Abstract
Background
To our knowledge, the effect of kidney function on successful aging has not been examined.
Methods:
We evaluated the relationship between cystatin C and aging success during a 6-year follow-up in the Cardiovascular Health Study, a community-based cohort of older adults (aged ≥65 years). Successful aging was defined as remaining free of cardiovascular disease, cancer, and chronic obstructive pulmonary disease and having intact physical and cognitive functioning. In adjusted analysis, an accelerated failure time model was used to evaluate the percentage reduction in successful years by level of cystatin C. A separate Cox proportional hazards model evaluated whether cystatin C was related to incident physical and cognitive disability.
Results:
A total of 2140 participants had cystatin C measured and were free of the previously mentioned conditions at baseline. Their mean age was 74 years. The mean cystatin C level, creatinine level, and estimated glomerular filtration rate were 1.06 mg/L, 0.93 mg/dL, and 78 mL/min/1.73 m2, respectively (to convert cystatin C to nanomoles per liter, multiply by 75; and to convert creatinine to micromoles per liter, multiply by 88.4). A total of 873 participants reached a first event in follow-up, 138 because of cognitive disability, 238 because of physical disability, 34 because of chronic obstructive pulmonary disease, 146 because of cancer, and 317 because of cardiovascular disease. The adjusted percentage reduction in successful life years in the highest vs the lowest quartile of cystatin C was 27% (95% confidence interval, 11%-39%). The highest vs lowest quartile of cystatin C also was independently associated with incident cognitive or physical disability (hazard ratio, 1.39; 95% confidence interval, 1.00-1.98).
Conclusion:
A higher cystatin C level, even within a range of relatively normal kidney function, was associated with unsuccessful aging.
Elderly persons are the fastest-growing subset of the US population, and age-associated increases in chronic disease and disability have led to a significant financial burden on health care.1 At the opposite end of the spectrum from disability is successful aging. Although there is no universally accepted definition of successful aging, one commonly used definition includes aging with lack of physical or cognitive impairment and without the development of major life-threatening chronic diseases.2,3 Unsuccessful aging is not inevitable, and evidence suggests that the aging process may be modified.4 The evaluation of risk factors for unsuccessful aging, in particular those that may be preventable, is, therefore, an important avenue of research.
Kidney function is known to decline with age; however, this age-related decline is highly variable5 and some older individuals have little change in kidney function while others have a rapid decline. Kidney dysfunction, as ascertained by elevated creatinine level, is also associated with inflammation and subclinical cardiovascular disease (CVD),6,7 2 factors that may promote unsuccessful aging; therefore, kidney dysfunction may be associated with unsuccessful aging.
A new serum measurement of kidney filtration, cystatin C, has allowed an advance in our understanding of the impact of kidney function on age-related health. Cystatin C is a cysteine proteinase inhibitor that is produced by nearly all human cells and released into the bloodstream, from which it is freely filtered by the kidney glomerulus and metabolized by the proximal tubule.8 Although the relative contribution of factors other than glomerular filtration rate (GFR) to serum cystatin C concentrations remains to be determined, the association of serum cystatin C with GFR seems to be independent of age, sex, and muscle mass, in contrast to serum creatinine.9-12 Whereas elevated serum creatinine levels detect only the small subset of elderly individuals with the most impaired kidney function who are at increased risk for CVD, cystatin C has a linear association with clinical CVD.13,14
The goal of this analysis was to evaluate the association of kidney function, as ascertained by cystatin C, with maintenance of successful aging in the Cardiovascular Health Study (CHS), a community cohort of older adults (aged ≥65 years).
METHODS
The CHS is a community-based longitudinal study of adults who were 65 years or older at baseline. The objective of the study was to evaluate risk factors for the development and progression of CVD.15 A main cohort of 5201 participants was recruited between June 10, 1989, and May 30, 1990, from 4 US communities (Sacramento County, California; Forsyth County, North Carolina; Washington County, Maryland; and Allegheny County, Pennsylvania).16 An additional 687 African American persons were recruited November 17, 1992, through June 11, 1993. Eligible participants were sampled from Medicare eligibility lists in each area. Subjects were excluded if they were institutionalized, required a proxy, were unable to provide informed consent, were planning to move out of the area within 3 years after recruitment, required a wheelchair in the home, were receiving hospice care, or were undergoing radiation or chemotherapy for cancer. Follow-up interviews for events were conducted semiannually at annual examinations and through interim 6-month telephone calls.
Institutional review board approval for the data collection procedures of the CHS was obtained at each of the 4 clinical sites and at the Data Coordinating Center (University of Washington). In addition, institutional review board approval for this study was obtained from Tufts–New England Medical Center.
DEFINITION OF SUCCESSFUL AGING
Maintenance of successful aging was defined as in prior studies and included remaining free of 3 major classes of disease (incident cancer, CVD, and chronic obstructive pulmonary disease [COPD]) and without a persistent physical disability or cognitive impairment.2,3 Because the goal of this analysis was to assess the risk of incident unsuccessful aging, we excluded participants with prevalent clinical CVD (n=1316), COPD (n=424), cancer (n=464), cognitive impairment (n=243), or physical disability (n=147) at baseline.
MEASUREMENT OF CYSTATIN C
Cystatin C was measured from frozen samples that were collected at the 1992 to 1993 visit of the CHS. Assays were performed on fasting samples that were stored at −70°C. Cystatin C was measured by using a BNII nephelometer (Dade Behring, Inc, Deerfield, Illinois) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, Inc).17 Polystyrene particles were coated with monoclonal antibodies to cystatin C that agglutinate in the presence of antigen (cystatin C) to increase the intensity of scattered light. The increase in scattered light is proportional to the amount of cystatin C in the sample. The assay range is from 0.195 to 7.330 mg/L (to convert to nanomoles per liter, multiply by 75); the reference range for young healthy persons is from 0.53 to 0.95 mg/L.18 The intra-assay coefficient of variation ranges from 2.0% to 2.8%, and the interassay coefficient of variation ranges from 2.3% to 3.1%. Among 61 healthy persons with 3 cystatin C measurements during a 6-month period, the intraindividual coefficient of variation was 7.7%.13
COVARIATES
Candidate covariates included demographic characteristics (age, sex, and race); traditional cardiovascular risk factors (hypertension, systolic and diastolic blood pressure, body mass index, smoking [former vs never and current vs never], diabetes mellitus [defined by use of an oral hypoglycemic agent or insulin or a fasting glucose level of ≥126 mg/dL {to convert to millimoles per liter, multiply by 0.0555}], and fasting levels of low-density lipoprotein cholesterol and high-density lipoprotein cholesterol); novel risk factors (C-reactive protein, hemoglobin, albumin, fibrinogen, and insulin levels); noninvasive tests of subclinical vascular disease (ankle-arm index [AAI] of <0.9, left ventricular hypertrophy on electrocardiography, and common and internal carotid intima-media thickness by ultrasonography); and a measure of lung function (forced expiratory volume in the first second of expiration).
OUTCOMES
Incident CVD was defined as angina, myocardial infarction, a cardiac revascularization procedure, congestive heart failure, stroke, or a transient ischemic attack.
Incident cancer was defined by hospitalization with a diagnosis of cancer using any International Classification of Diseases, Ninth Revision (ICD-9) discharge code identifying cancer other than nonmelanotic skin cancer.
Incident COPD was defined using hospital discharge codes 491, 492,and 493 for bronchitis, emphysema, and asthma, respectively.
Physical disability was ascertained using annual telephone or clinic interviews to determine the presence or severity of self-reported difficulty with 1 or more of 6 activities of daily living, including walking around the home, getting out of bed, eating, dressing, bathing, or using the toilet.15,19 Physical disability was defined as self-report of difficulty with any activity of daily living on 2 consecutive clinic visits or a single activity of daily living difficulty in a participant who subsequently died or was lost to follow-up.
Incident cognitive impairment was ascertained using annual telephone or clinic interviews to determine whether results on a clinic-administered cognitive function test indicated cognitive impairment. Cognitive impairment was defined according to previous criteria2,20 as a score lower than 80 on the 100-point modified Mini-Mental State Examination (MMSE)21 on 2 consecutive visits or lower than 80 on a single visit in a person with no follow-up measures on the MMSE. Consecutive visits are defined as 2 visits with data; for example, if a participant missed year 7, then years 6 and 8 would be considered consecutive for that participant. The date of decline is defined as the first of the 2 consecutive visits recording decline, to allow consistency for participants who declined at one visit but had no subsequent data. Both baseline MMSE score and decline in MMSE score in follow-up are associated with incident dementia; therefore, the outcome variable is clinically important.22
The last day of follow-up for this analysis was June 30, 1998, for hospitalizations and June 30, 1999, for clinic visits to allow for ascertainment of cognitive or physical disability through June 30, 1998.
STATISTICAL ANALYSES
We compared the distribution of potential covariates by quartiles of cystatin C. P values for linear trend across the quartile were calculated. Covariates were selected as candidates for multivariate analysis based on their potential to confound the association of cystatin C with successful aging. Candidate covariates for adjustment (previously listed) were retained in the final models if they modified the coefficient of cystatin C by 5% or more. Several other variables were forced into the models based on their known association with successful aging: age, sex, race, body mass index, smoking, C-reactive protein level, forced expiratory volume in the first second of expiration, common and internal carotid intima-media thickness, and hemoglobin level. Baseline MMSE was also included in the models because it intuitively predicts a future MMSE score of less than 80.22
An accelerated failure time model was used to evaluate the percentage reduction in successful years experienced by a participant with higher cystatin C levels compared with those with lower levels.23 In an accelerated failure time model, the parameter coefficients are interpreted as acting multiplicatively over time. Thus, the parameter reflects the rate of decline in successful life years. The β coefficients estimated from this model were converted to the percentage difference in time of successful life years through the following equation: (eβ−1)×100%. For a dichotomous variable, the exponential of the parameter estimate gives an estimate of expected successful life expectancy for persons with vs those without the characteristic. For continuous variables, this value indicates the percentage difference in (speed [or delay]) life expectancy time associated with a 1-U increment in the explanatory variable. For categorical variables, this value represents the percentage difference in (speed [or delay]) life expectancy for each level compared with the reference level. Positive values imply longer successful life expectancy years, and negative values imply shorter life expectancy. Deaths were censored as neutral in these analyses.
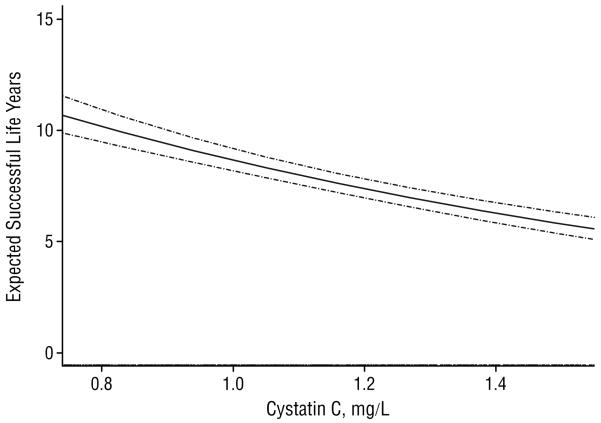
Analyses were performed using cystatin C as a continuous variable (per standard deviation change in cystatin C) and by quartile of cystatin C. Because there are no accepted cut points for cystatin C, we chose quartile cut points because they are relatively unbiased and frequently used in the medical literature. We also fitted a smooth function of cystatin C via splines using the accelerated failure model with a Weibull distribution. The middle 95% of the range is shown in this figure because outliers have a powerful influence on the shape of the spline. We then predicted the expected successful years of life using these estimates of the regression coefficients and the range of cystatin C from the original data.
Figure.
Smoothed spline of expected successful life years and baseline cystatin C level. Pointwise 95% confidence intervals (dashed lines) and a rug of the middle 95% of cystatin C measures are shown. To convert cystatin C to nanomoles per liter, multiply by 75.
DISABILITY ANALYSIS
Because our prior work has shown an association of cystatin C and CVD, a component of the composite outcome, we also evaluated a successful aging outcome that did not contain CVD. We analyzed cystatin C with incident cognitive impairment and physical disability using Cox proportional hazards analyses. In these analyses, participants who developed CVD, cancer, or COPD were censored at the time of these diagnoses.
GFR ANALYSES
While previous studies13,14 have demonstrated that cystatin C is more closely associated with incident heart failure and all-cause and cardiovascular-related mortality than estimated GFR, the association of estimated GFR with reduction in successful life years and incident disability (as previously defined) has not been evaluated. We, therefore, performed additional analyses evaluating whether estimated GFR is associated with reduction in successful life years and incident disability. These models adjusted for the same variables as the primary analyses. We calculated the estimated GFR from serum creatinine by using the modified 4-variable version of the Modification of Diet in Renal Disease formula.24 Creatinine was indirectly calibrated with the Cleveland Clinic laboratory—the core laboratory of the Modification of Diet in Renal Disease trial—as previously described.25,26
Results were considered statistically significant at P<.05. Statistical analyses were performed with commercially available software programs (SPSS 13.0.1 software for Windows [SPSS Inc, Chicago, Illinois] and Stata 9.2 for Windows [Stata Corp, College Station, Texas]).
RESULTS
A total of 2140 participants had cystatin C measured and were free of CVD, cancer, COPD, cognitive impairment, and physical disability at the 1992 to 1993 visit of the CHS. The mean age of the cohort was 74 years, 38.0% were men, and 12.0% had diabetes mellitus. The mean (median) cystatin C, creatinine, and estimated GFR were 1.06 (1.02) mg/L, 0.93 (0.89) mg/dL, and 78 (76) mL/min/1.73 m2, respectively. (To convert creatinine to micromoles per liter, multiply by 88.4.)
Participants with higher cystatin Clevels were more likely to be older and white and to have higher body mass index, fibrinogen, C-reactive protein, and insulin levels, a higher prevalence of electrocardiographic left ventricular hypertrophy, an AAI of 0.9 or less, and lower levels of high-density lipoprotein cholesterol and albumin (Table 1).
Table 1.
Baseline Characteristics by Quartile of Cystatin C
| Cystatin C Quartile, mg/La |
|||||
|---|---|---|---|---|---|
| Characteristic | ≤0.90 (n=554) |
0.91-1.01 (n=515) |
1.02-1.15 (n=526) |
≥1.16 (n=545) |
P Value for Trend |
| Age, y | 72 (4) | 73 (4) | 74 (5) | 76 (5) | < .001 |
| Male sexb | 155 (28.0) | 186 (36.1) | 239 (45.4) | 233 (42.8) | < .001 |
| African American raceb | 122 (22.0) | 85 (16.5) | 62 (11.8) | 46 (8.4) | < .001 |
| High school graduateb | 450 (81.2) | 412 (80.0) | 420 (79.8) | 401 (73.6) | .003 |
| Ever smokedb | 272 (49.1) | 239 (46.4) | 279 (53.0) | 285 (52.3) | .12 |
| Diabetes mellitusb | 69 (12.5) | 49 (9.5) | 59 (11.2) | 80 (14.7) | .18 |
| Hypertensionb | 170 (30.7) | 150 (29.1) | 195 (37.1) | 276 (50.6) | < .001 |
| Blood pressure, mm Hg | |||||
| Diastolic | 72 (12) | 72 (10) | 72 (11) | 71 (11) | .74 |
| Systolic | 134 (20) | 135 (21) | 136 (20) | 138 (23) | .001 |
| Hypertension medicationsb | 189 (34.1) | 166 (32.2) | 205 (39.0) | 297 (54.5) | < .001 |
| BMI | 25.9 (4.1) | 26.4 (4.1) | 27.0 (4.5) | 27.6 (4.8) | < .001 |
| Cholesterol, mg/dL | |||||
| Total | 212 (34) | 209 (36) | 209 (37) | 207 (41) | .05 |
| LDL | 128 (32) | 127 (31) | 129 (34) | 126 (35) | .67 |
| HDL | 60 (15) | 55 (14) | 53 (13) | 49 (12) | < .001 |
| Triglycerides, mg/dL | 122 (68) | 138 (74) | 139 (73) | 163 (97) | < .001 |
| Albumin, g/dL | 3.95 (0.27) | 3.90 (0.28) | 3.93 (0.25) | 3.89 (0.28) | .002 |
| Fibrinogen, mg/dL | 312 (57) | 318 (65) | 319 (59) | 338 (71) | < .001 |
| Hemoglobin, g/dL | 13.7 (1.8) | 13.7 (1.3) | 13.9 (1.3) | 13.7 (1.3) | .88 |
| Glucose, mg/dL | 107 (35) | 105 (29) | 105 (28) | 110 (41) | .17 |
| Insulin, μIU/mLc | 9.0 (6.0-12.0) | 9.0 (7.0-13.5) | 10.0 (7.0-15.0) | 11.0 (8.0-15.3) | < .001 |
| CRP, mg/Lc | 2.01 (0.97-4.52) | 2.27 (1.07-5.18) | 2.30 (1.12-4.87) | 3.33 (1.46-6.92) | < .001 |
| ECG LVHb | 20 (3.6) | 13 (2.5) | 29 (5.5) | 24 (4.4) | .16 |
| AAI < 0.9b | 26 (4.7) | 23 (4.5) | 44 (8.4) | 66 (12.1) | < .001 |
| Intima-media thickness, mm | |||||
| Common carotid | 1.02 (0.19) | 1.03 (0.19) | 1.06 (0.20) | 1.08 (0.22) | < .001 |
| Internal carotidc | 1.12 (0.93-1.49) | 1.11 (0.93-1.43) | 1.30 (1.02-1.65) | 1.29 (1.01-1.75) | < .001 |
| FEV1, L | 2.06 (0.58) | 2.07 (0.61) | 2.13 (0.62) | 1.99 (0.60) | .21 |
| MMSE score | 93.3 (4.9) | 93.2 (5.0) | 93.1 (4.9) | 92.2 (5.1) | < .001 |
| Creatinine, mg/dL | 0.77 (0.15) | 0.86 (0.18) | 0.95 (0.19) | 1.14 (0.50) | < .001 |
| Estimated GFR, mL/min/1.73 m2 | 92 (17) | 82 (15) | 75 (14) | 62 (15) | < .001 |
Abbreviations: AAI, ankle-arm index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRP, C-reactive protein; ECG, electrocardiographic; FEV1, forced expiratory volume in the first second of expiration; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVH, left ventricular hypertrophy; MMSE, Mini-Mental State Examination.
SI conversion factors: To convert albumin and hemoglobin to grams per liter, multiply by 10; creatinine to micromoles per liter, multiply by 88.4; CRP to nanomoles per liter, multiply by 9.524; cystatin C to nanomoles per liter, multiply by 75; fibrinogen to micromoles per liter, multiply by 0.0294; glucose to millimoles per liter, multiply by 0.0555; HDL, LDL, and total cholesterol to millimoles per liter, multiply by 0.0259; insulin to picomoles per liter, multiply by 6.945; and triglycerides to millimoles per liter, multiply by 0.0113.
Data are given as mean (SD) unless otherwise indicated.
Data are given as number (percentage) of each group.
Data are given as median (interquartile range).
The mean (median) follow-up was 4.3 (5.2) years. A total of 873 participants reached a first event in follow-up: 138 (15.8%) because of cognitive impairment, 238 (27.3%) because of physical disability, 34 (3.9%) because of COPD, 146 (16.7%) because of cancer, and 317 (36.3%) because of CVD. Patients in the higher quartiles of cystatin C were more likely to develop CVD, physical disability, and cognitive impairment (Table 2).
Table 2.
Frequency of Outcomes by Cystatin C Quartiles
| Cystatin C Quartile, mg/La |
|||||
|---|---|---|---|---|---|
| Outcome | ≤0.90 (n = 554) |
0.91-1.01 (n = 515) |
1.02-1.15 (n = 526) |
≥1.16 (n = 545) |
P Value for Trend |
| Total | 179 (32.3) | 181 (35.1) | 230 (43.7) | 283 (51.9) | < .001 |
| First failure type | |||||
| Cognitive impairment | 25 (4.5) | 28 (5.4) | 28 (5.3) | 57 (10.5) | < .001 |
| Physical disability | 50 (9.0) | 56 (10.9) | 74 (14.1) | 58 (10.6) | .002 |
| COPD | 10 (1.8) | 9 (1.7) | 5 (1.0) | 10 (1.8) | .64 |
| Cancer | 37 (6.7) | 30 (5.8) | 35 (6.7) | 44 (8.1) | .02 |
| CVD | 57 (10.3) | 58 (11.3) | 88 (16.7) | 114 (20.9) | < .001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
SI conversion factor: To convert cystatin C to nanomoles per liter, multiply by 75.
Data are given as number (percentage) of each group. The individual percentages may not sum to the total percentage because of rounding.
In unadjusted analysis, each SD (0.26 mg/L) increase in cystatin C was associated with a 20% (95% confidence interval [CI], 16%-23%) (P<.001) reduction in successful life years. In adjusted analysis, this relationship was somewhat attenuated (11% reduction; 95% CI, 5%-16%) (P<.001) per standard deviation increase. Similarly, in unadjusted analysis, higher quartiles of cystatin C were associated with a progressive reduction in successful life years (Table 3). The third and fourth quartiles of cystatin C were associated with a 25% and 27% reduction in successful life years, respectively (Table 3), in adjusted analysis. The risk in the highest quartile of cystatin C was comparable to that of an AAI of less than 0.9, electrocardiographic left ventricular hypertrophy, prevalent diabetes mellitus, and current smoking and stronger than a 5-year increase in age and being male, African American, or obese.
Table 3.
Unadjusted and Adjusted Reduction in Successful Life Years by Quartile of Cystatin C
| Risk Factor | Reduction in Successful Life Years, % (95% Confidence Interval) |
P Value |
|---|---|---|
| Unadjusted Model | ||
| Cystatin C quartile, mg/L | ||
| ≤0.90 | 0 [Reference] | NA |
| 0.91-1.01 | −9 (−24 to 10) | .34 |
| 1.02-1.15 | −28 (−40 to −14) | <.001 |
| ≥1.16 | −45 (−53 to −35) | <.001 |
|
| ||
| Adjusted Modela | ||
| Cystatin C quartile, mg/L | ||
| ≤ 0.90 | 0 [Reference] | NA |
| 0.91-1.01 | −14 (−29 to 4) | .12 |
| 1.02-1.15 | −25 (−38 to −11) | .002 |
| ≥1.16 | −27 (−39 to −11) | .001 |
| Age (per 5-y increase) | −17 (−23 to −11) | <.001 |
| Male sex | 4 (−13 to 24) | .66 |
| African American vs non-African American | 1 (−16 to 23) | .89 |
| BMI | ||
| <25 | 0 [Reference] | NA |
| 25-30 | −2 (−15 to 13) | .78 |
| >30 | −8 (−23 to 10) | .35 |
| Hypertension | −5 (−17 to 8) | .40 |
| LVH | −30 (−46 to −10) | .006 |
| Diabetes mellitus | −27 (−39 to −13) | <.001 |
| Smoking | ||
| Never | 0 [Reference] | NA |
| Former | −21 (−31 to −9) | .001 |
| Current | −36 (−48 to −21) | <.001 |
| AAI <0.9 | −24 (−38 to −7) | .007 |
Abbreviations: AAI, ankle-arm index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LVH, left ventricular hypertrophy; NA, data not applicable.
SI conversion factor: To convert cystatin C to nanomoles per liter, multiply by 75.
Adjusted for C-reactive protein level, hemoglobin level, Mini-Mental State Examination score, common and internal carotid intima-media thickness, and forced expiratory volume in the first second of expiration in addition to the other variables mentioned. Variables from Table 1 did not change the cystatin C coefficient by 5% and are, therefore, not presented. In addition, no other variables in Table1 were significantly associated with a reduction in successful life years.
Results of the spline analysis demonstrate a decrease in predicted successful life years as cystatin C increases (Figure).
DISABILITY ANALYSIS
During follow-up, 238 persons developed physical disability and 138 developed cognitive impairment. In unadjusted analysis, the highest quartile of cystatin C was associated with an approximately 2-fold risk of incident disability (Table 4). After multivariate adjustment, the upper 2 quartiles were each associated with an approximately 40% increase in risk of physical or cognitive disability compared with the lowest quartile.
Table 4.
Data for the Composite of Cognitive Impairment and Physical Disability in Unadjusted and Adjusted Analyses
| Risk Factor | Hazard Ratio (95% Confidence Interval) |
P Value |
|---|---|---|
| Unadjusted Model | ||
| Cystatin C quartile, mg/L | ||
| ≤0.90 | 1 [Reference] | NA |
| 0.91-1.01 | 1.31 (0.94-1.83) | .11 |
| 1.02-1.15 | 1.72 (1.26-2.37) | .001 |
| ≥ 1.16 | 1.98 (1.45-2.71) | <.001 |
|
| ||
| Adjusted Model a | ||
| Cystatin C quartile, mg/L | ||
| ≤ 0.90 | 1 [Reference] | NA |
| 0.91-1.01 | 1.31 (0.91-1.89) | .15 |
| 1.02-1.15 | 1.56 (1.09-2.23) | .02 |
| ≥1.16 | 1.39 (1.00-1.98) | .049 |
| Age (per 5-y increase) | 1.54 (1.34-1.76) | <.001 |
| Male sex | 0.66 (0.46-0.95) | .02 |
| African American race | 0.89 (0.46-1.29) | .54 |
| BMI | ||
| <25 | 1 [Reference] | NA |
| 25-30 | 1.51 (1.14-2.01) | .004 |
| >30 | 1.63 (1.14-2.34) | .007 |
| Hypertension | 0.96 (0.75-1.25) | .78 |
| LVH | 1.31 (0.78-2.19) | .31 |
| Diabetes mellitus | 1.38 (0.98-1.95) | .06 |
| Smoking | ||
| Never | 1 [Reference] | NA |
| Former | 1.21 (0.92-1.58) | .17 |
| Current | 1.73 (1.14-2.63) | .01 |
| AAI <0.9 | 1.19 (0.79-1.80) | .40 |
Abbreviations: See Table 3.
SI conversion factor: To convert cystatin C to nanomoles per liter, multiply by 75.
Adjusted for C-reactive protein level, hemoglobin level, Mini-Mental State Examination score, common carotid intima-media thickness, internal carotid intima-media thickness, and forced expiratory volume in the first second of expiration in addition to the other variables mentioned. Variables from Table 1 did not change the cystatin C coefficient by 5% and are, therefore, not included.
ANALYSIS USING ESTIMATED GFR
Glomerular filtration rate quartiles (1-4) were as follows: greater than 88.8, 76.4 to 88.8, 65.8 to 76.3, and less than 65.8 mL/min/1.73 m2, respectively. In adjusted analyses, in comparison with the first quartile, the second, third, and fourth quartiles were associated with an 8% increase (95% CI, − 10% to 30%; P=.40), a 9% increase (95% CI, −9% to 30%; P=.36), and a 16% decrease (95% CI, − 29% to 0%; P=.05), respectively, in successful life years. In the disability analysis, the second, third, and fourth quartiles were associated with a 0.90 (95% CI, 0.65-1.23; P=.49), 0.68 (95% CI, 0.49-0.94; P = .02), and 0.94 (95% CI, 0.68-1.29; P = .69) hazard of reaching the composite end point.
COMMENT
The results of this analysis suggest that cystatin C is associated with aging success and should be added to the list of risk factors for unsuccessful aging in older adults. The risk in the highest quartile of cystatin C was as strongly associated with unsuccessful aging as a decreased AAI, left ventricular hypertrophy, or prevalent diabetes mellitus, and demonstrated a stronger association than a history of hypertension, an increase in age of 5 years, and all the other potential covariates considered in the analysis. These results may have implications for defining patients at high risk for unsuccessful aging and investigating methods to delay the progress of unsuccessful aging.
A prior study3 from CHS identified behavioral factors (physical inactivity and smoking), CVD risk factors (diabetes mellitus, low high-density lipoprotein cholesterol, and higher blood pressure), and subclinical CVD (low AAI) as risk factors for unsuccessful aging. Another study2 from CHS with similar methods as the present analysis emphasized the importance of noninvasive measures of subclinical CVD to unsuccessful aging. Our study adds to this literature by identifying kidney dysfunction as one of the more important factors associated with unsuccessful aging. As in prior studies13,14 from CHS, we noted that cystatin C was more strongly associated with outcomes than estimated GFR. This likely reflects the fact that a low creatinine level may reflect either good kidney function or decreased muscle mass, the latter being a marker of malnutrition.
The exact mechanism through which kidney function promotes unsuccessful aging cannot be evaluated by this study, although the following are possibilities. First, kidney dysfunction may be a marker of poor health and may reflect the severity of subclinical vascular disease and the cumulative effects of cardiovascular risk factors, such as hypertension.27 Second, kidney function may mediate an increase in other risk factors for aging, such as anemia, insulin resistance, and inflammation.6,28-34 Third, kidney dysfunction may have direct links to unsuccessful aging as a result of inadequate glomerular filtration. For example, salt retention may promote hypertension, while phosphate retention may result in decreased compliance and vascular remodeling of the large blood vessels.
There are potential implications of these results. First, kidney function, even in a range that may be considered normal for aging, should be recognized as one of the major factors associated with unsuccessful aging in older adults. For example, the mean creatinine level and estimated GFR were 0.93 mg/dL and 78 mL/min/1.73 m2, respectively, values that in general would not be considered abnormal in older adults. One could, therefore, hypothesize that any increase in cystatin C reflects an erosion of kidney reserve and kidney reserve is essential for successful aging. Second, one could investigate whether individuals with higher cystatin C levels may be a subgroup in whom efforts to prevent unsuccessful aging should be focused. Third, it may be reasonable to evaluate whether methods to slow decline in kidney function (eg, tight blood pressure control or use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) promote successful aging.
There are several strengths of this analysis. These include a large community-based cohort of older adults with detailed ascertainment of risk factors and each of the outcomes and measures used to define successful aging. We have also used a statistical approach that allows meaningful interpretation and comparison of risk factors in terms of reduction in successful life years. Finally, to our knowledge, this is the first study to evaluate the relationship between cystatin C, a novel measure of kidney function, and the broad outcome of successful aging.
There are also several limitations. We acknowledge that cystatin C could potentially reflect another pathological process in addition to kidney function, although several studies9-12 have suggested that cystatin C is primarily a measure of kidney function. We also acknowledge that use of International Classification of Diseases, Ninth Revision (ICD-9), codes to define cancer and COPD outcomes may result in biases that could either overestimate or underestimate the association of cystatin C with these outcomes. Because this is an observational study, our results can only demonstrate associations rather than imply causation. The present analysis is based on a single measurement of cystatin C made late in life, and it is possible that change in cystatin C may be a better predictor of successful aging. Finally, although the Food and Drug Administration has approved cystatin C as a measure of kidney function, this test is not yet widely available and has not yet been standardized across laboratories.
In summary, cystatin C is associated with unsuccessful aging in older adults. Future studies in other elderly cohorts should evaluate whether cystatin C improves identification of high-risk older adults and whether interventions that reduce kidney function decline will translate into improved rates of aging success.
Acknowledgments
Funding/Support: This study was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute; by the National Institute of Neurological Disorders and Stroke; and by grant R01AG027002 from the National Institutes on Aging.
Role of the Sponsor: The funding bodies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Group Information: A list of participating Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org.
Financial Disclosure: None reported.
REFERENCES
- 1.Reed DM, Foley DJ, White LR, Heimovitz H, Burchfiel CM, Masaki K. Predictors of healthy aging in men with high life expectancies. Am J Public Health. 1998;88(10):1463–1468. doi: 10.2105/ajph.88.10.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman AB, Arnold AM, Naydeck BL, et al. Cardiovascular Health Study Research Group. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163(19):2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 3.Burke GL, Arnold AM, Bild DE, et al. CHS Collaborative Research Group. Factors associated with healthy aging: the Cardiovascular Health Study. J Am Geriatr Soc. 2001;49(3):254–262. doi: 10.1046/j.1532-5415.2001.4930254.x. [DOI] [PubMed] [Google Scholar]
- 4.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237(4811):143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 5.Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31(2):155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107(1):87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Fried LF, Crump C, et al. Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int. 2002;62(3):997–1004. doi: 10.1046/j.1523-1755.2002.00522.x. [DOI] [PubMed] [Google Scholar]
- 8.Use of cystatin C measurement in evaluating kidney function. Nephrology (Carlton) 2005;10(suppl 4):S157–S167. [Google Scholar]
- 9.Randers E, Kristensen JH, Erlandsen EJ, Danielsen H. Serum cystatin C as a marker of the renal function. Scand J Clin Lab Invest. 1998;58(7):585–592. doi: 10.1080/00365519850186210. [DOI] [PubMed] [Google Scholar]
- 10.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function: a review. Clin Chem Lab Med. 1999;37(4):389–395. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 11.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37(1):79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 12.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C: a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101(5):875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 14.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cardiovascular Health Study. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 17.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 18.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37(pt 1):49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 19.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987;(21):1–115. [PubMed] [Google Scholar]
- 20.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29(2):388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 22.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 23.Collett D. Modeling Survival Data in Medical Research. Chapman & Hall/CRC; Boca Raton, FL: 1999. [Google Scholar]
- 24.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39((2)(suppl 1)):S1–S266. [PubMed] [Google Scholar]
- 25.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular disease in the elderly. Kidney Int. 2003;63(3):1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 26.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 27.Sarnak MJ, Levey AS, Schoolwerth AC, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 28.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8(3):131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Roubenoff R, Rall LC. Humoral mediation of changing body composition during aging and chronic inflammation. Nutr Rev. 1993;51(1):1–11. doi: 10.1111/j.1753-4887.1993.tb03045.x. [DOI] [PubMed] [Google Scholar]
- 30.Cesari M, Penninx BW, Lauretani F, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):249–254. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- 31.Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women: should the criteria currently used to define anemia in older people be reevaluated? JAm Geriatr Soc. 2002;50(7):1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 32.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the Heart and Soul Study. J Am Soc Nephrol. 2004;15(11):2908–2915. doi: 10.1097/01.ASN.0000143743.78092.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fliser D, Pacini G, Engelleiter R, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53(5):1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 34.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6(3):295–299. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]