Abstract
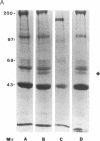
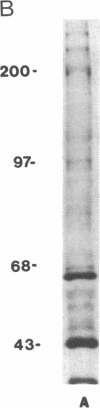
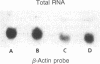
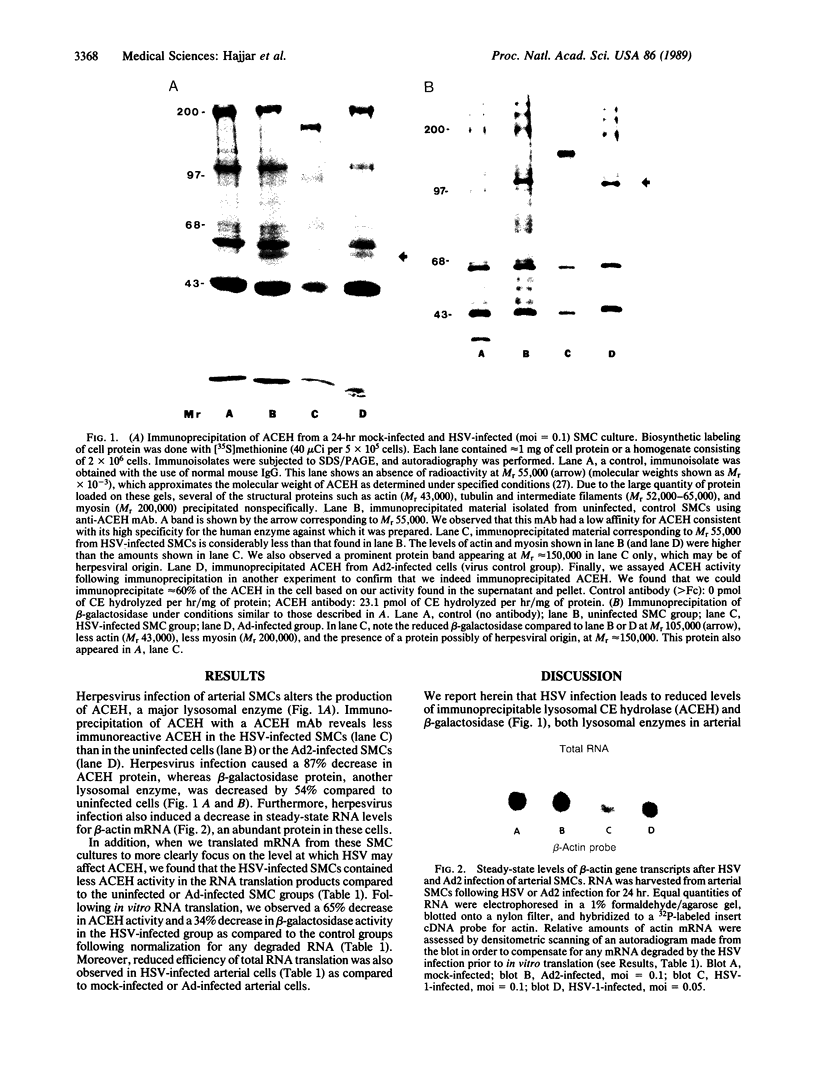
Herpes simplex viruses (HSVs) contain a function that can cause the degradation of host mRNA and mediate the shutoff of host protein synthesis. Previously, we observed that HSV infection causes a 40-fold increase in cholesteryl ester (CE) accretion in arterial smooth muscle cells due, in part, to a substantial decrease in CE hydrolysis. In studies reported herein, we found that HSV infection leads to reduced immunoprecipitable lysosomal (acid) CE hydrolase (ACEH) and beta-galactosidase, another lysosomal enzyme in vascular smooth muscle cells. The HSV-induced reduction was greater with respect to ACEH than beta-galactosidase. To determine whether degradation of host cellular mRNA or inhibition of cellular translation was responsible for decreased CE hydrolysis in HSV-infected smooth muscle cells, we utilized an in vitro translation system that permitted us to compensate for any mRNA degradation during viral infection. Reduced ACEH activity was observed in the total cellular RNA translation products of HSV-infected smooth muscle cells compared to uninfected cells owing to posttranscriptional modification. We conclude that the decrease in CE hydrolysis in HSV-infected smooth muscle cells is caused primarily by decreased ACEH synthesis and activity, which can contribute to CE accretion in these vascular cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L. In vitro translation of procollagen messenger RNAs. Methods Enzymol. 1987;144:84–95. doi: 10.1016/0076-6879(87)44174-8. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Li L., McClure D. B. Altered low density lipoprotein receptor regulation is associated with cholesteryl ester accumulation in Simian virus 40 transformed rodent fibroblast cell lines. In Vitro Cell Dev Biol. 1988 Apr;24(4):353–358. doi: 10.1007/BF02628838. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEAR J. H. Coxsackie virus infections of the newborn. Prog Med Virol. 1958;1:106–121. [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Fabricant C. G., Minick C. R., Fabricant J. Virus-induced atherosclerosis. Herpesvirus infection alters aortic cholesterol metabolism and accumulation. Am J Pathol. 1986 Jan;122(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fabricant C. G., Fabricant J. Altered cholesteryl ester cycle is associated with lipid accumulation in herpesvirus-infected arterial smooth muscle cells. J Biol Chem. 1985 May 25;260(10):6124–6128. [PubMed] [Google Scholar]
- Hajjar D. P. Herpesvirus infection prevents activation of cytoplasmic cholesteryl esterase in arterial smooth muscle cells. J Biol Chem. 1986 Jun 15;261(17):7611–7614. [PubMed] [Google Scholar]
- Hajjar D. P., Pomerantz K. B., Falcone D. J., Weksler B. B., Grant A. J. Herpes simplex virus infection in human arterial cells. Implications in arteriosclerosis. J Clin Invest. 1987 Nov;80(5):1317–1321. doi: 10.1172/JCI113208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Fowler S., de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res. 1980 Nov;21(8):961–969. [PubMed] [Google Scholar]
- Inglis S. C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982 Dec;2(12):1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayman B. A., Nishioka Y. Differential stability of host mRNAs in Friend erythroleukemia cells infected with herpes simplex virus type 1. J Virol. 1985 Jan;53(1):1–6. doi: 10.1128/jvi.53.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Petrie B. L., Dreesman G. R., Burek J., McCollum C. H., DeBakey M. E. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983 Sep 17;2(8351):644–647. doi: 10.1016/s0140-6736(83)92529-1. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Adam E., Melnick J. L. Association of herpesvirus/cytomegalovirus infections with human atherosclerosis. Prog Med Virol. 1988;35:21–42. [PubMed] [Google Scholar]
- Petrie B. L., Melnick J. L., Adam E., Burek J., McCollum C. H., DeBakey M. E. Nucleic acid sequences of cytomegalovirus in cells cultured from human arterial tissue. J Infect Dis. 1987 Jan;155(1):158–159. doi: 10.1093/infdis/155.1.158. [DOI] [PubMed] [Google Scholar]
- Platt E. J., Karlsen K., Lopez-Valdivieso A., Cook P. W., Firestone G. L. Highly sensitive immunoadsorption procedure for detection of low-abundance proteins. Anal Biochem. 1986 Jul;156(1):126–135. doi: 10.1016/0003-2697(86)90163-6. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Rosenbaum L. M. Human lysosomal acid lipase/cholesteryl ester hydrolase. Purification and properties of the form secreted by fibroblasts in microcarrier culture. J Biol Chem. 1985 Dec 5;260(28):15186–15193. [PubMed] [Google Scholar]
- Schek N., Bachenheimer S. L. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J Virol. 1985 Sep;55(3):601–610. doi: 10.1128/jvi.55.3.601-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D., Sanford B. A. Evidence for chronic viral infections in human arteries. Proc Soc Exp Biol Med. 1974 Nov;147(2):357–360. doi: 10.3181/00379727-147-38341. [DOI] [PubMed] [Google Scholar]
- Strom T., Frenkel N. Effects of herpes simplex virus on mRNA stability. J Virol. 1987 Jul;61(7):2198–2207. doi: 10.1128/jvi.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Yamashiroya H. M., Ghosh L., Yang R., Robertson A. L., Jr Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988 Jan;130(1):71–79. [PMC free article] [PubMed] [Google Scholar]