Abstract
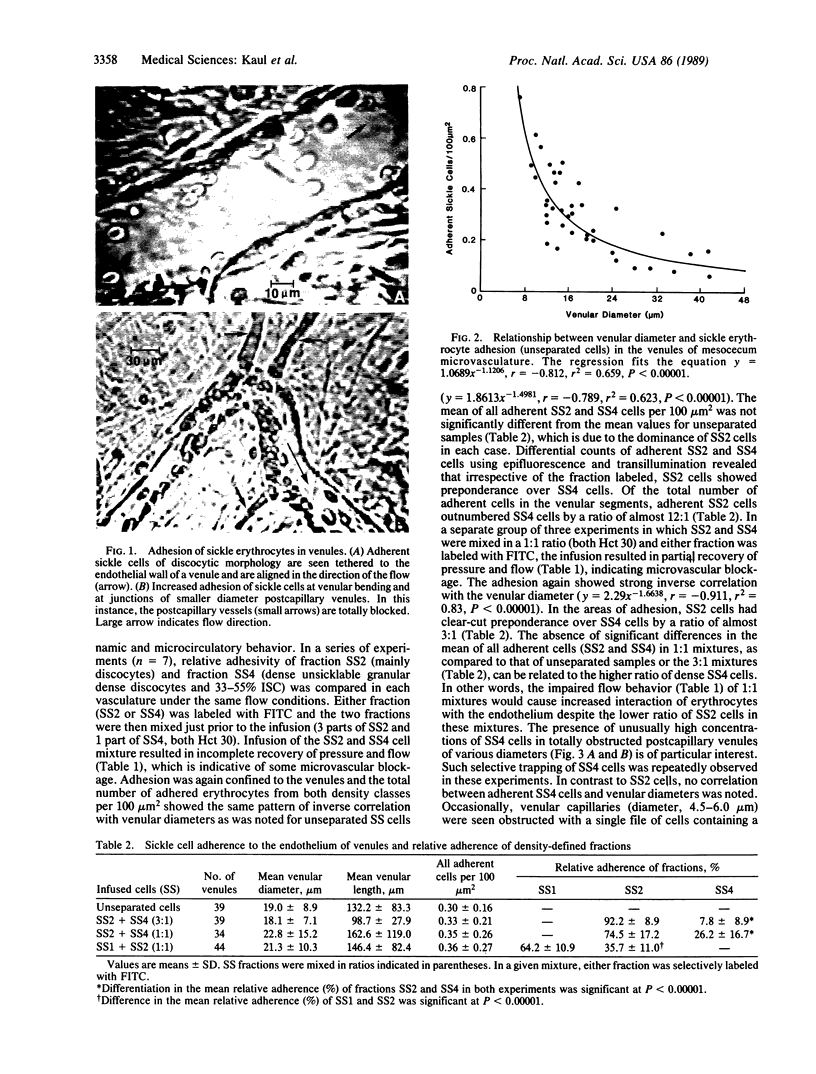
To understand the role of sickle cell adherence to the vascular endothelium in the pathophysiology of sickle cell anemia (SS) vasoocclusion, we have carried out a microcirculatory study utilizing the ex vivo mesocecum vasculature of the rat. A single bolus of washed oxy-normal (AA) erythrocytes or oxy-SS cells (unseparated or density-defined SS cell classes) was infused. Hemodynamic monitoring and intravital microscopic observations of the microvascular flow revealed higher peripheral resistance for SS erythrocytes and adherence of these cells exclusively to the venular endothelium but rare or no adherence of AA cells. The extent of adhesion was inversely correlated with venular diameters (r = -0.812; P less than 0.00001). The adhesion of SS erythrocytes is density-class dependent: reticulocytes and young discocytes (SS1) greater than discocytes (SS2) greater than irreversible sickle cells and unsicklable dense discocytes (SS4). Selective secondary trapping of SS4 (dense cells) is found in postcapillary venules where deformable SS cells are preferentially adhered. We conclude that in the oxygenated condition, vasoocclusion can be induced by two events: (i) random precapillary obstruction by a small number of SS4 cells; (ii) increased adhesion of SS1 and SS2 cells in the immediate postcapillary venules. A combination of precapillary obstruction, adhesion in postcapillary venules, and secondary trapping of dense cells may induce local hypoxia, increased polymerization of hemoglobin S, and rigidity of SS erythrocytes, thereby extending obstruction to nearby vessels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez S., Kaul D. K., Nagel R. L. Microvascular determinants of blood flow behavior and HbSS erythrocyte plugging in microcirculation. Blood Cells. 1982;8(1):127–137. [PubMed] [Google Scholar]
- Barabino G. A., McIntire L. V., Eskin S. G., Sears D. A., Udden M. Endothelial cell interactions with sickle cell, sickle trait, mechanically injured, and normal erythrocytes under controlled flow. Blood. 1987 Jul;70(1):152–157. [PubMed] [Google Scholar]
- Barabino G. A., McIntire L. V., Eskin S. G., Sears D. A., Udden M. Rheological studies of erythrocyte-endothelial cell interactions in sickle cell disease. Prog Clin Biol Res. 1987;240:113–127. [PubMed] [Google Scholar]
- Billett H. H., Kim K., Fabry M. E., Nagel R. L. The percentage of dense red cells does not predict incidence of sickle cell painful crisis. Blood. 1986 Jul;68(1):301–303. [PubMed] [Google Scholar]
- Burns E. R., Wilkinson W. H., Nagel R. L. Adherence properties of sickle erythrocytes in dynamic flow systems. J Lab Clin Med. 1985 Jun;105(6):673–678. [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- Fabry M. E., Benjamin L., Lawrence C., Nagel R. L. An objective sign in painful crisis in sickle cell anemia: the concomitant reduction of high density red cells. Blood. 1984 Aug;64(2):559–563. [PubMed] [Google Scholar]
- Fabry M. E., Nagel R. L. Heterogeneity of red cells in the sickler: a characteristic with practical clinical and pathophysiological implications. Blood Cells. 1982;8(1):9–15. [PubMed] [Google Scholar]
- Grabowski E. F. Sickle erythrocytes adhere to endothelial cell monolayers (ECM's) exposed to flowing blood. Prog Clin Biol Res. 1987;240:167–179. [PubMed] [Google Scholar]
- Hebbel R. P., Boogaerts M. A., Eaton J. W., Steinberg M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980 May 1;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979 Oct;54(4):872–876. [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Nagel R. L. Vaso-occlusion by sickle cells: evidence for selective trapping of dense red cells. Blood. 1986 Nov;68(5):1162–1166. [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Windisch P., Baez S., Nagel R. L. Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J Clin Invest. 1983 Jul;72(1):22–31. doi: 10.1172/JCI110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D. K., Nagel R. L., Baez S. Pressure effects on the flow behavior of sickle (HbSS) red cells in isolated (ex-vivo) microvascular system. Microvasc Res. 1983 Sep;26(2):170–181. doi: 10.1016/0026-2862(83)90068-7. [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Kovalcheck S., Zweifach B. W. The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ Res. 1978 Nov;43(5):738–749. doi: 10.1161/01.res.43.5.738. [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Sheikh N. U., Katz D. M. Intravital microscopy of capillary hemodynamics in sickle cell disease. J Clin Invest. 1987 Jul;80(1):117–127. doi: 10.1172/JCI113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky H. H., Usami S., Chien S. Human SS red cell rheological behavior in the microcirculation of cremaster muscle. Blood Cells. 1982;8(1):113–126. [PubMed] [Google Scholar]
- Matuhasi T., Usui M., Nariuchi H. Studies on the reactions between fluorescent blood cells and antifluorescein antibodies in vivo and in vitro. Ann N Y Acad Sci. 1971 Jun 21;177:467–480. doi: 10.1111/j.1749-6632.1971.tb35077.x. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Characterization of cellular determinants and plasma factors responsible for increased adherence of sickle cells to vascular endothelium. Prog Clin Biol Res. 1987;240:181–190. [PubMed] [Google Scholar]
- Mohandas N., Evans E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. J Clin Invest. 1985 Oct;76(4):1605–1612. doi: 10.1172/JCI112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Fabry M. E., Billett H. H., Kaul D. K. Sickle cell painful crises: a multifactorial event. Prog Clin Biol Res. 1987;240:361–380. [PubMed] [Google Scholar]
- Raventos-Suarez C., Kaul D. K., Macaluso F., Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3829–3833. doi: 10.1073/pnas.82.11.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D., La Celle P. L. Erythrocyte-endothelial cell adherence in sickle cell disorders. Blood. 1986 Nov;68(5):1050–1054. [PubMed] [Google Scholar]
- Wick T. M., Moake J. L., Udden M. M., Eskin S. G., Sears D. A., McIntire L. V. Unusually large von Willebrand factor multimers increase adhesion of sickle erythrocytes to human endothelial cells under controlled flow. J Clin Invest. 1987 Sep;80(3):905–910. doi: 10.1172/JCI113151. [DOI] [PMC free article] [PubMed] [Google Scholar]