that in many cases symptoms persist5,6 few patients are referred to a specialist.2,5 In primary care, diagnosis is usually based only on history and physical examination.
Treatment often consists of physiotherapy or local infiltration of a corticosteroid.3 Systematic reviews have shown that the effectiveness of these interventions remains questionable.7–9 Our objective was to compare the effectiveness of corticosteroid injections with physiotherapy on the treatment of painful stiff shoulder in a primary care setting.
Subjects and methods
Subjects
Consecutive patients who consulted one of 60 participating general practitioners were considered for participation. The main inclusion criteria were that patients had a painful restriction of glenohumeral mobility, were age 18 years or older, and gave informed consent. Patients were excluded if they had bilateral symptoms; if they had had treatment with corticosteroid injections or physiotherapy during the preceding six months; if they had contraindications to treatment; if they had had surgery, dislocation, or fractures in the shoulder area; if they had insulin-dependent diabetes mellitus, systemic disorders of the musculoskeletal system, or neurological disorders. Patients who met the selection criteria were referred to the research centre by their general practitioner. The study protocol was approved by the Ethics Committee of the University Hospital of the Vrije Universiteit Amsterdam.
At the research centre an independent observer, who was a trained physiotherapist, confirmed that all selection criteria had been met. The diagnosis of painful stiff shoulder (capsular syndrome) was made using the diagnostic guidelines for shoulder complaints issued by the Dutch College of General Practitioners3,4—that is, passive glenohumeral mobility must be painful and limited, lateral rotation must be relatively more restricted than abduction and medial rotation, and there must be no clear signs (painful arc, positive resistance tests, loss of power) that the shoulder pain was caused by another condition. After enrollment prognostic indicators and baseline values of outcome measures were assessed.
Randomisation
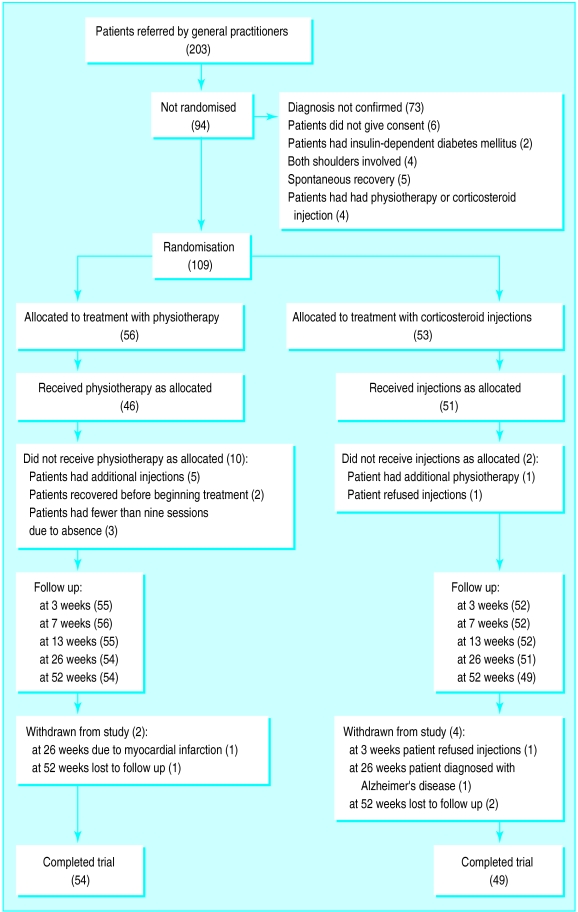
Patients were randomly allocated six weeks of either treatment with injections or physiotherapy (figure). The use of permuted blocks of four patients guaranteed nearly equal distribution of patients between the interventions. The random sequence of the blocks was generated using random number tables. Numbered, opaque, sealed envelopes containing the treatment allocation were prepared before the trial. After selection and baseline assessment an administrative assistant opened the next envelope in the appropriate stratum.
Interventions
Intra-articular injections of 40 mg triamcinolone acetonide were given by the general practitioners using the posterior route.10 Nearly all of the general practitioners had attended training in this technique before the study, although most had had previous experience with the technique. No more than three injections were given during the six weeks.
Physiotherapy consisted of 12 sessions of 30 minutes during which all patients received passive joint mobilisation and exercise treatment. Ice, hot packs, or electrotherapy could be used to reduce pain. Acupuncture and high velocity thrust manipulations were not allowed under the protocol. Ultrasound treatment was not used because it was not considered to be effective for this disorder. Treatment could be adjusted according to the severity of symptoms. Physiotherapists and general practitioners recorded details of treatment on standardised forms which included spaces for documenting deviations from protocol and adverse reactions. Adverse reactions were also recorded by patients on their own forms.
Patients were allowed to continue taking drugs for pain if they had started before enrollment; drugs could also be prescribed if pain was severe. All other interventions were to be avoided during the study.
Outcome assessment
The outcome of the intervention was assessed at 3 and 7 weeks. Additional follow up assessments were scheduled for 13, 26, and 52 weeks. The assessments at 13 and 52 weeks were by postal questionnaire only but contained all primary outcome measures.
Primary outcome measures
Patients were asked to score their improvement on a six point Likert scale. For the analysis of success rates for each treatment patients who rated themselves as having made a complete recovery or as having much improvement were counted as successes. Patients were asked to score the pain associated with their main complaint and the severity of their pain during the day and at night on a 100 mm visual analog scale; the score of 100 indicates very severe pain.11 Functional disability was evaluated with the shoulder disability questionnaire, a 16 item scale consisting of common situations that might cause shoulder pain.12,13 Scores on the questionnaire range from 0 to 100; 100 indicates severe disability.
Secondary outcome measures
After a standardised physical examination the independent observer scored the overall clinical severity of the disorder on a visual analog scale. Using the healthy shoulder as a reference, the observer measured the restriction of mobility during passive lateral rotation and glenohumeral abduction with a digital inclinometer (EDI-320, Cybex, Ronkonkoma, New York).14
Blinding
The independent observer did not know to which intervention a patient had been allocated. To optimise blinding the patient was instructed by the administrative assistant not to reveal any information about their treatment. In all patients the actual or potential injection site was covered with gauze. Immediately after each examination the observer was asked to guess to which intervention the patient had been assigned.
Statistical analysis
The changes in scores of symptoms over time were calculated for each patient by subtracting the results at baseline from those at follow up. The differences in the changes in symptom scores between the two groups were computed with 95% confidence intervals. The principal analysis was performed on an intention to treat basis. In an alternative analysis all patients who had not been treated according to protocol during the intervention period were excluded; these were cases of non-compliance with treatment and violation of protocols. Statistical analysis of the differences in improvement between the groups over time was done using a multivariate analysis of variance (repeated measurements design); this analysis included the results of outcome assessments at each follow up (at baseline, 3, 7, 13, 26, and 52 weeks).15
Calculations of sample size were based on the ability to detect a clinically important difference in success rate of 25% between the two groups. We assumed a success rate of 40% in the group having the least successful treatment and thus estimated the target sample size at 60 patients in each group (two tailed, α=0.05, β=0.20).
Results
Patient flow and follow up
A total of 109 out of 203 patients referred by their general practitioners were enrolled in the trial. Most of the exclusions (73/94) were made because the independent observer could not confirm capsular syndrome as the main cause of shoulder pain. Other probable causes of pain were diagnosed as rotator cuff tendinitis, subacromial bursitis, and dysfunction of the cervical spine. Twenty one patients were excluded for other reasons (figure).
One patient withdrew from the study immediately after randomisation, refusing to have any injections. A total of six patients (5.5%) withdrew from the study, four of whom reported complete recovery before withdrawal. All patients who withdrew from the study were included in the statistical analysis until withdrawal.
Characteristics of patients
Fifty three patients were allocated to treatment with injections and 56 patients to physiotherapy. Despite randomisation there were some differences between the intervention groups in regard to sex, the onset of pain, involvement of the dominant side, concomitant neck pain, previous episodes of shoulder pain, baseline severity of the main complaint, and rating of the pain at night (table 1).
Table 1.
Baseline characteristics of patients with painful stiff shoulder by treatment received. Values are numbers (percentages) unless indicated otherwise
| Patients treated with corticosteroid injection (n=53) | Patients treated with physiotherapy (n=56) | |
|---|---|---|
| Mean (SD) age (years) | 57.3 (10.2) | 60.2 (10.7) |
| Women | 25 (47) | 33 (59) |
| Concomitant neck pain | 24 (45) | 32 (57) |
| Previous episodes of shoulder pain | 23 (43) | 17 (30) |
| Duration of current episode (months): | ||
| <1 | 7 (13) | 6 (11) |
| ⩾1-3 | 21 (40) | 26 (46) |
| >3-6 | 13 (24) | 9 (16) |
| >6-12 | 8 (15) | 9 (16) |
| >12 | 4 (8) | 6 (11) |
| Acute onset | 9 (17) | 15 (27) |
| Precipitating cause: | ||
| Overuse or strain | 7 (13) | 7 (13) |
| Minor injury | 8 (15) | 7 (13) |
| Unknown | 35 (66) | 34 (61) |
| Dominant shoulder affected | 18 (34) | 25 (45) |
| Course of symptoms preceding enrollment: | ||
| Improved | 4 (8) | 7 (13) |
| Stable | 34 (64) | 35 (65) |
| Deteriorated | 15 (28) | 12 (22) |
| Median (interquartile range) rating of severity as measured on visual analog scale*: | ||
| Associated with main complaint | 86 (74 to 93) | 78 (62 to 87) |
| Of pain during the day | 49 (28 to 66) | 48 (31 to 65) |
| Of pain at night | 52 (11 to 75) | 43 (16 to 78) |
| According to observer | 56 (30 to 65) | 53 (37 to 60) |
| Median (interquartile range) rating of shoulder disability† | 69 (56 to 81) | 69 (56 to 85) |
| Median (interquartile range) degree of restriction of range of motion‡: | ||
| Lateral rotation | 22 (8 to 31) | 19 (8 to 34) |
| Glenohumeral abduction | 40 (25 to 50) | 35 (22 to 48) |
Range of scores is 0-100; 100 indicates very severe pain.
Range of scores on shoulder disability questionnaire is 0-100; 100 indicates severe disability.11 12
Restriction of range of motion as compared to healthy shoulder; measured by the independent observer.
Interventions
Twenty five patients (48%) allocated to receive injections had three injections. The mean number of injections was 2.2 (SD 0.8). All patients allocated to physiotherapy received passive joint mobilisation and exercise treatment. Additional electrotherapy was used in 41 patients and ice or hot packs in 33.
At baseline, the use of pain medication was evenly distributed between the two groups; 15 patients in each group used paracetamol (acetaminophen) or non-steroidal anti-inflammatory drugs. The number of patients needing additional treatment after six weeks and the types of treatment received are shown in table 2. Additional treatment was given more often to patients allocated to physiotherapy (75% v 42%).
Table 2.
Number (percentage) of patients with painful stiff shoulder needing treatment for residual pain or disability at seven week follow up (treatment no longer restricted to interventions as described by protocol)
| Additional treatment | Patients
|
||
|---|---|---|---|
| All (n=108)* | Treated with corticosteroid injection (n=52)* | Treated with physiotherapy (n=56) | |
| None | 44 (41) | 30 (58) | 14 (25) |
| Paracetamol or non-steroidal anti-inflammatory drugs | 6 (5) | 2 (4) | 4 (7) |
| Corticosteroid injection | 16 (15) | 6 (11) | 10 (18) |
| Physiotherapy | 27 (25) | 8 (15) | 19 (34) |
| Corticosteroid injections and physiotherapy | 13 (12) | 5 (10) | 8 (14) |
| Arthroscopic surgery | 2 (2) | 1 (2) | 1 (2) |
One patient withdrew from the study after three weeks so no information on additional treatment was available.
The observer correctly guessed the allocated treatment for 65 (60%) out of 108 patients after 7 weeks and for 51 (48%) out of 105 after 26 weeks. The frequency of correct guesses was similar in both groups (30/52 (58%) for patients having injections and 35/56 (63%) for those having physiotherapy at 7 weeks).
Outcome
The mean improvement in outcome measures at each point of follow up is shown in table 3. Using the intention to treat analysis we found a statistically significant difference between the groups which favoured treatment with corticosteroid injections. In a multivariate analysis differences in prognosis at baseline had little influence on the outcome of the study (data not shown).
Table 3.
Mean (SD) improvement in outcome measures in patients with painful stiff shoulder and differences between groups by treatment at different points in follow up
| Patients treated with corticosteroid injection* | Patients treated with physiotherapy* | Mean (95% CI) difference between groups | P value | |
|---|---|---|---|---|
| Improvement in rating of severity† | ||||
| Associated with main complaint: | ||||
| 3 weeks | 32 (26) | 17 (21) | 15 (6 to 24) | (0.071)‡ |
| 7 weeks | 58 (28) | 32 (29) | 26 (15 to 37) | |
| 13 weeks | 66 (28) | 47 (33) | 19 (7 to 31) | |
| 26 weeks | 63 (31) | 54 (33) | 9 (−3 to 22) | |
| 52 weeks | 70 (24) | 59 (30) | 11 (1 to 23) | |
| Of pain during the day: | ||||
| 3 weeks | 22 (20) | 10 (15) | 12 (5 to 18) | < 0.001 |
| 7 weeks | 35 (20) | 23 (24) | 12 (4 to 21) | |
| 13 weeks | 36 (26) | 27 (31) | 9 (−3 to 20) | |
| 26 weeks | 32 (25) | 32 (28) | 0 (−10 to 10) | |
| 52 weeks | 38 (23) | 35 (26) | 3 (−7 to 13) | |
| Of pain at night: | ||||
| 3 weeks | 21 (26) | 9 (23) | 12 (2 to 21) | 0.015 |
| 7 weeks | 36 (28) | 22 (30) | 14 (3 to 25) | |
| 13 weeks | 37 (33) | 28 (36) | 9 (−4 to 23) | |
| 26 weeks | 34 (36) | 33 (41) | 1 (−13 to 17) | |
| 52 weeks | 37 (33) | 35 (39) | 2 (−12 to 16) | |
| As rated by observer: | ||||
| 3 weeks | 13 (17) | 0 (18) | 13 (6 to 20) | <0.001 |
| 7 weeks | 24 (20) | 9 (20) | 15 (7 to 22) | |
| 26 weeks | 29 (24) | 27 (27) | 2 (−9 to 11) | |
| Improvement in rating of shoulder disability§ | ||||
| 3 weeks | 19 (27) | 6 (22) | 13 (4 to 23) | 0.024 |
| 7 weeks | 39 (27) | 14 (27) | 25 (14 to 35) | |
| 13 weeks | 38 (31) | 28 (32) | 10 (−2 to 22) | |
| 26 weeks | 45 (30) | 33 (34) | 12 (0 to 25) | |
| 52 weeks | 42 (33) | 38 (34) | 4 (−10 to 17) | |
| Improvement in degree of restriction of range of motion | ||||
| External rotation: | ||||
| 3 weeks | 6 (14) | –3 (12) | 9 (3 to 14) | 0.002 |
| 7 weeks | 13 (16) | –2 (14) | 15 (9 to 20) | |
| 26 weeks | 16 (18) | 7 (21) | 9 (1 to 16) | |
| Abduction: | ||||
| 3 weeks | 2 (12) | –3 (13) | 5 (0 to 9) | 0.065 |
| 7 weeks | 4 (11) | –1 (14) | 5 (0 to 10) | |
| 26 weeks | 9 (12) | 7 (17) | 2 (−3 to 8) | |
At 3 and 13 weeks there is one missing value in each group. At 7 weeks there is one missing value in the injection group. At 26 weeks there are one missing value in the injection group and two in the physiotherapy group. At 52 weeks there are four missing values in the injection group and one in the physiotherapy group.
Pain as rated on visual analog scale in which scores range from 0-100; 100 indicates very severe pain.
The change in scores for this outcome measure show a non-gaussian distribution. Non-parametric testing results in statistically significant differences at 3, 7, 13, and 52 weeks
As rated on shoulder disability questionnaire in which scores range from 0-100; 100 indicates severe disability.11 12
At 7 weeks 40 (77%) out of 52 patients treated with injections were considered to be treatment successes compared with 26 (46%) out of 56 treated with physiotherapy (difference between groups 31%, 95% confidence interval 14% to 48%). The difference in improvement was in the same direction for all outcome measures; these differences were statistically significant (multivariate analysis of variance) for most outcome measures but not for restriction of abduction and severity of the main complaint. The change in scores for the main complaint had a non-gaussian distribution. Non-parametric testing (Mann-Whitney U test) indicated that there was a significantly greater improvement in the main complaint among those treated with corticosteroids at 3, 7, 13, and 52 weeks. Table 3 shows that the differences between the groups were mainly due to the comparatively fast relief of symptoms occurring among those receiving corticosteroids. At assessment at 26 and 52 weeks there were comparatively small differences between the groups.
An alternative analysis was conducted which excluded 12 patients who were not treated according to protocol. For all outcome measures the results were similar to those in the intention to treat analysis. At 7 weeks treatment was considered to be successful in 39 (77%) out of 51 patients receiving injections and in 22 (48%) out of 46 for those treated with physiotherapy.
Adverse reactions
Mild adverse reactions, mainly increased pain after treatment, were reported by more than 50% (62/108) of all patients (table 4). Few adverse reactions occurred after physiotherapy. Adverse reactions to corticosteroids were particularly frequent in women; facial flushing was reported by nine and irregular menstrual bleeding by six women, two of whom were postmenopausal.
Table 4.
Frequency of adverse reactions to treatment for painful stiff shoulder. Values are number of occurrences unless indicated otherwise
| Patients treated with injection (n=57)* | Patients treated with physiotherapy (n=57)† | |
|---|---|---|
| No of patients having any adverse reaction | 30 (53%) | 32 (56%) |
| Pain after treatment: | ||
| Lasting ⩽1 day | 9 | 17 |
| Lasting >2 days | 16 | 13 |
| Facial flushing | 9 | 1 |
| Irregular menstrual bleeding | 6‡ | 0 |
| Fever reported by patient | 4 | 1 |
| Skin irritation | 1 | 2 |
| Other reaction | 6§ | 4¶ |
Includes 52 patients treated according to protocol and 5 patients treated with both interventions.
Includes 56 patients treated according to protocol and one patient treated with both interventions.
Two of these women were postmenopausal.
Reactions included sweating, fatigue, dry mouth, dizziness, and headache.
Reactions included slight swelling, tingling, and radiating pain.
Discussion
This paper describes a randomised trial in a primary care setting that compared two common interventions, corticosteroid injections and physiotherapy, for treatment of painful stiff shoulder. The analysis done on an intention to treat basis and an alternative analysis that excluded patients whose treatment deviated from the protocol showed that corticosteroid injections were superior to physiotherapy in terms of the success of treatment; improvement in degree of lateral rotation; improvement in clinical severity; and in relief of the main complaint, pain, and disability. We decided against performing an analysis of the long term results by treatment actually received as this would have produced a biased outcome. The reasons for concluding or modifying treatment were, after all, strongly related to the results of the allocated intervention.16
Four earlier trials compared the effectiveness of corticosteroid injections with physiotherapy for shoulder pain.17–20 Three trials with relatively small study populations (fewer than 25 patients per intervention group) were unable to show significant differences between the treatments. These studies used a single injection17,19 or a different type of corticosteroid.18,19 Only one trial was conducted in a primary care setting and this trial reported significant differences between the treatments.20 In that study treatment was considered successful after five weeks for 35 (75%) out of 47 patients treated with injections and for seven (20%) out of 35 treated with physiotherapy. Corticosteroid treatment consisted of multiple injections. Passive mobilisation was not permitted for patients allocated to physiotherapy, a practice that is not compatible with everyday practice. To enhance the external validity of our trial and to facilitate implementation of the findings in clinical practice we tried to ensure that the interventions used resembled those carried out in primary care.
In this study injections were administered by general practitioners. Inaccurate placement of intra-articular injections is reported to occur often, even among trained rheumatologists.21,22 Recent studies report a better response to treatment after accurately placed injections.22,23 Despite the inevitable uncertainty about placement in our study, many of our patients had a good response to the corticosteroid injections administered by their general practitioner.
Adverse reactions were generally mild but were sometimes troublesome, particularly in women receiving corticosteroid injections. Surprisingly, published reports of irregular menstrual bleeding after corticosteroid injection are scarce. One letter that we identified described this side effect as a frequent occurrence, especially in women not taking oral contraceptives.24 These observations should be investigated further. Doctors and patients should be aware of the possibility of irregular menstrual bleeding after corticosteroid injection so that women are not needlessly made anxious or subjected to diagnostic procedures; however, women and their doctors should be aware that postmenopausal bleeding may be a sign of cancer of the endometrium or cervix.
This randomised trial showed that corticosteroid injections administered by general practitioners for treatment of painful stiff shoulder are superior to physiotherapy. Differences between the intervention groups were mainly due to the comparatively quick relief of symptoms occurring in patients treated with injections. Injections may be preferable to physiotherapy in the initial treatment of painful stiff shoulder.
Figure.
Randomisation of patients in and withdrawal of patients from the trial
Acknowledgments
We thank Marianne Ellermeijer, Monique Heemskerk, and Michel van Aarst for their work during data collection; and all participating general practitioners and physiotherapists.
Footnotes
Funding: Netherlands Organisation for Scientific Research and the Fund for Investigative Medicine of the Health Insurance Council.
Conflict of interest: None.
References
- 1.Croft P. Soft tissue rheumatism. In: Silman AJ, Hochberg MC, editors. . Epidemiology of the rheumatic diseases. Oxford: Oxford Medical Publications; 1993. pp. 375–421. [Google Scholar]
- 2.Lamberts H, Brouwer HJ, Mohrs J. Reason for encounter-, episode- and process-oriented standard output from transition project: part I. Amsterdam: Department of General Practice/Family Medicine, University of Amsterdam; 1991. [Google Scholar]
- 3.Van der Windt DAWM, Koes BW, De Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics and management. Ann Rheum Dis. 1995;54:959–964. doi: 10.1136/ard.54.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker JF, de Jongh L, Jonquière M, Mens J, Oosterhuis WW, Poppelaars A, et al. Standaard Schouderklachten. [Practice guidelines for shoulder complaints.] Huisarts en Wetenschap. 1990;33:196–202. [Google Scholar]
- 5.Van der Windt DAWM, Koes BW, Boeke AJP, Devillé W, De Jong BA, Bouter LM. Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract. 1996;46:519–523. [PMC free article] [PubMed] [Google Scholar]
- 6.Croft P, Pope D, Silman A. The clinical course of shoulder pain: prospective cohort study in primary care. BMJ. 1996;313:601–602. doi: 10.1136/bmj.313.7057.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Heijden GJMG, Van der Windt DAWM, Kleijnen J, Koes BW, Bouter LM. Steroid injections for shoulder disorders: a systematic review of randomised clinical trials. Br J Gen Pract. 1996;46:309–316. [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Heijden GJMG, Van der Windt DAWM, de Winter AF. Physiotherapy for soft tissue shoulder disorders: a systematic review of randomised clinical trials. BMJ. 1997;315:25–30. doi: 10.1136/bmj.315.7099.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled clinical trials of interventions for painful shoulder: selection criteria, outcome assessment, and efficacy. BMJ. 1998;316:354–360. doi: 10.1136/bmj.316.7128.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs LG, Barton MA, Wallace WA, Ferrousis J, Dunn NA, Bossingham DA. Intra-articular distension and corticosteroids in the management of capsulitis of the shoulder. BMJ. 1991;302:1498–1501. doi: 10.1136/bmj.302.6791.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analog scale. Pain. 1983;16:87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 12.Van der Heijden GJMG. Maastricht: Datawyse/University Press Maastricht; 1996. Shoulder disability questionnaire: design and responsiveness of a functional status measure. In: Shoulder disorder treatment: efficacy of ultrasound and electrotherapy; pp. 79–91. [Google Scholar]
- 13.Van der Windt DAWM, Van der Heijden GJMG, Koes BW, De Winter AF, Devillé W, Bouter LM. The responsiveness of the shoulder disability questionnaire. Ann Rheum Dis. 1998;57:82–87. doi: 10.1136/ard.57.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heemskerk MAMB, Van Aarst M, Van der Windt DAWM. De reproduceerbaarheid van het meten van de passieve beweeglijkheid van de schouder met de EDI-320 digitale hoekmeter. [The reproducibility of the Cybex EDI-320 digital goniometer for the measurement of the passive glenohumeral range of motion.] Ned Tijdschr Fysiother. 1997;107:146–149. [Google Scholar]
- 15.Norusis MJ. SPSS/PC+ advanced statistics manual. Chicago: Statistical Product and Service Solutions; 1992. [Google Scholar]
- 16.Lee YJ, Ellenberg JH, Hirtz DG, Nelson KB. Analysis of clinical trials by treatment actually received: is it really an option? Stat Med. 1991;10:1595–1605. doi: 10.1002/sim.4780101011. [DOI] [PubMed] [Google Scholar]
- 17.Dacre JE, Beeney N, Scott DL. Injections and physiotherapy for the painful stiff shoulder. Ann Rheum Dis. 1989;48:322–325. doi: 10.1136/ard.48.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulgen DY, Binder AI, Hazleman BL, Dutton J, Roberts S. Frozen shoulder: prospective clinical study with an evaluation of three treatment regimens. Ann Rheum Dis. 1984;43:353–360. doi: 10.1136/ard.43.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PN, Lee M, Haq AMMM, Longton EB, Wright V. Periarthritis of the shoulder: trial of treatments investigated by multivariate analysis. Ann Rheum Dis. 1974;33:116–119. doi: 10.1136/ard.33.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314:1320–1325. doi: 10.1136/bmj.314.7090.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones A, Regan M, Ledingham J, Pattrick M, Manhire A, Doherty M. Importance of placement of intra-articular steroid injections. BMJ. 1993;307:1329–1330. doi: 10.1136/bmj.307.6915.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56:59–63. doi: 10.1136/ard.56.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White AET, Tuite JD. The accuracy and efficacy of shoulder injections in restrictive capsulitis. J Orthop Rheumatol. 1996;9:37–40. [Google Scholar]
- 24.De Wolf AN, Mens JMA. Kan een injectie met corticosteroïden leiden tot menstruatiestoornissen? [Can corticosteroid injection cause menstrual bleeding?] Vademecum. 1994;12:7. [Google Scholar]