Abstract
Sweating during exercise, especially during exercise in the heat, leads to sodium and water losses, and the quantity of these losses depends upon the intensity and duration of the activity, genetic predisposition and conditioning of the individual, and environmental factors. In athletes, adequate sodium intake is necessary to maintain fluid balance during training and competition. To ensure the precise regulation of volume and osmolality of body fluids, a number of integrated neural and hormonal systems have evolved to control thirst and sodium appetite. These systems respond to stimuli that arise from a deficit of fluid arising in both the intracellular and extracellular fluid compartments or to systemic hypertonicity. Thirst is highly sensitive to increases in plasma sodium concentration and osmolality, requiring only a 2%–3% increase to induce feelings of thirst. A larger change in plasma volume (10%) is required to induce thirst if there is no concomitant change in plasma sodium concentration. If plain water is used to replenish body water, plasma volume is preferentially restored over the interstitial and intracellular fluid space, suppressing plasma sodium concentration and removing the dipsogenic drive long before total body fluid has been restored. During or after dehydrating exercise, sodium ingestion helps to maintain and restore plasma volume and osmolality by continuing thirst sensation (thus drinking) and also by increasing body fluid retention. A high sodium meal or intravascular hypertonic saline infusion may cause transient osmotically mediated blood pressure increases, but in healthy people, acute sodium ingestion does not cause sustained hypertension. The purpose of this review is to provide evidence that acute increases in sodium are an intrinsic part of the thirst response during and after exercise, and that blood pressure increases associated with hypertonicity appear to be short lived.
INTRODUCTION
A number of scientific bodies, such as the National Institutes of Health, American Heart Association (AHA), and Institute of Medicine, as well as many physicians and researchers, focus upon the negative effects of chronically high sodium intake and so recommend low sodium diets to the general population. For example, on its Web site, the AHA recommends reducing sodium from the diet, with a goal of “less than 2300 mg·d−1.” The popular press and the food and weight-loss industries have used these recommendations to market any number of low-sodium diets, suggesting that all people would benefit from reducing sodium in their diets. The justification for these recommendations is that sodium ingestion is associated with both direct and hormone-induced effects on the brain and the kidney tubules, resulting in water retention, which may cause sustained hypertension. Indeed, excessive or chronically high sodium in the diet can increase the risk of hypertension in some groups of individuals, especially those with metabolic syndrome and salt-sensitive hypertension. However, long-term exercise can result in significant sodium losses through sweating, and thus sodium needs to be replaced in the diet (20). This sodium loss occurs in both elite and recreational athletes, and is more extreme during exercise in hot environments. Therefore, dietary recommendations for physically active individuals need to take these potentially large sodium losses into account. While a large acute sodium load may lead to transient, osmo- and volume receptor-mediated increases in blood pressure, in healthy people, acute sodium ingestion does not cause sustained hypertension. Moreover, sodium is an essential component of most athletes’ diets because sodium ingestion helps restore total body water and fluid-electrolyte balance by replacing sweat sodium losses. The purpose of this review is to describe the thirst and cardiovascular responses to acute increases in plasma tonicity and to provide evidence that in active individuals the addition of sodium to the diet contributes to the maintenance of thirst and fluid balance during training.
SODIUM STIMULATES THIRST AND CONTRIBUTES TO FLUID REPLACEMENT
Acute Effects of Sodium Ingestion upon Thirst
To ensure the precise regulation of volume and osmolality of body fluids, a number of integrated neural and hormonal systems have evolved to control thirst and sodium appetite. These systems respond to stimuli that arise from a deficit of fluid in both the intracellular and extracellular fluid compartments or to systemic hypertonicity (in humans, plasma osmolality is primarily a function of plasma sodium concentration or “tonicity” of the blood). Osmotic stimuli are sensed by osmo-Na+ receptors, which are nerves that are responsible for detecting the concentration of the interstitial fluid. Plasma osmolality elevation is the most potent stimulus of thirst, with only a 2%–3% change in osmolality required to induce thirst in humans (24,25,27). Experiments in the 1940s using arterial injections to deliver hypertonic saline directly to the brains of rats provided the first indication of the existence of osmotic mechanisms, or osmoreceptors, that control arginine vasopressin (AVP) release (29). Later studies supported the involvement of the anterior/preoptic hypothalamus in the regulation of thirst and drinking (See Fig. 1). Nerves that are responsible for detecting changes in plasma or blood volume, called volume-pressure receptors, are located in the superior and inferior vena cava and the atria of the heart. These baroreceptors detect changes in stretch that occur in response to alterations in central blood volume. “Volumetric” thirst mechanisms are initiated when intravascular fluid volume falls by greater than 10%. The acute changes in central volume or blood sodium concentration initiate a global response, including adjustments in thirst and sodium appetite, sympathetic nervous system activity, the renin-angiotensin-aldosterone system, as well as AVP and atrial natriuretic peptide secretion (1,15). See McKinley (15) and Antunes-Rodrigues et al. (1) for excellent in-depth reviews on central thirst mechanisms and body fluid regulation.
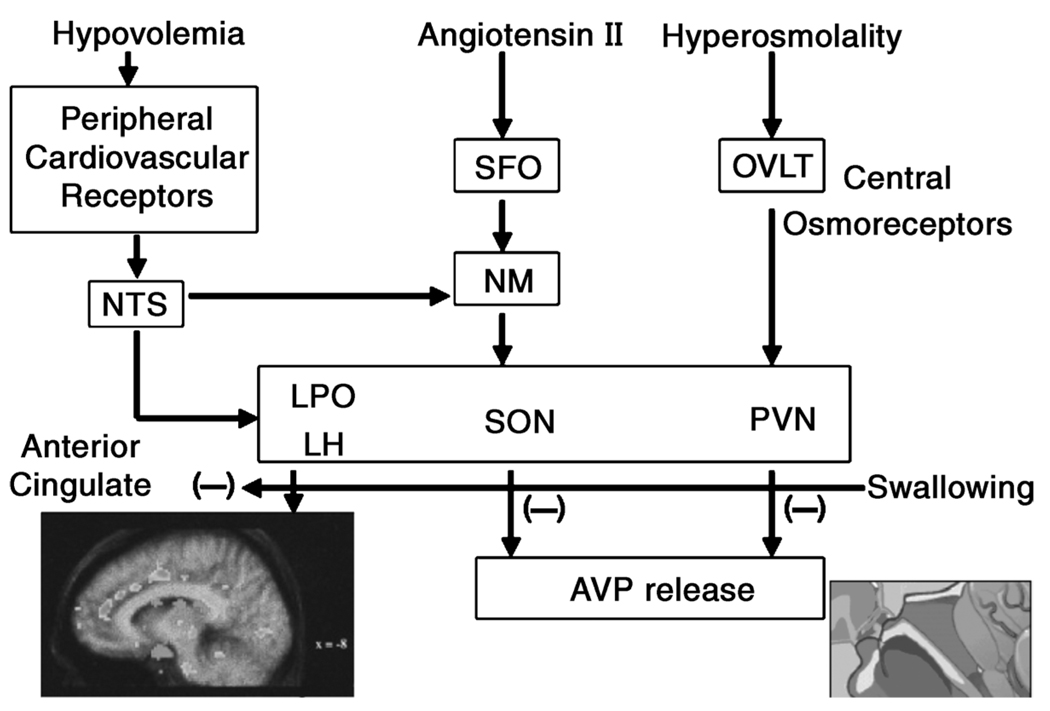
Figure 1.
Schematic of central regulation of body fluid regulation in response to acute changes in sodium and volume. The OVLT (organum vasculosum of the lamina terminalis) is a circumventricular organ located outside the blood-brain barrier (BBB), in the anteroventral part of the third ventricle that is an essential component of the osmotic thirst sensation pathway. Osmotic information from the OVLT is transmitted neurally to the hypothalamus and ultimately results in thirst, drinking, and arginine vasopressin (AVP) release. The median preoptic nucleus (NM) is responsible for initiating drinking in response to volumetric and angiotensinergic thirst stimulation. Through these mechanisms, the NM is used by both the subfornical organ (SFO) and the nucleus of the solitary tract (NTS), structures at the center of sodium appetite and thirst regulation. Changes in volume are initiated by the kidneys and stimulated by angiotensin outside the BBB. The SFO sends a message across the BBB to the NM, which then initiates volumetrically controlled thirst and drinking responses. Atrial baroreceptors also send a signal to the NTS. In addition to stimulating thirst, angiotensin stimulates the SFO and causes fluid regulating hormones to be secreted by the pituitary and adrenal glands, increases blood pressure, and eventually causes the kidney to stop secreting sodium and water, which, in turn, decreases both salt appetite and water intake. The paraventricular (PVN) and supraoptic nuclei (SON), both located in the hypothalamus, signal the release of AVP by the posterior pituitary. Thus both the PVN and SON also represent important structures involved in the control of water and sodium regulation because AVP has powerful effects upon both blood pressure and free water retention. Finally, the anterior cingulate cortex is involved in the relay of neural signals between the right and left hemispheres of the brain. This part of the brain is important for decision-making and plays an important role in sensing thirst and initiating drinking (9). Figure 1 by G.W. Mack (with permission). (Reprinted from Denton, D., R. Shade, F. Zamarippa, et al .Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc. Natl. Acad. Sci. U. S. A. 96:2532–2537, 1999. Copyright © 1999 National Academy of Sciences. Used with permission.)
In humans, strong and positive correlations (>0.90) exist between plasma osmolality and both thirst and plasma AVP concentration (P[AVP]) (6,24,25,27). The slopes of these relationships are used to assess osmotic control of thirst and AVP release; a steeper slope is interpreted as heightened sensitivity of central osmoreceptors that stimulate the cognitive sensation of thirst and cause the release of AVP from the hypothalamus and posterior pituitary. A lower intercept indicates an earlier osmotic threshold for the cognitive sensation of thirst hypothalamic release of AVP.
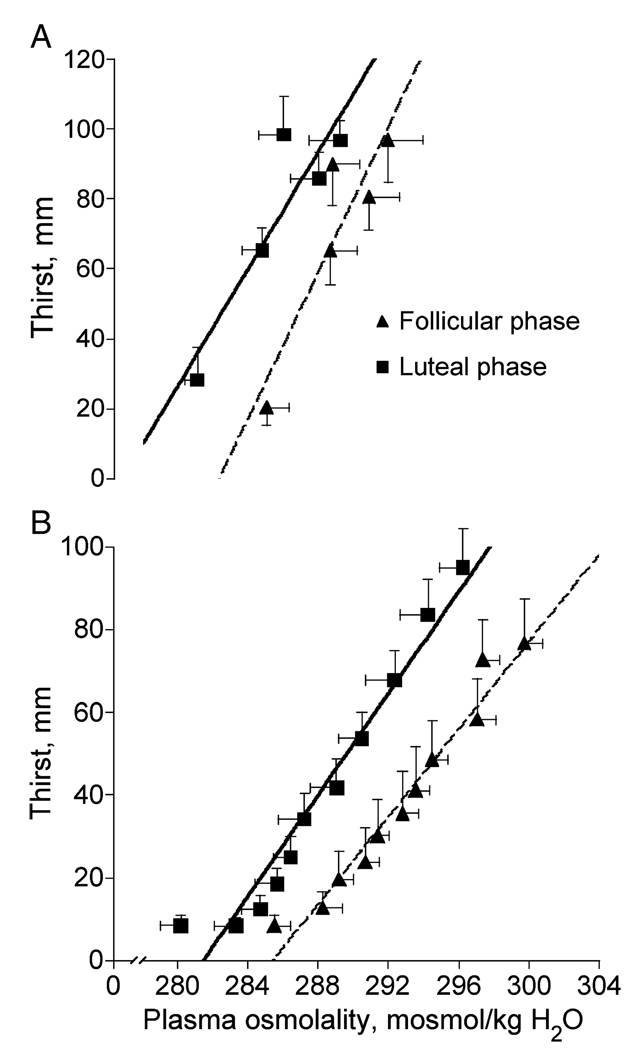
We have examined the relative contributions of osmotic and volume receptors to thirst using a number of different techniques designed specifically to stimulate either osmotic or volume receptors (24,25,27). For example, dehydration, induced by sweating during exercise or heat stress, leads to increases in plasma osmolality and serum sodium concentrations because sweat is hypotonic to plasma and water moves quickly out of the vascular space. The increase in plasma osmolality occurs concomitant with plasma volume loss, stimulating thirst (Fig. 2A). Because more water than sodium is lost through sweating, dehydration during exercise usually results in a hyperosmotic hypovolemia. This hyperosmotic hypovolemia is associated with water loss from both intracellular and extracellular fluid compartments. The use of graded dehydration to study acute thirst response to an increase in sodium concentration in the blood is important because it is a primary physiological response to exercise. However, exercise-induced dehydration does not isolate the independent contributions of osmo-Na+receptors to the overall thirst response because both osmo- and volume receptors are affected (13,17,26).
Figure 2.
Osmotic regulation of thirst during exercise-induced dehydration (A) (26) and hypertonic saline infusion (B) (6) in the early follicular and mid-luteal phase of the menstrual cycle in young women.
Measurement of Cognitive Thirst Sensation in Humans
When measuring thirst perceptions in humans, we use a visual-analogue rating scale (14,24,25). The subjects respond to the question, “How thirsty do you feel right now?” by marking on a line 180 mm in length with intersecting lines anchored at 0 mm for “not at all” and at 125 mm for “extremely thirsty.” This scale has been used extensively for psychophysical assessments in both older and younger subjects and corresponds well to physiological determinants of thirst, such as plasma osmolality (6,25).
To address thirst responses to sodium ingestion, and to better study the effects of a sodium load independent of volume depletion, we have used hypertonic (3% NaCl) saline infusion to examine osmoreceptor input into thirst sensation (6). The plasma hypertonicity associated with hypertonic saline infusion induces a powerful and linear thirst response (Fig. 2B) without a concomitant decrease in plasma volume. Hypertonic saline infusion leads to a powerful thirst drive because plasma osmolality increases by as much as 16–20 mOsm·kg−1H2O during a 2-h infusion (Fig. 2B). Hypertonic saline infusion also causes a large intravascular fluid expansion (20%) as water is drawn from cells in response to the increased osmotic pressure in fluid surrounding them, hence a hyperosmotic hypervolemia. Thus hypertonic saline infusion induces opposing inputs from osmo- and volume reflexes (25), although the large thirst response indicates that the osmotic stimulus predominates under these conditions.
Thermoneutral head-out water immersion combined with dehydration has been used to determine the independent inputs of extracellular volume or osmotic factors to thirst sensation and the drive for drinking (24,31). During head-out water immersion, hydrostatic pressure drives approximately 700 ml into the intrathoracic space, increasing cardiac filling pressure and heart volume (12). This central blood volume expansion is associated with increased stroke volume concomitant with decreases in AVP and other fluid-regulating hormones, as well as increased urine output, without altering plasma composition (24,31). In dehydrated young men and women, the central volume expansion activates cardiac volume (stretch) receptors, which results in attenuated thirst and drinking despite continuing dehydration and elevations in plasma osmolality (24,31). It is interesting to note that in similar studies, dehydrated older subjects showed similar thirst responses to changes in osmotic stimuli (24,25) but did not respond to the central plasma volume expansion induced by head-out water immersion (24). Taken together, these data suggest that thirst deficits reported in older people (13) are related to changes in volumetric, rather than osmotic, thirst mechanisms.
Sodium Loads During Exercise and Recovery from Exercise
During and after exercise-induced dehydration, sodium ingestion plays an important role in maintaining the level of fluid in the body. As described previously, thirst sensation is highly sensitive to changes in sodium within the vascular system. However, even when plasma sodium concentration is high, the act of drinking rapidly suppresses thirst before any significant change in blood tonicity, probably through an overriding oropharyngeal reflex (See Fig. 1). During recovery from dehydrating exercise, for example, in a more sustained fashion, drinking plain water causes rapid plasma sodium concentration and plasma volume restoration, even while extra- and intracellular dehydration continues (2,30). Thus, unless a conscious effort is made to do so, athletes may not consume enough fluids to avoid the negative consequences of a fluid deficit (e.g., overheating) during exercise because of this early removal of the dipsogenic drive or “involuntary dehydration.” Both during (2,30) and after (17) dehydrating exercise, adequate sodium ingestion can be used to counteract this involuntary dehydration and will maintain or restore plasma volume and osmolality more completely. The recent American College of Sports Medicine Position Stand “Exercise and Fluid Replacement” (20) provides specific information on the sweat sodium lost during various activities, and it is clear from the Position Stand that athletes can lose as much as 5 g sodium in sweat during a single high-intensity workout. Sodium ingestion not only replaces some of the sodium lost in sweat (30), but continues osmotic thirst stimulation and a dipsogenic drive and enhances ad libitum drinking (2). These mechanisms for increasing total body sodium lead to a more complete restoration of body fluids (17) as well as improved performance (30). A series of experiments by Nose and colleagues demonstrated an important role for sodium ingestion in rehydration after approximately 2.5% dehydrating exercise (16,17). These studies demonstrate that water from inside cells was mobilized to maintain extracellular fluid volume during dehydrating exercise, and the loss of extracellular fluid volume was a direct function of the sweat sodium loss (17). In these subjects, when only water was provided during the first 60 min of rehydration (a more dynamic part of rehydration), the plasma was rapidly and preferentially restored over the other fluid compartments, concomitant with lower plasma sodium concentration. Because thirst sensation is sensitive to plasma sodium concentration, this rapid restoration of plasma tonicity and volume removed the dipsogenic drive before total body water was restored. Through ad libitum water drinking over the entire 180-min recovery period, subjects restored only 48% of their lost total body water and only 36% of their extracellular fluid. However, despite these continued body water deficits, plasma volume was preferentially restored by 90% over this same recovery period. When given sodium tablets along with the water, these subjects drank more and restored 69% and 84% of total and extracellular fluid, respectively, over the 180-min recovery period (17). Concomitant with a slight plasma volume expansion over the recovery period, ingesting sodium was associated with continued elevation of plasma osmolality and sodium concentration, which resulted in greater ad libitum water intake and more complete body fluid recovery. In contrast, the water-only trial was associated with an early removal of the dipsogenic drive, leading to incomplete recovery of body fluids in this post-exercise period. These findings by Nose et al. (17) are similar to more recent data using sports drinks during exercise (2,30).
In summary, thirst and drinking are tightly controlled through complex central mechanisms (Fig. 1). Plasma sodium concentration is the most potent thirst stimulus at rest, as well as during and after exercise. Moreover, sodium ingestion increases intravascular sodium concentration and will counteract involuntary dehydration by enhancing thirst and drinking (2,17,30), leading to a more complete restoration of body fluids (17).
SODIUM IS AN IMPORTANT COMPONENT FOR CARDIOVASCULAR CONTROL
Acute Effects of Sodium Ingestion upon Cardiovascular Function
An acute sodium load, such as a hypertonic saline infusion, can lead to transient increases in blood pressure. The sympathetic nervous system plays an important role in these short-term blood pressure responses via osmotic, angiotensinergic, and volume mechanisms (5) (see Fig. 3). The transient increases in either systemic or local osmolality and subsequent stimulation of sympathetic outflow during infusion of hypertonic saline have been described in numerous animal models (3,28). In general, these studies have indicated that increases in plasma tonicity resulting from sodium infusion stimulate the blood pressure regulatory system via central osmoreceptor input to sympathetic nerves. In the human studies described later in this article, we used hypertonic (3% NaCl) saline infusion to stimulate the sodium and volume regulatory systems rather than sodium ingestion. This method permits a large sodium stimulus, and also permits much greater control over the levels of sodium in the plasma. The osmotic (20 mOsm·kg−1 H2O) and sodium (8 mEq·L−1) loads induced by 120 min of hypertonic saline infusion transiently increased systolic blood pressure under most conditions (6,22,27) (Fig. 4). In our studies, we did not measure sympathetic nervous system activity, but more recent studies have demonstrated greater sensitivity of baroreflex control of sympathetic outflow (muscle sympathetic nerve activity [MSNA]) concomitant with moderate increases in mean arterial pressure during mild plasma osmolality increases (3 mOsm·kg−1 H2O) (7). Consistent with these findings, a more substantive and longer-lasting hypertonic saline infusion (increases in plasma osmolality of 10 mOsm·kg−1 H2O) produced even greater sympathetic outflow concomitant with increases in both systolic and mean arterial pressure, and blood pressure increased as a function of sympathetic outflow in the later stages of the infusion (10). Moreover, sympathetic outflow and other more direct effects upon cardiovascular function were moderated by the extent of plasma volume contraction (8) or expansion (7,10). This sensitivity to plasma volume status supports a role for baroreceptors in the blood pressure responses in such instances as dehydration (plasma volume loss) (8) and hypertonic saline infusion (plasma volume expansion) in much the same way as in thirst sensation.
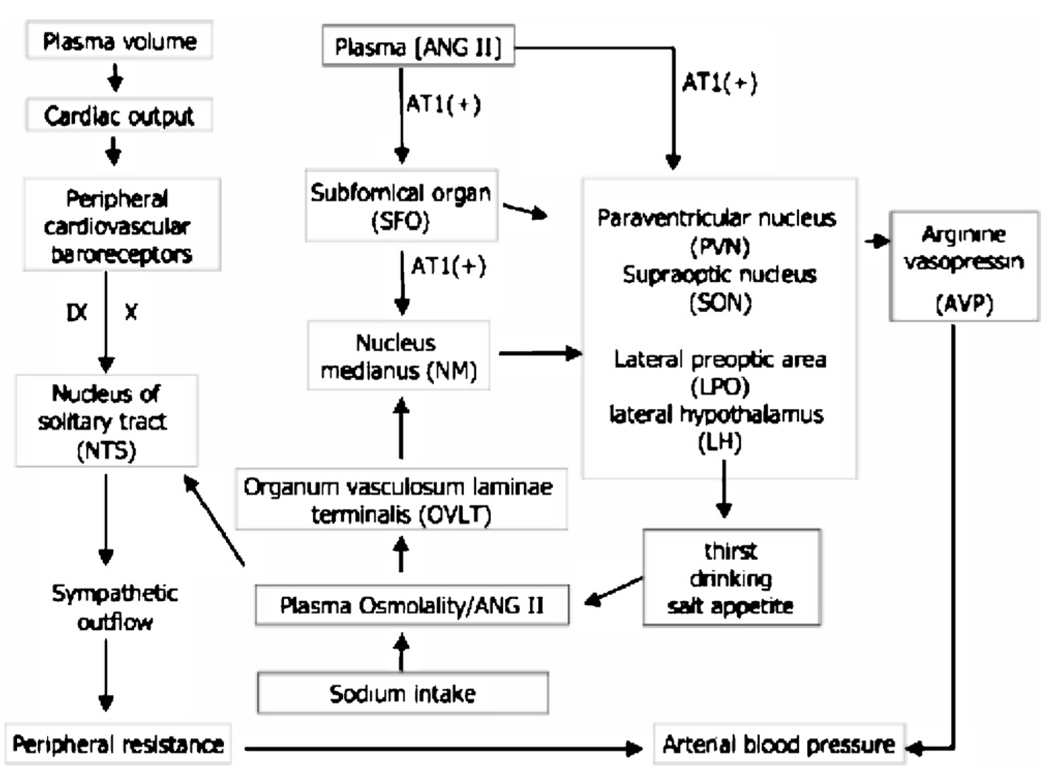
Figure 3.
Schematic diagram of the forebrain neurocircuitry and peripheral inputs underlying the influences of osmotic and peripheral angiotensin II (Ang II) on sympathetic outflow and the baroreflex. Similar to mechanisms described for thirst sensation, neurons within the forebrain circumventricular organ, the OVLT, detect changes in plasma osmolality or blood tonicity. This information is relayed onto neurons in the median nucleus (NM) and nucleus of the solitary tract (NTS). Parvoand magnocellular neurons in the paraventricular (PVN) and supraoptic (SON) nuclei within the hypothalamus are then activated, leading to the release of the pressor hormone arginine vasopressin (AVP). These hypothalamic structures (e.g., the SFO) also use an angiotensinergic pathway, meaning they are also directly sensitive to changes in local and peripheral changes in Ang II via AT1 receptors. Arterial baroreflexes located in the carotid sinus and aortic arch sense transient changes in pressure and central volume. Mechanoreceptors sensitive to stretch in the arteries relay information regarding the absolute level and the change in blood pressure to the central nervous system (via the vagus [X] and glossopharyngeal [IX] cranial nerves). Baroreflexes send signals to the NTS, and neurons in the NTS relay the processed input to central sites (LPO and LH, the lateral preoptic nucleus and lateral hypothalamus), which then mediate vagal and sympathetic outflow to the heart and blood vessels. The sympathetic outflow leads to rapid adaptation of local blood vessels to the altered volumes and pressures, causing autonomic neural responses that prevent large fluctuations in blood pressure. In response to blood pressure increases, a key element of sympathoinhibition is the attenuation of the chronic sympathoexcitatory effects of circulating Ang II, as well as other hormones, such as arginine vasopressin and norepinephrine.
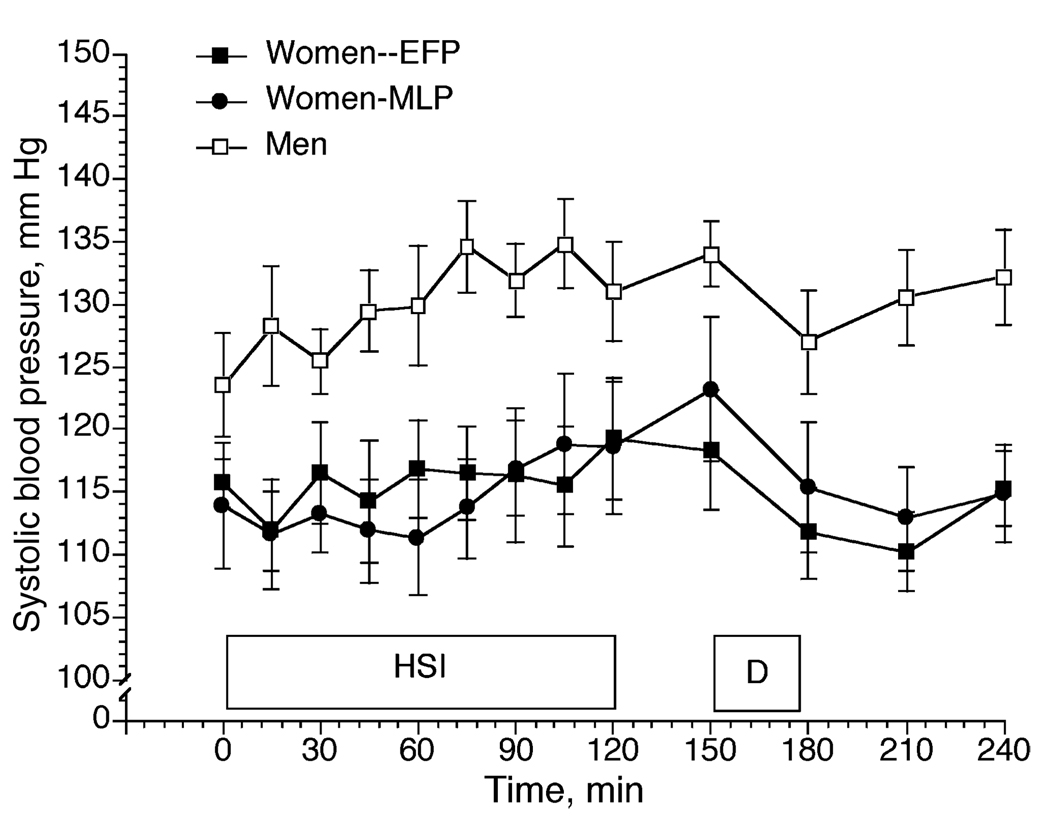
Figure 4.
3% NaCl infusion (HSI) in women during the early follicular (EFP) and mid-luteal (MLP) phases of the menstrual cycle, and in men. D = drinking (15mL·kg−1 body weight). Data from Stachenfeld et al. (27).
Hormonal Responses to Acute Increases in Blood Sodium
Although early increases in blood pressure during sodium loading are associated with changes in baroreceptor control of sympathetic outflow (7), stimulation of the pituitary hormone AVP also may contribute to the acute blood pressure increases during sodium loads. Arginine vasopressin, the primary hormone sensitive to changes in plasma osmolality, is one of the most powerful vasoconstrictors in the body and is also the primary hormone in control of renal free water clearance. While AVP is sensitive to both volume and osmotic stimuli, it is most profoundly and also linearly sensitive to changes in the osmotic content of the plasma (Fig. 5). Moreover, blood pressure changes appear to mirror those of AVP during hypertonic saline infusion (10,27), dehydration (26), and drinking (26,27). Thus AVP may contribute to the increase in arterial blood pressure during these substantive and longer-lasting hypertonic saline infusions.
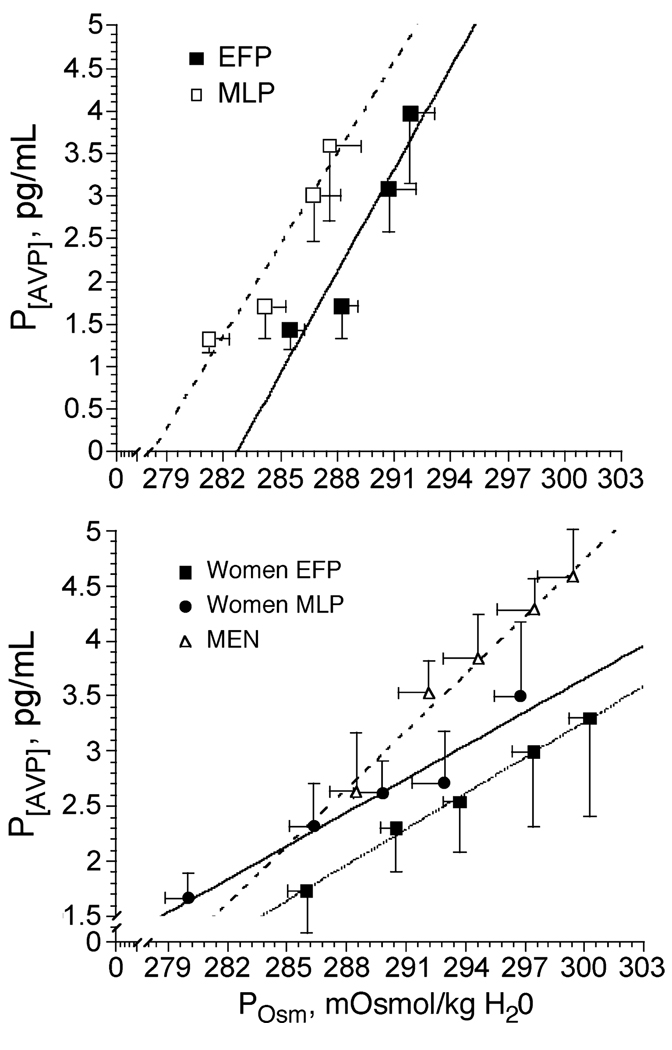
Figure 5.
Osmotic regulation of arginine vasopressin (P[AVP]) during dehydration in women in the early follicular (EFP) and mid-luteal (MLP) phases of the menstrual cycle (top). Data from Stachenfeld et al. (26). Osmotic regulation of arginine vasopressin (P[AVP]) during 3% NaCl infusion in women during the EFP and MLP phases of the menstrual cycle, and in men (bottom). Data from Stachenfeld et al. (27).
Whether sex differences exist in osmotic regulation of AVP during exercise remains unclear, but during hypertonic saline infusion, we observed a greater P[AVP]-POsm slope in men compared with women, indicating greater sensitivity of systems regulating AVP synthesis and/or release in response to changes in plasma osmolality (See Fig. 5, bottom graph). This greater osmotic sensitivity for AVP in the men was associated with greater resting and greater systolic blood pressure during the infusion (See Fig. 4). In addition, during hypertonic saline infusion the increases in systolic blood pressure were transient in the women, with blood pressure recovering within 30 min after the end of the infusion. In contrast, in the men, blood pressure recovery was considerably slower than that of the women, even though plasma osmolality and sodium concentration had been restored to preinfusion levels (27). The cause for the sex differences was not clear from our data, but estrogens and androgens cross the blood-brain barrier, and vasopressin-producing cells in the paraventricular and supraoptic nuclei have binding sites for both of these steroid hormones that affect osmotic regulation of AVP (18,19). Estrogens and androgens also modulate hypothalamic AVP release indirectly through catecholaminergic neurons that bind estradiol and testosterone and project to the paraventricular and supraoptic nuclei (11) so sex differences in blood pressure responses also may be mediated by sympathetic inputs to the brain.
Renin-Angiotensin-Aldosterone System
Dehydrating exercise and increases in plasma sodium also are associated with renin-angiotensin-aldosterone system stimulation (16,23) as a function of local sympathetic stimulation to the kidney (4), increases in plasma osmolality, and loss of plasma volume (13,21,26). During rehydration within the first 60 min after exercise, this system is rapidly suppressed as plasma volume and osmolality are restored (16,26). Indeed, there are close relationships between changes in plasma volume and changes in plasma renin activity and aldosterone concentration, and these relationships remain intact whether water or sodium is ingested during the rehydration period (16,26). Moreover, the selective restoration of plasma volume serves to depress these hormones even when the body is deprived still of sodium lost in sweat (16). Thus the renin-angiotensin-aldosterone system during recovery from exercise is driven primarily by factors related to the maintenance of plasma volume and is unlikely to be associated with any changes in arterial pressure even when sodium is provided during rehydration.
CONCLUSION
Acute sodium intake increases thirst, sympathetic output, and transient blood pressure. Therefore, there are situations (e.g., multiple training sessions per day) where an athlete may need to be proactive regarding water and electrolyte replacement to avoid excessive deviations in fluid and electrolyte balance. Fortunately, humans have evolved thirst and sodium appetite mechanisms to facilitate restoration of fluid and electrolyte balance when several hours of recovery are available. Sodium ingestion is particularly important during long-term exercise and the period after exercise when sodium-mediated renal fluid retention and the continuation of an osmotically driven dipsogenic drive (17) are essential to the maintenance and restoration of total body water, extracellular fluid, plasma volume, and osmolality. Exact amounts of sodium required to replace losses depend upon the type of exercise, the environment, intensity, and important individual differences. While the American College of Sports Medicine recent position stand “Exercise and Fluid Replacement” provides guidelines (20), each individual must determine the extent to which he or she loses salt or water during exercise and replace it accordingly (20). During or after exercise of 2–4 h in length, body water and electrolytes will remain compromised until most of the water and electrolytes lost in sweat are replaced. The thirst and renal mechanisms in humans will accomplish the replacement of total body water over time, but athletes must pay special attention because they continue to lose water and electrolytes with each exercise bout. While acute sodium loads are associated with moderate, transient increases in blood pressure, these increases are unlikely to lead to long-term hypertension except in those individuals with particular sensitivity to sodium.
Acknowledgments
The author acknowledges the intellectual contributions of Drs. Ethan Nadel (posthumous), Gary Mack, and Wendy Calzone, M.S., the technical assistance of Cheryl Leone, M.A., the clinical support of Drs. Hugh Taylor and Celso Silva, and the cooperation of the volunteer subjects. This work was supported, in part, by National Heart Lung Blood Institute R01 HL62240 and R01 HL71159. This work was also supported, in part, by the U.S. Army Medical and Research and Materiel Command under contract DAMD17-96-C-6093. The views, opinions, and findings contained in this report are those of the author and should not be construed as an official Department of Defense position, policy, or decision unless so designated by other documentation. In conduct of research where humans are the subjects, the investigators adhered to the policies regarding the protection of human subjects as prescribed by 45 CFR 46 and 32 CFR 219 (Protection of Human Subjects). All protocols were also approved by the Human Investigation Committee at Yale School of Medicine.
References
- 1.Antunes-Rodrigues J, de Castro M, Elias LL, et al. Neuroendocrine control of body fluid metabolism. Physiol. Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- 2.Baker LB, Munce TA, Kenney WL. Sex differences in voluntary fluid intake by older adults during exercise. Med. Sci. Sports Exerc. 2005;37:789–796. doi: 10.1249/01.mss.0000162622.78487.9c. [DOI] [PubMed] [Google Scholar]
- 3.Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin. Exp. Pharmacol. Physiol. 2005;32:426–432. doi: 10.1111/j.1440-1681.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 4.Brooks VL, Osborne JW. Hormonal-sympathetic interactions in long-term regulation of arterial pressure: an hypothesis. Am. J. Physiol. 1995;268:R1343–R1358. doi: 10.1152/ajpregu.1995.268.6.R1343. [DOI] [PubMed] [Google Scholar]
- 5.Brooks VL, Scrognin K, McKeigh DF. The interaction of angiotensin II and osmolality in the generation of sympathetic tone during changes in dietary salt intake. An hypothesis (Review) Ann. N. Y. Acad. Sci. 2001;940:380–394. doi: 10.1111/j.1749-6632.2001.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 6.Calzone WL, Silva C, Keefe DL, Stachenfeld NS. Progesterone does not alter osmotic regulation of AVP. Am. J. Physiol. 2001;281:R2011–R2020. doi: 10.1152/ajpregu.2001.281.6.R2011. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Eisenach JH, Joyner MJ, et al. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2456–H2460. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- 8.Charkoudian N, Halliwill JR, Morgan BJ, et al. Influences of hydration on post-exercise cardiovascular control in humans. J. Physiol. 2003;552:635–644. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denton D, Shade R, Zamarippa F, et al. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farquhar WB, Wenner MM, Delaney EP, et al. Sympathetic neural responses to increased osmolality in humans. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2181–H2186. doi: 10.1152/ajpheart.00191.2006. [DOI] [PubMed] [Google Scholar]
- 11.Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- 12.Lange L, Lange S, Echt M, Gauer OH. Heart volume in relation to body posture and immersion in a thermo-neutral bath. Pfugers Arch. 1974;352:219–226. doi: 10.1007/BF00590487. [DOI] [PubMed] [Google Scholar]
- 13.Mack GW, Weseman CA, Langhans GW, et al. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J. Appl. Physiol. 1994;76:1615–1623. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 14.Marks LE, Stevens JC, Bartoshuk LM, et al. Magnitude-matching: the measurement of taste and smell. Chem. Senses. 1988;13:66–87. [Google Scholar]
- 15.McKinley MJ, Cairns MJ, Denton DA, et al. Physiological and pathophysiological influences on thirst. Physiol. Behav. 2004;81:795–803. doi: 10.1016/j.physbeh.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 16.Nose H, Mack GW, Shi X, Nadel ER. Involvement of sodium retention hormones during rehydration in humans. J. Appl. Physiol. 1988;65:332–336. doi: 10.1152/jappl.1988.65.1.332. [DOI] [PubMed] [Google Scholar]
- 17.Nose H, Mack GW, Shi X, Nadel ER. Role of osmolality and plasma volume during rehydration in humans. J. Appl. Physiol. 1988;65:325–331. doi: 10.1152/jappl.1988.65.1.325. [DOI] [PubMed] [Google Scholar]
- 18.Sar M, Stumpf WE. Distribution of androgen target cells in rat forebrain and pituitary after [3H]-dihydrotestosterone administration. J. Steroid Biochem. 1977;8:1131–1134. doi: 10.1016/0022-4731(77)90063-2. [DOI] [PubMed] [Google Scholar]
- 19.Sar M, Stumpf WE. Simultaneous localization of [3H]estradiol and neurophysin I or arginine vasopressin in hypothalamic neurons demonstrated by a combined technique of dry-mount autoradiography and immunohistochemistry. Neurosci. Lett. 1980;17:179–184. doi: 10.1016/0304-3940(80)90081-6. [DOI] [PubMed] [Google Scholar]
- 20.Sawka MN, Burke LM, Eichner ER, et al. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. SportsExerc. 2007;39:377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 21.Sawka MN, Wenger CB. Human Performance Physiology and Environmental Medicine at Terrestrial Extremes. In: Pandolf KB, Sawka MN, Gonzales RR, editors. Human Performance Physiology and Environmental Medicine at Terrestrial. Indianapolis, IN: Benchmark; 1988. pp. 97–151. [Google Scholar]
- 22.Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am. J. Physiol. 1998;274:R187–R195. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- 23.Stachenfeld NS, Gleim GW, Zabetakis PM, Nicholas JA. Fluid balance and renal response following dehydrating exercise in well-trained men and women. Eur. J. Appl. Physiol. 1996;72:468–477. doi: 10.1007/BF00242277. [DOI] [PubMed] [Google Scholar]
- 24.Stachenfeld NS, Mack GW, DiPietro L, Nadel ER. Mechanism for attenuated thirst in aging: role of central blood volume receptors. Am. J. Physiol. 1997;272:R148–R157. doi: 10.1152/ajpregu.1997.272.1.R148. [DOI] [PubMed] [Google Scholar]
- 25.Stachenfeld NS, Mack GW, Takamata A, et al. Thirst and fluid regulatory responses to hypertonicity in older adults. Am. J. Physiol. 1996;271:R757–R765. doi: 10.1152/ajpregu.1996.271.3.R757. [DOI] [PubMed] [Google Scholar]
- 26.Stachenfeld NS, Silva CS, Keefe DL, et al. Effects of oral contraceptives on body fluid regulation. J. Appl. Physiol. 1999;87:1016–1025. doi: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 27.Stachenfeld NS, Splenser AE, Calzone WL, et al. Sex differences in osmotic regulation of AVP and renal sodium handling. J. Appl. Physiol. 2001;91:1893–1901. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- 28.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol. Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 29.Verney E. The antidiuretic hormone and the factors which determine its release. Proc. Soc. Lond. B. Biol. 1947;135:25–105. [PubMed] [Google Scholar]
- 30.Vrijens DM, Rehrer NJ. Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat. J. Appl. Physiol. 1999;86:1847–1851. doi: 10.1152/jappl.1999.86.6.1847. [DOI] [PubMed] [Google Scholar]
- 31.Wada F, Sagawa S, Miki K, et al. Mechanism of thirst attenuation during head-out water immersion in men. Am. J. Physiol. 1995;268:R583–R589. doi: 10.1152/ajpregu.1995.268.3.R583. [DOI] [PubMed] [Google Scholar]