Abstract
A network of connections is established as neural circuits form between neurons. To make these connections, neurons initiate asymmetric axon outgrowth in response to extracellular guidance cues. Within the specialized growth cones of migrating axons, F-actin and microtubules asymmetrically accumulate where an axon projects forward. Although many guidance cues, receptors, and intracellular signaling components required for axon guidance have been identified, the means through which the asymmetry is established and maintained is unclear. We discuss recent studies in invertebrate and vertebrate organisms that define a signaling module comprising UNC-6(netrin), UNC-40(DCC), PI3K, Rac, and MIG-10(lamellipodin) and we consider how this module could establish polarized outgrowth in response to guidance cues.
Introduction
The formation of neural circuits requires that proper connectivity is established between neurons during development. Failure to achieve correct connectivity results in a dysfunctional nervous system, which may be associated with disorders such as Autism and Down Syndrome [1, 2]. In addition to its importance in understanding developmental disorders, an understanding of how neural connectivity is established will also be important for efforts to improve treatment for nerve connections damaged by injury or neurodegenerative diseases [2, 3].
An early step in the establishment of connectivity involves the growth and guidance of axons. Axon growth is led by the growth cone, a specialized structure that sits at the tip of growing axons. The growth cone is guided by extracellular guidance cues, including the slits, netrins, ephrins, and semaphorins, wnts, sonichedgehog, and BMPs. These guidance cues are secreted from a source and activate receptors on the growth cone, causing it to migrate towards or away from the source [4-7]. In response to guidance cues, asymmetric accumulation of F-actin and microtubules occurs in the growth cone. The growth cone then migrates in the direction of F-actin and microtubule accumulation [8-13]. Asymmetry in several other processes has also been observed during the guidance response, including exocytosis, calcium signaling, and protein translation, suggesting that they also play a role in growth cone guidance [14-18].
A key question is how directional information provided by an extracellular gradient is transduced to generate asymmetry within the growth cone. Recent work has identified several proteins that are asymmetrically activated or localized within growth cones including: Src, ERMs, Cofilin and MIG-10 [19-22]. This review focuses on MIG-10 (Lamellipodin) and a signaling module that also comprises UNC-6(netrin), UNC-40(DCC), PI3K, and Rac. Recent studies suggest, 1) the interaction between the UNC-6 guidance cue and the UNC-40 receptor establishes asymmetry within neurons, 2) interactions between Rac and PI3K can amplify shallow extracellular gradients into a sharp intracellular signal to direct migration, and 3) MIG-10 links Rac and PI3K to proteins that promote actin-based protrusive activity. Therefore this signaling module is positioned to transmit the directional information provided by the graded distribution of the extracellular guidance cue to the internal cellular machinery that promotes directed outgrowth.
The cytoplasmic signaling molecule MIG-10(lamellipodin) is asymmetrically localized in response to guidance cues
One striking example of polarization is the cytoplasmic signaling molecule MIG-10, an ortholog of vertebrate lamellipodin, which has been implicated in the response to the guidance cues SLT-1(Slit) and UNC-6(netrin) [23-27]. MIG-10(lamellipodin) contains several protein interaction domains and is thought to form an organizing center for a signaling complex that promotes axon outgrowth [24, 27, 28]. Overexpression of MIG-10 in the AVM neuron of C. elegans in the absence of guidance cues causes the growth of multiple misguided processes, suggesting that MIG-10 has an outgrowth-promoting activity. Addition of the UNC-6(netrin) or Slt-1(Slit) guidance cue results in the formation of a single process with enhanced guidance, indicating that the MIG-10 outgrowth-promoting activity can be polarized and oriented by these guidance cues [27]. Consistent with these observations, in neurons that respond to the UNC-6 cue, there is an UNC-6-dependent asymmetric localization of the MIG-10 protein to the site of axon outgrowth (Figures 1A-B) [23]. The sharp polarization of MIG-10 is remarkable in that it appears to amplify the directional information imparted from what is thought to be shallow gradients of the guidance cues.
Figure 1.
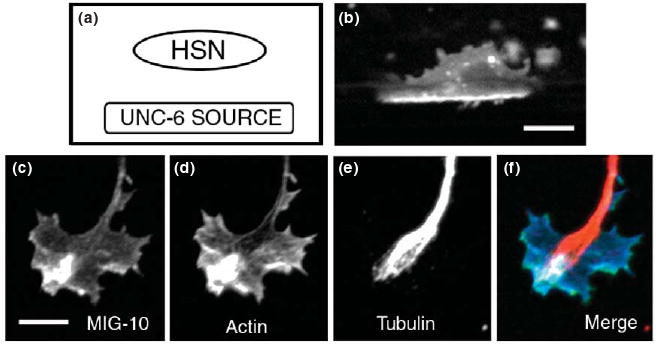
Polarization of MIG-10(lamellipodin) in response to guidance cues in C. elegans and cultured chick growth cones. MIG-10(lamellipodin) is a cytoplasmic adaptor protein that is thought to serve as an organizing center for a signaling complex that regulates actin dynamics [20, 24, 27, 28]. (a) UNC-6(netrin) is secreted from sources ventral to the HSN neuron. (b) MIG-10∷GFP localization in the HSN neuron during the third larval (L3) stage, just prior to axon extension. During the L3 stage MIG-10∷GFP becomes asymmetrically localized to the ventral side of the HSN neuron. This ventral localization of MIG-10:GFP requires UNC-6(netrin), which is secreted from sources ventral to the HSN neuron [23]. (c-f) Asymmetric localization of MIG-10∷GFP in the growth cone of a cultured cortical neuron double stained for F-actin and microtubules. Figure is modified from Ref [20]. Scale bars represent 5 μm.
MIG-10 can also be polarized within the growth cone of cultured neurons, where its localization correlates with asymmetric accumulations of F-actin and microtubules (Figures 1C-E) [20]. MIG-10(lamellipodin) can interact with proteins that promote actin polymerization. Therefore, the asymmetric localization of MIG-10(lamellipodin) might promote polarized actin polymerization, which may in turn promote asymmetric accumulation of microtubules. Experiments using live imaging of actin, microtubules, and lamellipodin in cultured vertebrate growth cones may address this model.
Guidance receptors are asymmetrically localized in response to guidance cues
Studies of the HSN neuron in C. elegans indicate that the UNC-40(DCC) receptor, becomes asymmetrically localized at the ventral edge of the cell where the axon will form, in response to UNC-6(netrin). The axon migrates ventrally towards the source of UNC-6. Loss-of-function mutations that affect UNC-6 or the UNC-6 receptor UNC-40 cause the failure of leading edge formation and the late development of an axon that is subsequently misguided [23]. MIG-10 is not required for the asymmetric localization of UNC-40, suggesting that the polarization of UNC-40 is an early response to the UNC-6 cue.
Recent work on cultured rat neurons indicates that GABA receptors are also polarized during an attractive response to GABA [29]. In response to a gradient of GABA and prior to growth cone turning, GABA receptors redistribute asymmetrically towards the GABA source. The redistribution of GABA receptors occurs as a result of a microtubule-dependent lateral movement of the receptors within the membrane. Together, these data suggest that the attractive response of growth cones towards GABA is mediated by the redistribution of GABA receptors to the side of the growth cone closest to the source of GABA.
The mechanisms that mediate asymmetric localization of receptors are not yet well understood. One mechanism may involve transport along microtubules, as has been observed in the case of GABA receptors in cultured rat neurons [29]. Studies with cultured Xenopus neurons have revealed another mechanism that involves asymmetric recruitment of guidance receptors into lipid rafts, specialized cholesterol and glycosphingolipid-rich membrane microdomains [30, 31]. For example, in growth cones that are undergoing attraction towards BDNF, the trkB receptors for BDNF are associated with lipid rafts that become localized to the side of the growth cone that is closest to the source of BDNF. A third possible mechanism involves the regulation of receptor trafficking by the Rho GTPases. In C. elegans, Rac can regulate guidance receptors by affecting their subcellular localization [32, 33]. Likewise, a recent study on cultured vertebrate neurons has indicated that Rho can regulate the recruitment of the netrin receptor DCC to the plasma membrane [34].
Guidance receptors mediate multiple signaling events
Axon guidance may be a multistep process, with the axon guidance receptors involved in separate yet functionally linked signaling events to orient and promote axon outgrowth activity [27, 35]. In the first step, the association of the guidance cue ligand enables signaling that causes the asymmetric localization of receptors to the cell membrane where axon outgrowth will occur. This localization denotes the ability of the neurons to sense the guidance cue gradient. In the second step, the guidance receptors promote axon outgrowth in the direction that was set up by the asymmetric localization. As migrating axons encounter different environments, new signals may negatively regulate these steps to control morphological changes that occur during turning, branch formation, and synaptogenesis [34]
The asymmetric localization of guidance receptors likely triggers asymmetric activation of downstream signaling events that promote outgrowth (Figures 2-3). Key signaling pathways downstream of guidance receptors include the Rac GTPase and phosphoinositide 3-kinase signaling pathways [24, 36, 37]. These signaling pathways are of particular importance in understanding polarization of axon outgrowth in the growth cone because studies of chemotactic cell migration in neutrophils have indicated that interactions between these pathways are important for the establishment of polarity [38]. Although the Rac and phosphoinositide signaling pathways have been implicated downstream of multiple guidance receptors, here we focus on events downstream of the netrin receptor UNC-40(DCC).
Figure 2.
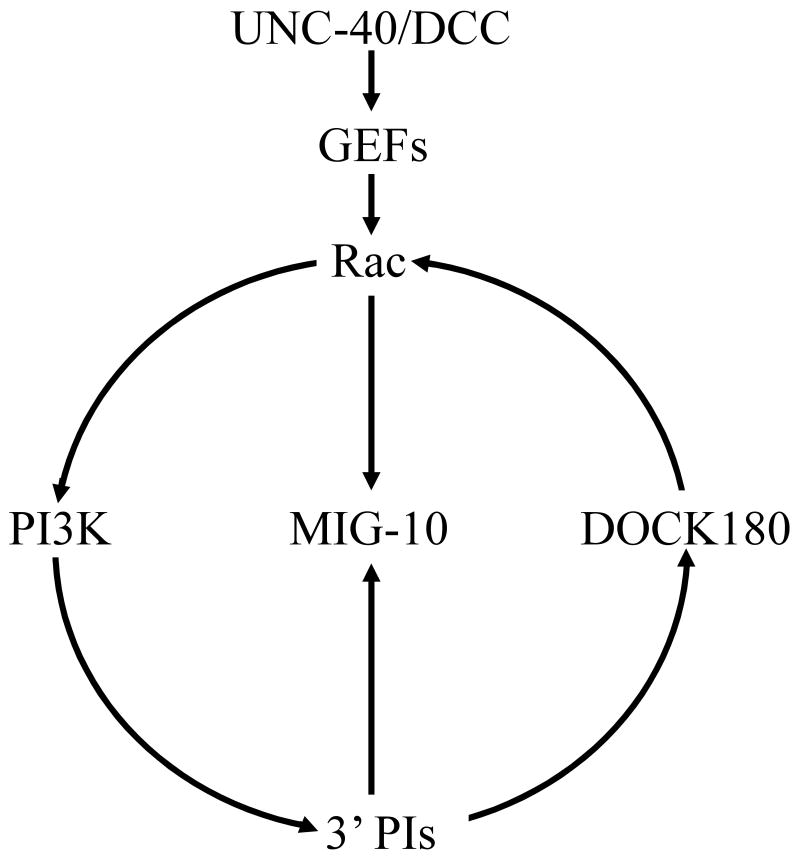
UNC-40(DCC) signaling in neuronal growth cones might activate a positive-feedback loop that includes Rac and 3′-phosphorylated phosphoinositides (3′ PIs) [38, 52]. Guanine nucleotide exchange factors (GEFs), including DOCK180 and Trio, link UNC-40(DCC) activation to Rac activation [44, 45]. Activated Rac binds to and stimulates phosphoinositide 3-kinase (PI3K) activity, thus generating 3′ PIs [38]. 3′ PIs then recruit DOCK180, a Rac GEF, which restarts the cycle by activating Rac [67]. The organizing adaptor protein MIG-10(lamellipodin) is recruited by binding to both activated Rac and the phosphoinositide PtdIns(3,4)P2, a 3′ PI. Thus, MIG-10(lamellipodin) might spatially link this positive-feedback loop to actin polymerization.
Figure 3.
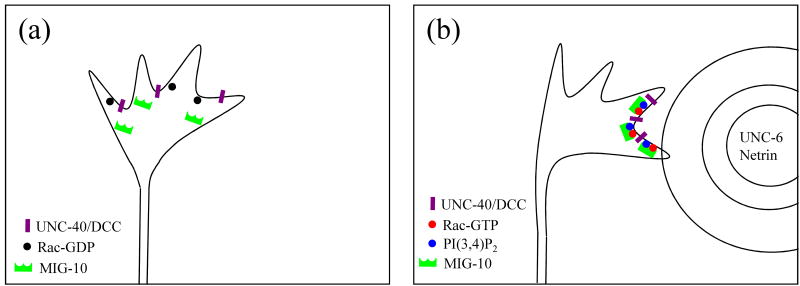
Model for asymmetric localization of MIG-10(lamellipodin) in a growth cone during axon guidance. (a) In the absence of an UNC-6(netrin) gradient, the UNC-40(DCC) receptor is distributed uniformly. (b) In the presence of a gradient of UNC-6(netrin), UNC-40(DCC) becomes polarized to the side of the growth cone closest to the source of UNC-6(netrin) [23]. This asymmetric localization of UNC-40(DCC) may cause asymmetric activation of Rac and asymmetric localization of PtdIns(3,4)P2. MIG-10(lamellipodin) binds to activated Rac and PtdIns(3,4)P2, thus causing asymmetric localization of MIG-10(lamellipodin) [20, 23, 28]. Asymmetric localization of MIG-10(lamellipodin) causes asymmetric actin-based protrusive activity, thereby causing growth cone turning.
The Rac GTPase is activated downstream of UNC-40(DCC)
Genetic studies in C. elegans have shown that Rac functions downstream of UNC-40(DCC) to mediate the response to UNC-6(netrin) [39]. Consistent with these findings, biochemical studies on vertebrate cultured neurons have indicated that Rac is activated in response to UNC-40(DCC) receptor signaling [40-43]. The activation of Rac is mediated by an association of UNC-40(DCC) with two guanine nucleotide exchange factors (GEFs), UNC-73(Trio) and CED-5(DOCK180) [44, 45], which are responsible for switching the inactive GDP-bound Rac GTPase to the active GTP-bound form. These observations suggest that asymmetric localization of the UNC-40(DCC) receptor leads to asymmetric activation of Rac.
It is also known that guidance cues can generate Ca2+ gradients in the growth cone and that localized elevation of Ca2+ can trigger activation of Rac and Cdc42 [14, 46]. The effect of Ca2+ on Rac and Cdc42 activation requires protein kinase C (PKC), a calcium-sensitive kinase. The downstream targets of PKC in this process remain to be identified, but are likely to include regulators of Rac and Cdc42, such as GEFs, GAPs, and GDIs. These observations suggest that calcium-sensitive kinases can regulate Rac activation, linking asymmetric calcium signals to asymmetric activation of Rac.
3′-phosphoinositide phosphorylation downstream of UNC-40(DCC) signaling
Studies of cultured Xenopus neurons have indicated that phosphoinositide 3-kinase (PI3K) is required for the response to UNC-6(netrin) [37]. Consistent with this observation, genetic studies in C. elegans indicate that AGE-1, an ortholog of PI3K, functions downstream of UNC-40(DCC) to mediate the response to UNC-6(netrin) [24].
PI3K phosphorylates phosphoinositides at the 3′ position to produce PtdIns(3,4,5)P3 and PtdIns(3,4)P2. Although the role of 3′ PIs in axon guidance has not been fully tested, studies of chemotaxis in neutrophils, Dictyostelium, and fibroblasts provide insight into their likely function [47-51]. 3′ PIs are polarized in response to gradients of chemoattractants, such that they are localized to the side of the cell closest to the source of chemoattractant. Polarization can be induced by the direct delivery of exogenous 3′ PIs, indicating that polarity is generated at the level, or downstream, of 3′ PIs [52].
3′ PIs and the Rho GTPases have been implicated as part of an internal cellular ‘compass’ for eukaryotic chemotaxis [38, 53]. Genetic, pharmacological, and biochemical experiments have placed PI3K both upstream and downstream of Rac in neutrophil chemotaxis [38, 50, 54, 55]. These observations could be explained by postulating a positive-feedback loop between Rac and 3′ PIs. There is evidence for such a loop; polarity in neutrophils is stimulated by direct delivery of exogenous 3′ PIs and inhibitors of PI3K or Rho GTPases can block this effect [52]. As PI3K and Rac have both been implicated in axon guidance, it is likely that this positive-feedback loop is also important in generating polarity in neuronal growth cones (Figure 2).
Asymmetric localization of effectors for Rac and 3′ PIs
Asymmetric localization of 3′ PIs and activated Rac can lead to asymmetric recruitment of effectors for these molecules (Figure 3). Effectors are proteins that bind to and mediate the downstream effects of activated Rac and 3′ PIs. Below, we discuss the effectors for Rac and 3′ PIs that have been implicated in the response to UNC-6(netrin).
MIG-10(lamellipodin) is an asymmetrically localized effector for Rac and PtdIns(3,4)P2
Recent work has demonstrated that MIG-10 functions as an effector for Rac during axon guidance. Biochemical experiments have shown that the Ras Association-Plekstrin Homology (RAPH) domain of MIG-10, and its vertebrate ortholog lamellipodin, interact specifically with activated Rac. Genetic experiments have indicated that MIG-10 functions downstream of Rac to mediate the response to UNC-6(netrin) [20]. Consistent with these observations, the asymmetric localization of MIG-10 is dependent upon Rac. Together, these data suggest that asymmetric activation of Rac recruits asymmetric MIG-10(lamellipodin).
The asymmetric localization of MIG-10(lamellipodin) might also be controlled by interaction with the 3′ PI PtdIns(3,4)P2 [23]. Biochemical experiments have indicated that lamellipodin binds to PtdIns(3,4)P2 [28], and genetic experiments have shown that AGE-1(PI3K) and DAF-18(PTEN) (a dual-specificity phosphatase for proteins and lipids) are required for the asymmetric localization of MIG-10[23]. The AGE-1(PI3K) kinase and the DAF-18(PTEN) phosphatase are responsible for the phosphorylation and dephosphorylation of 3′ PIs, respectively. Together, these data suggest that asymmetrically localized PtdIns(3,4)P2 recruits MIG-10(lamellipodin) enabling it to become localized asymmetrically.
MIG-10, and its vertebrate ortholog lamellipodin, can regulate actin polymerization to promote formation of filopodia and lamellipodia, suggesting that asymmetric localization of MIG-10(lamellipodin) results in the asymmetric formation of lamellipodia and filopodia. RNAi knockdown experiments in cultured cells have shown that loss of lamellipodin impairs formation of lamellipodia [28, 56]. Conversely, overexpression of MIG-10 or lamellipodin in cultured cells causes an increase in the formation of lamellipodia [27, 28]. In neurons, loss of MIG-10 function impairs the formation of filopodia in response to UNC-6(netrin) [24]. The effect of MIG-10 and lamellipodin on actin dynamics is mediated, in part, through an interaction with UNC-34(enabled) [24, 27, 28], an actin-regulatory protein that can influence filopodial and lamellipodial dynamics [25]. However, loss of MIG-10 or lamellipodin function causes defects that are more severe than those observed after loss of UNC-34 or enabled [20, 28], indicating that MIG-10 and lamellipodin must also signal through other regulators of actin polymerization.
Other effectors for Rac and 3′3′ PIs in axon guidance
The WAVE complex is an effector for Rac that has been implicated in axon guidance (Table 1). When activated by Rac, the WAVE complex interacts with monomeric actin and the ARP2/3 complex to promote actin nucleation [57-59]. Loss of WAVE complex function causes axonal defects in Drosophila, including ectopic midline crossing in the CNS and ectopic branching of motor neurons [60, 61]. Loss of WAVE complex function also causes defects in axon guidance in the PDE neuron of C. elegans [59]. The localization and activation of the WAVE complex during axon guidance have not been studied. However, by analogy to MIG-10, Rac might asymmetrically recruit and/or activate the WAVE complex in response to guidance cues.
Table 1.
Rac effectors in axon guidance.
| Guidance Pathways | Effector | Downstream Targets |
|---|---|---|
| Netrin, Slit | MIG-10 | Enabled, ? |
| Netrin, Slit | PAK | LIM Kinase, MLC Kinase |
| ? | WAVE Complex | ARP 2/3 Complex |
The p21-activated kinase (PAK) proteins are also effectors for Rac that have been implicated in guidance (Table 1) [62-64]. The PAK proteins function downstream of Rac activation by phosphorylating LIM kinase and myosin light chain kinase (MLCK) kinase, both regulators of actin dynamics [65]. Genetic and biochemical studies in Drosophila and C. elegans have indicated that the PAK kinases are involved in axon guidance and that they function in parallel to MIG-10(lamellipodin) [20, 62, 64]. Although the localization of the PAK proteins has not been examined during the guidance response, we hypothesize that these proteins are asymmetrically localized and/or activated in response to guidance cues.
DOCK180 might act as an effector for 3′ PIs during axon guidance. DOCK180 is a GEF activator of Rac that binds to and functions downstream of PtdIns(3,4,5)P3 during cell migration [66, 67], suggesting that DOCK180 mediates a connection between the Rac and phosphoinositide signaling pathways. Genetic studies have indicated that CED-5, the C. elegans ortholog of DOCK180, functions in axon guidance [68]. Consistent with this observation, a recent study has found that DOCK180 functions in the response to UNC-6(netrin) [45]. In addition to binding to PtdIns(3,4,5)P3, DOCK180 also associates with UNC-40(DCC). This association might be mediated indirectly through the interaction between DOCK180 and PtdIns(3,4,5)P3. Consistent with this idea, both UNC-40(DCC) and PtdIns(3,4,5)P3 are found within lipid rafts [69-71]. Alternatively, it is possible that DOCK180 binds to both UNC-40(DCC) and PtdIns(3,4,5)P3. The relationships between PtdIns(3,4,5)P3, DOCK180 and UNC-40(DCC) are an important area for future investigations.
Polarization model for axon guidance: implications for understanding attractive and repulsive guidance
The recent findings discussed here suggest that guidance cues cause the asymmetric localization of signaling complexes and the polarization of axon outgrowth. Similar to events required for chemotaxis of other cell types, axon guidance may require functionally linked signaling events that work synergistically to direct movement when neurons sense gradients of extracellular guidance cues. Although growth cones can respond to shallow gradients of extracellular guidance cues [72], in order for a directional response to occur it is thought that shallow gradients of extracellular guidance cues must be amplified into a steep intracellular signaling gradient [73]. This gradient amplification may be manifested by the asymmetric localization of signaling complexes, such as those involving UNC-40 and MIG-10 in the neuron.
The establishment of polarization may be analogous to that which occurs in other cell types during events such as asymmetric cell division, epithelial polarity development, and cell migrations. For example, in axon guidance, the asymmetric activation of Rac causes polarized recruitment of MIG-10 [20]. During asymmetric cell division, establishment of epithelial polarity and cell migration, asymmetric activation of the GTPase Cdc42 causes polarized recruitment of the adaptor protein Par6, a cytoplasmic polarity protein [74-76]. Thus, in each context, the Rho family GTPase promotes polarity by asymmetrically recruiting an effector molecule.
By considering that the asymmetric localization of signaling complexes can lead to polarized axon outgrowth, the concepts of attractive and repulsive axon guidance can be considered in a different manner. Currently, it is commonly proposed that attractive guidance cues promote actin-based protrusive activity, whereas repulsive guidance cues inhibit actin-based protrusive activity [8]. Therefore, it was surprising when activation of Rac, which promotes actin-based protrusive activity, was implicated in both attractive and repulsive guidance responses [39, 40, 62, 77]. However, within the context of a polarization model, attraction might be considered as being the result of extracellular cues polarizing Rac activation to one side of the growth cone, whereas repulsion might be considered as the result of Rac activation being polarized to the opposite side of the growth cone. Thus, whether a cue induces an ‘attractive’ or ‘repulsive’ response may be dictated by the relative asymmetric localization of signaling complexes, rather than the activation of opposing cytoskeletal effectors.
While we have described here the outgrowth promoting activity of MIG-10(lamellipodin) in response to UNC-6(netrin) attraction, MIG-10 is also involved in the repulsive response to SLT-1 [27]. Although it has not been possible to visualize the localization of MIG-10 in response to SLT-1(Slit), it is likely to be polarized to the side of the growth cone furthest from the source of SLT-1. This hypothesis is supported by the observation that MIG-10 overexpression enhances axon outgrowth away from SLT-1 sources [27]. Future experiments examining the localization of MIG-10 or lamellipodin during repulsive responses may provide more direct evidence for this hypothesis.
The idea that attraction and repulsion depend on the relative localization of signaling complexes is also supported by two recent studies of cultured Xenopus neurons. The first study found that actin translation is required for guidance and that during attractive guidance, actin is asymmetrically translated on the side of the growth cone closest to the source of guidance cue. Conversely, during repulsive guidance, actin is asymmetrically translated on the opposite side of the growth cone [18]. The second study found that phosphorylated cofilin is localized to one side of the growth cone during an attractive response and to the opposite side during a repulsive response [22].
Concluding remarks
Here, we have reviewed recent evidence suggesting that signaling complexes become asymmetrically localized in response to guidance cues. The asymmetric localization of receptors causes asymmetric activation of Rac and localization of 3′ PIs (Figure 3). This in turn causes the asymmetric recruitment and/or activation of effectors for Rac and 3′ PIs. These effectors can promote actin-based protrusive activity, thereby causing the polarized outgrowth of the growth cone.
Future investigations are likely to determine how asymmetry is established in the growth cone. In particular, it will be important to determine how the guidance receptors become asymmetrically localized. This process might involve positive-feedback loops between Rac, phosphoinositides, and the guidance receptors. Additional likely contributors include microtubules, lipid rafts, calcium signaling and membrane trafficking pathways. In addition, it will be important to further characterize the roles of Rac and 3′ PI effectors in modulating actin dynamics. Together these future studies are likely to provide a more complete understanding of growth cone guidance that will impact our understanding of normal axon development and of diseases that affect the nervous system.
Acknowledgments
We thank Elizabeth Ryder for comments on the manuscript. This work was supported by National Institutes of Health grants F32NS468402 (C.C.Q.) and R01NS033156 (W.G.W). This work was also supported by grants from the New Jersey Commission on Spinal Cord Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Yaron A, Zheng B. Navigating their way to the clinic: emerging roles for axon guidance molecules in neurological disorders and injury. Dev Neurobiol. 2007;67:1216–1231. doi: 10.1002/dneu.20512. [DOI] [PubMed] [Google Scholar]
- 3.Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilton JK. Molecular mechanisms of axon guidance. Dev Biol. 2006;292:13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Garbe DS, Bashaw GJ. Axon guidance at the midline: from mutants to mechanisms. Crit Rev Biochem Mol Biol. 2004;39:319–341. doi: 10.1080/10409230490906797. [DOI] [PubMed] [Google Scholar]
- 6.Round J, Stein E. Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa S, Thomas JB. Secreted cell signaling molecules in axon guidance. Curr Opin Neurobiol. 2004;14:45–50. doi: 10.1016/j.conb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58:92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- 9.Lin CH, Forscher P. Cytoskeletal remodeling during growth cone-target interactions. J Cell Biol. 1993;121:1369–1383. doi: 10.1083/jcb.121.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor TP, Bentley D. Accumulation of actin in subsets of pioneer growth cone filopodia in response to neural and epithelial guidance cues in situ. J Cell Biol. 1993;123:935–948. doi: 10.1083/jcb.123.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pak CW, et al. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- 12.Sabry JH, et al. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou FQ, Cohan CS. How actin filaments and microtubules steer growth cones to their targets. J Neurobiol. 2004;58:84–91. doi: 10.1002/neu.10278. [DOI] [PubMed] [Google Scholar]
- 14.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 15.Leung KM, et al. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tojima T, et al. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- 17.Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao J, et al. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- 19.Mintz CD, et al. ERM proteins regulate growth cone responses to Sema3A. J Comp Neurol. 2008;510:351–366. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn CC, et al. CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr Biol. 2008;18:808–813. doi: 10.1016/j.cub.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suter DM, et al. Microtubule dynamics are necessary for SRC family kinase-dependent growth cone steering. Curr Biol. 2004;14:1194–1199. doi: 10.1016/j.cub.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Wen Z, et al. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler CE, et al. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, et al. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 25.Drees F, Gertler FB. Ena/VASP: proteins at the tip of the nervous system. Curr Opin Neurobiol. 2008;18:53–59. doi: 10.1016/j.conb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killeen MT, Sybingco SS. Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Quinn CC, et al. UNC-6/netrin and SLT-1/slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr Biol. 2006;16:845–853. doi: 10.1016/j.cub.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Krause M, et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7:571–583. doi: 10.1016/j.devcel.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Bouzigues C, et al. Asymmetric redistribution of GABA receptors during GABA gradient sensing by nerve growth cones analyzed by single quantum dot imaging. Proc Natl Acad Sci U S A. 2007;104:11251–11256. doi: 10.1073/pnas.0702536104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golub T, et al. Spatial and temporal control of signaling through lipid rafts. Curr Opin Neurobiol. 2004;14:542–550. doi: 10.1016/j.conb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Guirland C, et al. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 32.Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci. 2007;10:161–168. doi: 10.1038/nn1835. [DOI] [PubMed] [Google Scholar]
- 33.Watari-Goshima N, et al. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007;10:169–176. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- 34.Moore SW, et al. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development. 2008;135:2855–2864. doi: 10.1242/dev.024133. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni G, et al. CLEC-38, A Transmembrane Protein with C-Type Lectin-Like Domains, Negatively Regulates UNC-40-Mediated Axon Outgrowth and Promotes Presynaptic Development in Caenorhabditis elegans. J Neurosci. 2008;28:4541–4550. doi: 10.1523/JNEUROSCI.5542-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundquist EA. Rac proteins and the control of axon development. Curr Opin Neurobiol. 2003;13:384–390. doi: 10.1016/s0959-4388(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 37.Ming G, et al. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 38.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gitai Z, et al. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- 40.Li X, et al. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J Biol Chem. 2002;277:37788–37797. doi: 10.1074/jbc.M205428200. [DOI] [PubMed] [Google Scholar]
- 41.Li X, et al. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J Biol Chem. 2002;277:15207–15214. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]
- 42.Shekarabi M, Kennedy TE. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol Cell Neurosci. 2002;19:1–17. doi: 10.1006/mcne.2001.1075. [DOI] [PubMed] [Google Scholar]
- 43.Shekarabi M, et al. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briancon-Marjollet A, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, et al. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin M, et al. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J Neurosci. 2005;25:2338–2347. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funamoto S, et al. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 48.Haugh JM, et al. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meili R, et al. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Servant G, et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F, et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- 52.Weiner OD, et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 54.Benard V, et al. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 55.Hawkins PT, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 56.Lafuente EM, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Eden S, et al. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 58.Innocenti M, et al. Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol. 2004;6:319–327. doi: 10.1038/ncb1105. [DOI] [PubMed] [Google Scholar]
- 59.Shakir MA, et al. The Arp2/3 activators WAVE and WASP have distinct genetic interactions with Rac GTPases in C. elegans axon guidance. Genetics. 2008;179 doi: 10.1534/genetics.108.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schenck A, et al. WAVE/SCAR, a multifunctional complex coordinating different aspects of neuronal connectivity. Dev Biol. 2004;274:260–270. doi: 10.1016/j.ydbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Zallen JA, et al. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol. 2002;156:689–701. doi: 10.1083/jcb.200109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan X, et al. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–127. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- 63.Hing H, et al. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 64.Lucanic M, et al. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development. 2006;133:4549–4559. doi: 10.1242/dev.02648. [DOI] [PubMed] [Google Scholar]
- 65.Linseman DA, Loucks FA. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Front Biosci. 2008;13:657–676. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- 66.Cote JF, et al. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol. 2005;7:797–807. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lundquist EA, et al. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 2001;128:4475–4488. doi: 10.1242/dev.128.22.4475. [DOI] [PubMed] [Google Scholar]
- 69.Arcaro A, et al. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell Signal. 2007;19:1081–1092. doi: 10.1016/j.cellsig.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005;169:151–165. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Mouton C, et al. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosoff WJ, et al. A new chemotaxis assay shows the extreme sensitivity of axons to molecular gradients. Nat Neurosci. 2004;7:678–682. doi: 10.1038/nn1259. [DOI] [PubMed] [Google Scholar]
- 73.Mortimer D, et al. Growth cone chemotaxis. Trends Neurosci. 2008;31:90–98. doi: 10.1016/j.tins.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Atwood SX, et al. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J Cell Sci. 2007;120:3200–3206. doi: 10.1242/jcs.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burridge K, Doughman R. Front and back by Rho and Rac. Nat Cell Biol. 2006;8:781–782. doi: 10.1038/ncb0806-781. [DOI] [PubMed] [Google Scholar]
- 76.Hutterer A, et al. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Schmucker D. Downstream of guidance receptors: entering the baroque period of axon guidance signaling. Neuron. 2003;40:4–6. doi: 10.1016/s0896-6273(03)00603-2. [DOI] [PubMed] [Google Scholar]