Abstract
Development of primary graft dysfunction (PGD) is associated with poor outcomes after transplantation. We hypothesized that Receptor for Advanced Glycation End-products (RAGE) levels in donor lungs is associated with the development of PGD. Furthermore, we hypothesized that RAGE levels would be increased with PGD in recipients after transplantation.
We measured RAGE in bronchoalveolar lavage fluid (BALf) from 25 donors and 34 recipients. RAGE was also detected in biopsies (TBBX) from recipients with and without PGD.
RAGE levels were significantly higher in donor lungs that subsequently developed sustained PGD vs transplanted lungs that did not display PGD. Donor RAGE level was a predictor of recipient PGD (odds ratio = 1.768 per 0.25 ng/ml increase in donor RAGE level). In addition, RAGE levels remained high at 14 days in those recipients that developed severe graft dysfunction.
Recipients may be at higher risk for developing PGD if they receive transplanted organs that have higher levels of soluble RAGE prior to explantation. Moreover, the clinical and pathologic abnormalities associated with PGD post-transplantation are associated with increased RAGE expression. These findings also raise the possibility that targeting the RAGE signaling pathway could be a novel strategy for treatment and/or prevention of PGD.
Keywords: Primary Graft Dysfunction, Lung transplantation, Reperfusion injury, Receptor for advanced glycation end products, RAGE
INTRODUCTION
Primary graft dysfunction (PGD) typically follows procurement and transplantation of the lung and is the major cause of acute mortality following lung transplantation (1–4). Since there is no effective therapy, mortality can be as high as 43% (2, 5). There are no proven medical treatments for PGD and identification of additional biomarkers that could accurately identify organs that will develop this serious condition is needed (6).
PGD is the end result of a series of lung injuries that may occur before or after the declaration of donor brain death, or during the transplant process, and/or during or after the initiation of reperfusion (7). These injuries are difficult to predict using current standards based on gas exchange, chest radiograph, or bronchoscopic findings. Conversely, it is very conceivable that many potentially useable donor lungs may go unutilized due to the lack of accurate evaluation methods that could determine their suitability for transplantation (8). In conjunction with data showing patient outcome from recipients of lungs not meeting all of the usual pre-transplant clinical criteria, the validity of the clinical criteria used for screening donor organs should be called into question (9). Measurement of biological markers that could predict development of PGD may be useful in predicting alveolar epithelial function/dysfunction (10). Such a prediction may be useful in determining which donor lungs are primed for acute lung injury (ALI) and subsequent development of PGD following ischemia and reperfusion.
Alveolar type I epithelial cells (AT1) comprise more than 95% of the internal surface of the lung (11), and damage to AT1 cells is an important histologic feature of PGD (12). Receptor for Advanced Glycation End Products (RAGE), a protein highly expressed by AT1 cells (13, 14), has been demonstrated to be a marker for severity of ALI in animal and human studies (15–18). Studies have demonstrated that RAGE is released into the alveolar space by injurious and inflammatory stimuli in rat and human studies (11, 15–22). In addition, a recent study showed that elevated plasma levels of RAGE are associated with PGD following lung transplantation (23).
Functional and biochemical assessment of alveolar epithelial injury in donor lungs prior to lung transplantation may be useful clinically. PGD is characterized by diffuse alveolar infiltrates in the lung allograft occurring between 6–96 hours after reperfusion, along with a PaO2/FiO2 ratio < 300mmHg and poor lung compliance. Histologic manifestations of PGD include alveolar epithelial injury in the lung allograft that persists beyond the initial 48 hours of transplantation in the absence of other identifiable causes of allograft dysfunction (24). Furthermore, the current definition of PGD, despite being sensitive in detecting poor graft function, is not specific in determining if PGD is the cause of poor oxygenation or responsible for the abnormal radiograph.
In conclusion, despite more than two decades of cumulative experience with lung transplantation, we still are unable to accurately predict which lung allograft recipients will develop primary graft failure (25). Therefore, we hypothesized that increased RAGE expression by lung donors and lung recipients correlates with the development of PGD and that high RAGE levels are indicative of graft dysfunction.
PATIENTS AND METHODS
Donor and Recipient Characteristics
The subjects in this study were consecutive cases of lung transplant recipients with their corresponding lung donors. Of these 35 donors, biological specimens were available from 25. Lung donors were recruited from brain-dead patients consented for lung donation. Clinical characteristics such as gender, age, cause of death, race, and PaO2/FiO2 ratios, chest radiograph, bronchoscopic findings, and culture data were recorded for 35 lung donors.
Explanted lungs were preserved using Perfadex (Vitrolife, Goteborg, Sweden). The graft ischemia time was recorded for all lung transplants.
Recipients listed at our institution received transplants according to their clinical priorities and independent of the study results. Clinical data were available for 35 lung transplant recipients: biological samples were collected from 34. PGD treatment involved administration of diuretics, prolonged mechanical ventilation with adjusted FiO2 and positive end-expiratory pressure, and inhaled NO (6) as required. This study was approved by the institutional review board at Emory University.
Assessment of Graft Function in Recipients
PGD was scored from 0 to 3 using chest radiographs and PaO2/FiO2 ratios according to the International Society for Health and Lung Transplantation guidelines, at regular or specified intervals during the first 96 hours post lung transplant (24). For the purpose of this study, recipients were assigned to groups based on their clinical characteristics. Patients who did not develop PGD at any time point during the 96 hours were classified as PGD 0. Other patients were grouped as ‘PGD 1’, or ‘PGD 2/3’ if they fit the standard clinical classifications for a 24 hour period. In addition to the standard classifications of PGD that utilizes clinical data at 1 time-point, we included a group of patients with severe PGD who met the above clinical criteria (grade 2 and 3) for two consecutive time-points. For the purpose of this study, this group was designated ‘2[PGD 2/3]’. Prior to extubation, all recipients received a bronchoscopy to assess bronchial anastomosis and BALf was obtained and submitted for culture. In addition, TBBX/BALf was obtained at regular intervals post-transplantation (2 weeks, 1, 2, 3, 6, 9, and 12 months) as part of surveillance bronchoscopy.
Transplant Immunosuppression Protocols
All patients received a standard immunosuppression protocol as described previously (26).
Transplant Infection Prophylaxis
All recipients received antibiotics up to five days after surgery, and subsequent antibiotic therapy length was determined based on final donor culture as previously described (27).
Collection of broncho-alveolar lavage fluid (BALf)
At the time of organ evaluation, BALf was performed following general inspection of the tracheobronchial tree and installation of 30–50 ml of room temperature normal saline. Normally, the BALf is obtained from a “wedged position” at the right middle lobe or lingula. The recovery volumes were close to 15 to 20 cc. The recovered fluid was centrifuged immediately to remove alveolar macrophages and any particulate matter, and transported on ice back to our institution. Upon arrival, the samples were then placed in storage at −70°C until subsequent analyses.
RAGE ELISA
Soluble RAGE levels (sRAGE) in bronchoalveolar lavage fluid (BALf) from donor lungs prior to procurement and recipients post-transplant (day 14) was measured using a commercially-available ELISA kit following the manufacturer’s instructions (R&D Systems, Minneapolis, MN). A total of 34 recipient bronchoscopic samples were analyzed for RAGE content. Out of the 34 recipient BALf samples analyzed, 25 corresponding donor samples were available for analyses. sRAGE levels are reported as ng per ml of BALf. As no reliable normalization methods exist, sRAGE levels were not normalized to parameters such as urea or protein.
Immuno-histochemical Analyses of RAGE Expression
To further investigate the potential correlation of RAGE expression with incidence of PGD, we performed immuno-histochemical analyses of cell membrane-bound RAGE in TBBX obtained through routine surveillance approximately two weeks after transplantation. Sections from TBBX were deparaffinized using standard techniques and then subjected to an antigen-retrieval step by heating under pressure to ∼120°C for 7 minutes in antigen retrieval solution (Dako Cyotomation). Samples were permeabilized with 0.05% Tween-20 and 0.05% Triton X-100, and blocked with streptavidin followed by biotin (Streptavidin / Biotin blocking kit; Vector Laboratories) in the presence of human IgG (Jackson ImmunoResearch) prior to incubation with primary antibody. Subsequently sections were incubated in 0.5 µg/ml goat polyclonal anti-RAGE primary antibody (R&D Systems) overnight at 4°C in the presence of human IgG. Endogenous peroxide activity was quenched with 3% H2O2. Samples were then incubated with biotinylated donkey anti-goat F(ab’)2 fragments for 1 hour. Bound biotinylated secondary antibodies were coupled to horseradish peroxidase-streptavidin (Vectastain ABC kit; Vector Laboratories) and color was developed with 3,3’-diaminobenzidine tetrahydro-chloride substrate (Vector Laboratories). Sections were counterstained with hematoxylin to visualize nuclei. To control for non-specific staining, sections were incubated with goat polyclonal IgG in place of the primary anti-RAGE antibody. Relative immuno-histochemical staining between groups was determined by quantifying staining in the tissue. Briefly, multiple photomicrographs were imported into Photoshop 7.0 (Adobe Systems Inc.). A color sampler tool was used to gate representative shades of brown diaminobenzidine staining. To determine the stain density of each field, the area of the field containing the selected color stain was selected, the remaining background was removed, and the resulting image was imported into Scion Image Beta 4.0.2 (Scion Corporation, Frederick, MD). The density of staining was averaged and calculated for each section.
Statistical Analyses
The assumptions of normal parametric tests (ANOVA, t-tests) were not met due to small sample sizes and non-normality, and therefore the corresponding nonparametric tests were employed. RAGE levels in donors and recipients were compared across the 4 PGD grades using the Kruskal-Wallis test. The Spearman rank correlation coefficient (rs) was used to determine the association between the donor RAGE levels and PGD grade and recipient RAGE levels and PGD grade. Two approaches were used to evaluate donor RAGE level as a predictor of recipient PGD. First, the risk of recipient PGD was modeled as a function of donor RAGE by using logistic regression. Logistic regression provided an estimate of the odds ratio and its 95% confidence interval as a measure of the degree of association between outcome (Recipient PGD grade 0 or any PGD) and donor RAGE. The goodness of fit of the model was assessed by the Hosmer-Lemeshow statistic. In addition, the Box-Tidwell transformation was used to assess the linearity of RAGE on the log odds scale (28). Second, the performance of donor RAGE was summarized with classification measures such as sensitivity, specificity and the area under the curve (AUC) of a receiver operating characteristic curve. Descriptive statistics were reported as medians, ranges and counts.
RESULTS
Donor characteristics
The study group included clinical data on 35 donors but biological samples were available from 25 donors. Clinical characteristics of donors are shown in Table 1. Age, sex, race, cause of death, selection criteria, and ischemic times for normal recipients versus PGD recipients were similar. The most common radiographic (CXR) finding in donors was mild perihilar congestion and atelectasis. The BALf culture was positive in 54% of the subjects in both groups. Of the donors that presented with very high RAGE BALf levels (greater than the median), 4 of them had ‘normal’ CXR (2 of these had some atelectasis), 1 had mild pulmonary edema, and 1 had a right pneumothorax (with chest tube placement prior to procurement). For a complete radiological description of PGD 0 donors versus PGD+ve donors (PGD 1, PGD 2/3, and 2[PGD 2/3], see Table 1.
Table 1.
Clinical characteristics of donors (n=35) that lead to recipients without (PGD −) or with PGD (PGD +)
| Donor | PGD − | PGD + |
|---|---|---|
| Mean Age ± SEM | 26 ± 11 | 36 ± 14 |
| Sex | 9/14 (64%) M | 9/21 (42%) M |
| 5/14 (36%) F | 12/21 (58%) F | |
| Race | 8/14 White (57 %) | 11/21 White (48%) |
| 4/14 Black (29%) | 8/21 Black (38%) | |
| 1/14 Hispanic (7%) | 2/21 Hispanic (10%) | |
| 1/14 Other (7%) | 0/21 other | |
| PaO2 ± SEM | 472 ± 67 | 458 ± 66 |
| Chest Radiograph at Time of procurement |
Edema 0/14 | Edema 3/21 9 (14%) |
| Contusion 1/14 (7%) | Contusion 2/21 (9.5%) | |
| Focal Air space 1/14 (7%) | Focal Air space 2/21(9.5%) | |
| Normal 12/14 (86%) | Normal 14/21 (67%) | |
| Positive bronchoscopy culture |
7/14 | 12/21 |
| Cause of Death | Traumatic 8/14 | Traumatic 10/21 |
| Non-traumatic 6/14 | Non-traumatic 11/21 | |
| Smoking history (≥10 pk yr) | 4/14 | 5/21 |
| Total Donors | 14 | 21 |
In nine cases, biopsies were obtained from donor lungs that were downsized prior to implantation. Histological analyses of these nine biopsies revealed normal lung architecture or existing focal changes consistent with either acute bronchiolitis, thomboemboli in pulmonary vessels, pneumonitis, atelectasis, focal alveolar damage, focal alveolar hemorrhage, and cystic changes. The outcome of recipients whose donor lungs were evaluated histologically was as follows: 5 (cystic changes, hemorrhage, thromboemboli, emphysema, and normal lung histology) led to PGD 2/3 that persisted for 48 hours or more (2[PGD 2/3]); 2 (thromboemboli and subpleural fibrosis) led to PGD 2/3 that resolved in 24 hours; and 2 (atelectasis / hemorrhage / pneumonitis, and atelectasis / hemorrhage / thromboemboli) led to PGD 0.
Donor BALf soluble RAGE levels
Donor BALf RAGE levels correlated with the severity of PGD
We first compared RAGE levels as a function of severity of PGD in the recipients. BALf RAGE levels in donor lungs that did not develop PGD post-transplantation were significantly lower compared to donor lungs that developed any clinical signs of PGD (data not shown). RAGE levels in 25 donors increased across PGD grades (P = 0.016, Figure 1). Additionally there was a correlation between donor RAGE level and PGD grade (rs = 0.61, P < 0.001). Donor RAGE levels for the 4 groups increase with severity of PGD: PGD 0 (median = 0.18 ng/mL, IQR = 0.20 ng/mL); PGD 1 (median = 0.26 ng/mL, IQR = 1.06 ng/mL); PGD 2/3 (median = 1.13 ng/mL, IQR = 0.69 ng/mL); and 2[PGD 2/3] (median = 1.75 ng/mL, IQR = 2.66 ng/mL).
Figure 1. Donor BALf RAGE levels increased with the severity of PGD.
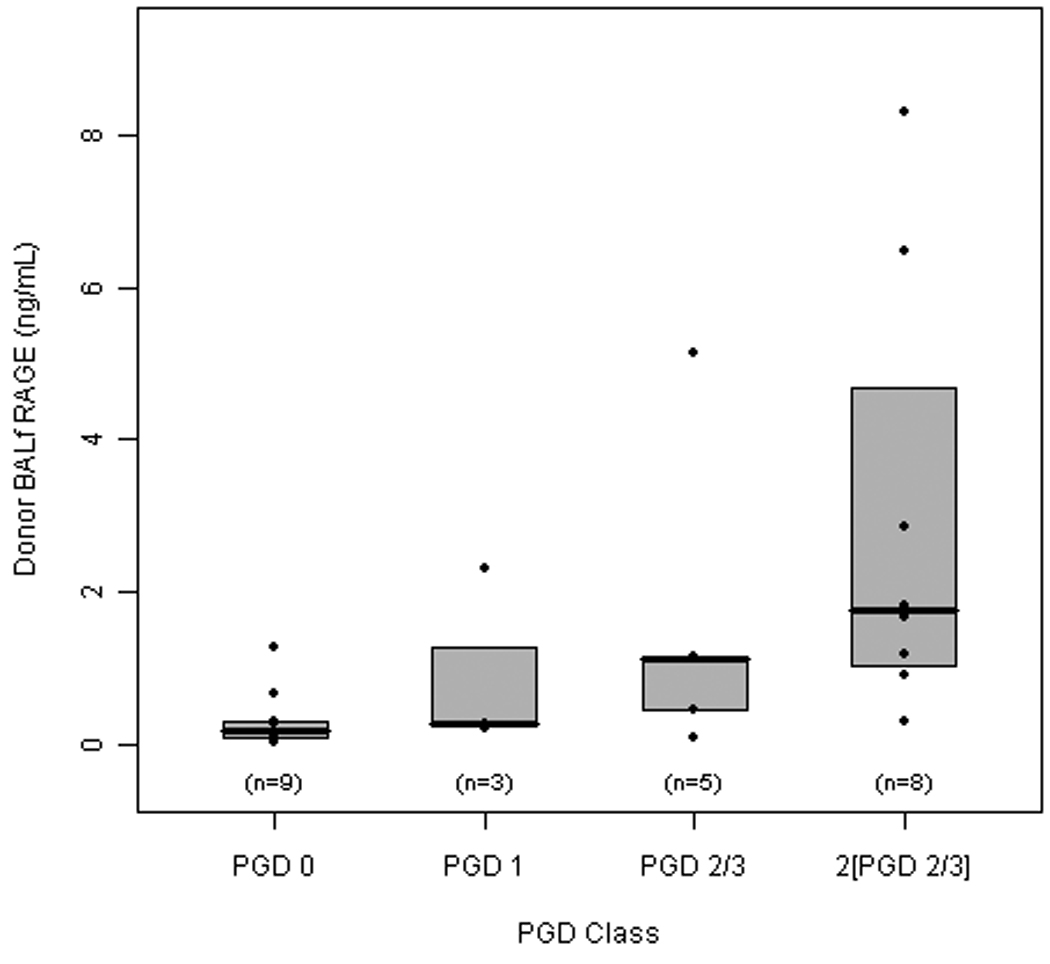
Box plot of the median and interquartile range and individual data values for donor RAGE by PGD severity. The middle line within the box represents the median, the top line represents the 75th percentile and the bottom line represents the 25th percentile. All analyses focused on RAGE levels according to PGD severity. RAGE levels did not differ between donor lungs that did not develop PGD (PGD 0), PGD 1, or PGD 2/3 groups. Donor lungs that did not develop PGD had a RAGE level that was significantly different from 2[PGD 2/3] (p = 0.008).
It is important to mention that the subgroup of donors with abnormal histology at the time of transplantation had adequate donor selection criteria, but had high RAGE levels in their BALF, and correlated with patients that subsequently developed PGD (data not shown).
Donor RAGE level was a predictor of recipient PGD (odds ratio (OR) = 1.768 per 0.25 ng/ml increase in donor RAGE level; 95% confidence interval for the OR: 1.028 to 3.041). Based on the logistic regression model, the estimated risk of recipient PGD with a donor RAGE level of 0.75 ng/ml was 40% [the calculated risk of recipient PGD is: ew/(1 + ew), where w = 3.0059 × 0.75 – 2.6717].
The sensitivity and specificity estimates for donor RAGE values of 0.38 ng/ml were 80% and 78%, respectively. The accuracy of donor RAGE level depended on how well the donor RAGE value separated recipients with and without PGD. The area under the receiver operating characteristic (ROC) curve (AUC) is a measure of test accuracy. An AUC of 1.0 represents a perfect test; and an AUC of 0.5 represents discrimination no better than chance (the diagonal line on the Figure 2). The AUC measures the discrimination, that is, the ability of the donor RAGE level to correctly classify recipients as with or without PGD. Using recipients who did not have PGD (PGD 0) and those who had persistent PGD (2[PGD 2/3]), the estimated area under the curve (AUC) of the ROC curve was 0.94 (Figure 3). The AUC estimate for donor RAGE reflected the proportion (0.94) of recipient pairs for which the logistic regression model assigned a higher probability to a recipient who had PGD 2/3 at two consecutive timepoints (2[PGD 2/3]) than to a recipient who did not have PGD (PGD 0).
Figure 2. Donor RAGE levels predicted the development of PGD in recipients.

Using recipients who did not have PGD (PGD 0) and those who had persistent PGD (2[PGD 2/3]), donor RAGE levels predicted development of recipient severe PGD (odds ratio = 2.1 per 0.25 ng/ml increase in donor RAGE level; P = 0.04). For a donor RAGE value of 0.75 ng/ml, the sensitivity and specificity estimates were 88% and 89%, respectively. The estimated area under the curve (AUC) of the receiver operating characteristic curve was 0.94.
Figure 3. Recipient BALf RAGE levels correlated with PGD grade.
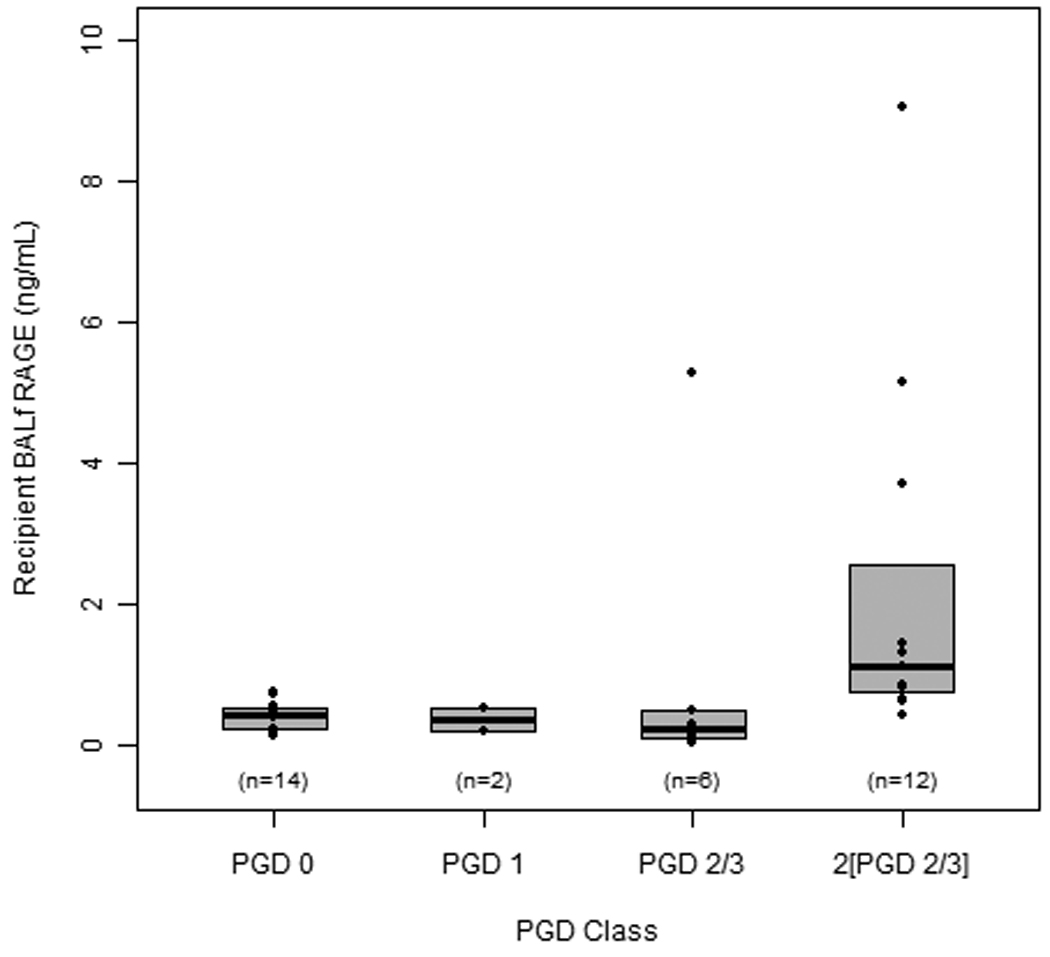
RAGE levels in recipient lungs that did not develop PGD (‘none’) were compared to levels in lungs that developed PGD based on severity (‘mild’ = PGD 1; ‘moderate’ = PGD 2/3; ‘severe’ = PGD 2/3 at two consecutive time points). RAGE levels did not differ between lungs that did not develop PGD (none) and ‘mild’ or ‘moderate’ PGD groups. However, those patients that did not develop PGD had a RAGE level that was significantly different from severe PGD (p = 0.008).
Recipient Characteristics
At our center, 44 lung transplants were performed utilizing lungs from donors who met brain death criteria between February 2007 and July 2008. Of the 44, this study included 35 recipients with clinical data, and 34 biological samples for analyses. The main indications for transplant were distributed between patients with pulmonary fibrosis (IPF) (34%) and patients with emphysema-COPD (48%). Although 42.9% (9/21) of the recipients with PGD had an IPF diagnosis and 21.4% (3/14) of the recipients without PGD had IPF, this difference was not statistically significant (P = 0.28 by Fisher’s exact test) (Table 2). 86% of lung transplants were bilateral and the remainder was unilateral lung transplants. 2 out of 35 (∼6%) of patients received re-transplants (Table 2). Of the single transplants performed, our center did not receive the contralateral lung from the same donor and as a result, we do not have data pertaining to the outcome of those transplants at other centers. The recipients' postoperative course was followed for 12 months from the time of transplantation, or until death as part of our ongoing surveillance protocol.
Table 2.
Clinical characteristics in recipients (N = 35) without (PGD −) and with PGD (PGD +)
| Recipient | PGD − | PGD + |
|---|---|---|
| Mean Age ± SEM | 56 ± 6 | 55 ± 11 |
| Sex | 10/14 (71%) M | 11/21 (53%) M |
| 4/14 (29%) F | 10/21 (47%) F | |
| Diagnosis | COPD (9/14) | COPD (8/21) |
| IPF (3/14) | IPF (9/21) | |
| Sarcoidosis (1/14) | OB (1/21) | |
| OB (1/14) | CF (1/21) | |
| Other (2/21) | ||
| Type of Lung transplant | 12 Bilateral | 18 Bilateral |
| 1 Single | 2 Single | |
| 1 Single retransplant | 1 Single retransplant | |
| Ischemia Time Left Lung ± SEM | 257 min ± 22 | 281 min ± 20 |
| Ischemia Time Right Lung ± SEM | 260 min ± 19 | 294 min ± 16 |
| Graft Function | PGD 0 (14) | PGD 1 (2) |
| PGD 2/3 (6) | ||
| PGD 2[PGD 2/3] (12) | ||
| ICU Days (mean) | PGD 0 (4.9) | PGD 1 (6) |
| PGD 2/3 (7.7) | ||
| 2 [ PGD 2–3] (12.5) | ||
| Ventilator Days (mean) | PGD 0 (2.2) | PGD 1 (4.3) |
| PGD 2/3 (3.4) | ||
| 2 [ PGD 2–3] (7) | ||
| Mortality (1 yr post transplant) | 0 | 3 |
Assessment of Graft Function in recipients
Using the consensus definition, PGD grades 0, 1, 2, and 3 at 48 hours post-transplantation occurred in 41%, 6%, 17 %, and 34 % of patients, respectively.
Recipient soluble BALf RAGE levels
BALf RAGE levels in recipient lungs that did not develop PGD post-transplantation were significantly lower compared to RAGE in recipient lungs that developed any clinical signs of PGD (data not shown). RAGE levels in 34 recipients increased across PGD grades (P=0.02; Figure 3). Additionally there was a correlation between recipient RAGE level and PGD grade (rs =0.66, P < 0.001). Furthermore, RAGE levels remained high in 8 recipients that developed severe graft dysfunction (2[PGD 2/3]; median = 1.37 ng/ml) compared to 9 recipients who did not display clinical signs of PGD (PGD 0; median = 0.39 ng/ml, P = 0.008).
Immuno-histological analyses revealed increased RAGE in recipient TTBX
Clinical PGD correlated with findings of diffuse alveolar damage (DAD) on two week TBBX, whereas the absence of clinical PGD was not associated with DAD. Tissue was obtained from patients without (PGD 0; n = 8) and with severe PGD (2[PGD 2/3]; n = 8). Consistent with previous studies (13, 14), RAGE expression by alveolar epithelial cells was evident in TBBX from recipients without PGD (Figure 4A). Digital image analyses of RAGE immuno-histochemical staining revealed nearly three-fold increase in RAGE expression in biopsies that were obtained from patients with 2[PGD 2/3] (p = 0.0078; Figure 4B).
Figure 4. Immuno-histological analyses revealed increased RAGE in recipient TTBX.
(A) Representative immuno-staining for RAGE in TTBX from recipients without PGD (no PGD; top panels) or recipients with severe PGD (bottom panels). RAGE protein is expressed in normal TTBX; however, staining intensity is increased in TTBX from patients with severe PGD. (B) Densitometric analyses of immuno-histochemical staining. TTBX from recipients with severe PGD display ∼3-fold increased staining compared to recipients without PGD. Micrographs are representative of TTBX from 8 post-transplant recipients for each group. Sections were counterstained with hematoxylin to visualize nuclei. Isotype control antibodies (Ctrl IgG) were used to control for non-specific antibody binding. Bar = 100 µm.
DISCUSSION
We found that RAGE levels prior to organ recovery or after transplantation were associated with subsequent PGD. In addition, we found that donor RAGE levels were significantly higher in recipients with 2[PGD 2/3] (PGD grade 2/3 at two consecutive timepoints) versus recipients with PGD grade 0 (Figure 1). These data may prove to be useful in evaluating donor lungs, since donor lung RAGE levels may predict the development of PGD in the recipient. Furthermore, we found that RAGE levels in the BALf of LTx recipients correlated with those recipients whose graft dysfunction was severe and persistent. Densitometric analyses of immuno-histochemical staining of transbronchial biopsies revealed increased abundance of membrane-bound RAGE in patients with 2[PGD 2/3]. The data presented here may represent an additional predicative parameter for the development of severe PGD. In addition, BALf RAGE levels may be useful as a biochemical marker of significant and persistent graft dysfunction after LTx.
In this study, we described a population of recipients that exhibit a more severe clinical phenotype of PGD after lung transplantation. We categorized these recipients as having ISHLT-defined PGD grade 2 or 3 at two consecutive timepoints (2[PGD 2/3]). Due to the fact that this group is not currently defined by ISHLT guidelines, this classification of PGD severity may be considered exploratory. In addition, a major limitation of this study is that the small sample size of 25 donors and 35 recipients prevented additional covariate adjustment (both donor and recipient characteristics) through multivariable analyses. Thus, our predictive analyses are preliminary and must be interpreted with caution. A multicenter prospective study is necessary to ensure the statistical power necessary to investigate the relationship between donor BALf RAGE and severity of PGD, and adjust for complex donor-recipient interactions involving demographic and co-existing conditions associated with PGD occurrence. Our work is suggestive of a relationship between RAGE levels and PGD but a larger sample size is necessary to better develop this initial predictive model. After the model is developed, a validation study is necessary to ensure protection against unrecognized problems and limitations. Furthermore, although more recipients with PGD had an IPF diagnosis vs patients without PGD, this probably represents a clinically important difference that we cannot currently detect as statistically important due to the small sample size and limited statistical power to detect an association of the magnitude. Lastly, due to limitations in the number of available samples from donors and recipients, future studies are required to accurately investigate any possible correlation between donor BALf RAGE values and less severe grades of PGD (e.g. PGD grade 1 or 2 at one timepoint).
The results presented here are consistent with those in previous studies wherein biological markers have been utilized to help identify the risk for subsequent lung injury in the LTx recipient (29, 30). Fisher et al., found that IL-8 cytokine levels in BALf could be used in clinical practice as an objective indicator of donor-lung suitability (29). In addition, VEGF levels in the lung donor have been reported to be associated with increased risk for the development of PGD in the recipient (30). Earlier biomarkers such as changes in mRNA profiles may also be associated with outcomes post-transplantation. Anraku et al. used microarray analyses of lung tissue to identify four genes associated with oxidative stress or ischemic injury that were upregulated in donor lungs that developed PGD post-transplantation (31). Using quantitative real-time PCR, Kaneda et al. demonstrated that the mRNA profile of cytokines such as IL-6 was the most predictive of mortality after lung transplantation (32). A similar microarray profile revealed differential expression of genes involved in signaling, apoptotic, and stress pathways between donor lungs that developed PGD post-transplantation and those that did not (33). Interesting, there was a correlation between high metallothionein 3 expression and freedom from PGD after transplantation (31).
The use of BALf RAGE levels as a predictor of underlying lung injury in lung recipients has a physiologic basis as RAGE mRNA and protein are expressed most prominently in the lungs (14, 21). Furthermore, increased serum RAGE levels were found to predict ICU outcomes in patients following LTX (21) and a recent study showed that serum RAGE levels are higher in lung transplant recipients with PGD, compared to non-PGD recipients (23). RAGE is expressed on the basal membrane of AT1 cells, and rat and human studies have shown that soluble RAGE is released by injurious stimuli into the alveolar space where it may serve to modulate the inflammatory response (14, 34). As it pertains to the donor lung, we hypothesize that soluble RAGE levels may identify if multiple insults to the lung prior to organ procurement increases susceptibility for subsequent development of PGD in the recipient. This hypothesis is consistent with the fact that since AT1 cells comprise more than 95% of the internal surface of the alveoli, damage to these cells is an important feature of injured lung. In support of this contention, AT1-specific proteins have been used as markers of the severity of acute respiratory distress syndrome in animal and human studies (34, 35).
In addition to its role as a marker of alveolar epithelial injury, recent evidence provides an important functional role for RAGE in lung injury. Using an animal model of ischemia reperfusion injury to the lung, the degree of injury was attenuated when RAGE was neutralized or ablated (20). RAGE levels were also found to be associated with altered lung liquid clearance (11). These data are consistent with the notion that patients with poor alveolar epithelial function have impaired fluid clearance, and worse prognosis after a severe acute lung injury (36). Furthermore, Briot et al. showed that in donor lungs that were rejected for transplantation, the rate of alveolar fluid clearance correlated inversely with RAGE levels in the BALf (15).
In conclusion, the current study describes data on RAGE levels in BALf and lung tissue from a well-characterized cohort of lung donors and LTx recipients. For recipients who developed PGD, BALf RAGE levels were increased in their corresponding donors. In addition, those recipients who developed severe and persistent PGD also had elevated RAGE levels in their BALf and lung tissue. These data are indicative of underlying epithelial injury occurring prior to procurement, during, and after the LTx. These findings suggest RAGE is a promising target for further investigation as a useful biological marker of alveolar epithelial injury in donor lungs prior to LTx and perhaps in patients with PGD after LTx.
Acknowledgements
The authors wish to thank Dr Steven R. Duncan at the University of Pittsburgh for his assistance editing the manuscript, LifeLink Foundation (organ procurement organization) and Jennifer Smith (Department of Pathology, Emory University Hospital) for their assistance.
Funding sources: F32 NRSA to POM (5F32AA016262), Emory University Research Council award to AP (2006073), Emory Alcohol Lung and Biology Center (NIH NIAAA, 1P50AA13575701)
Abbreviations
- PGD
Primary graft dysfunction
- RAGE
Receptor for advanced glycation end-products
- LTx
Lung transplantation
- BALf
Broncho-alveolar fluid
- TBBX
Transbronchial biopsy
- CXR
Chest radiograph
- ALI
Acute Lung Injury
- IPF
Idiopathic pulmonary fibrosis
References
- 1.Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal NP, Kaiser LR, et al. Primary graft failure following lung transplantation. Chest. 1998;114(1):51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 3.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69(6):1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 4.Khan SU, Salloum J, O'Donovan PB, Mascha EJ, Mehta AC, Matthay MA, et al. Acute pulmonary edema after lung transplantation: the pulmonary reimplantation response. Chest. 1999;116(1):187–194. doi: 10.1378/chest.116.1.187. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24(10):1489–1500. doi: 10.1016/j.healun.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 8.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360(9333):619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- 9.Angel LF, Levine DJ, Restrepo MI, Johnson S, Sako E, Carpenter A, et al. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006;174(6):710–716. doi: 10.1164/rccm.200603-432OC. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med. 1999;159(3):980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 11.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L52–L59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton CM, Iversen M, Milman N, Zemtsovski M, Carlsen J, Steinbruchel D, et al. Outcome of lung transplanted patients with primary graft dysfunction. Eur J Cardiothorac Surg. 2007;31(1):75–82. doi: 10.1016/j.ejcts.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Katsuoka F, Kawakami Y, Arai T, Imuta H, Fujiwara M, Kanma H, et al. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem Biophys Res Commun. 1997;238(2):512–516. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- 14.Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9(2):165–174. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 15.Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest. 2009;135(2):269–275. doi: 10.1378/chest.08-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant. 2007;26(7):675–680. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63(12):1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths MJ, McAuley DF. RAGE: a biomarker for acute lung injury. Thorax. 2008;63(12):1034–1036. doi: 10.1136/thx.2008.101493. [DOI] [PubMed] [Google Scholar]
- 19.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1427–L1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 20.Sternberg DI, Gowda R, Mehra D, Qu W, Weinberg A, Twaddell W, et al. Blockade of receptor for advanced glycation end product attenuates pulmonary reperfusion injury in mice. J Thorac Cardiovasc Surg. 2008;136(6):1576–1585. doi: 10.1016/j.jtcvs.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Tasaka S, Shiraishi Y, Fukunaga K, Yamada W, Seki H, et al. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med. 2008;178(4):356–362. doi: 10.1164/rccm.200707-1069OC. [DOI] [PubMed] [Google Scholar]
- 23.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma Levels of Receptor for Advanced Glycation End-Products(sRAGE), Blood Transfusion, and Risk of Primary Graft Dysfunction. Am J Respir Crit Care Med. 2009 Aug 6; doi: 10.1164/rccm.200901-0118OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 25.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24(10):1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 26.Pelaez A, Lyon GM, Force SD, Ramirez AM, Neujahr DC, Foster M, et al. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant. 2009;28(1):67–71. doi: 10.1016/j.healun.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik PM, Lyon GM, 3rd, Ramirez A, Lawrence EC, Neujahr DC, Force S, et al. Dapsone-induced hemolytic anemia in lung allograft recipients. J Heart Lung Transplant. 2008;27(11):1198–1202. doi: 10.1016/j.healun.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley and Sons; 1989. [Google Scholar]
- 29.Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, et al. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163(1):259–265. doi: 10.1164/ajrccm.163.1.2005093. [DOI] [PubMed] [Google Scholar]
- 30.Krenn K, Klepetko W, Taghavi S, Paulus P, Aharinejad S. Vascular endothelial growth factor increases pulmonary vascular permeability in cystic fibrosis patients undergoing lung transplantation. Eur J Cardiothorac Surg. 2007;32(1):35–41. doi: 10.1016/j.ejcts.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Anraku M, Cameron MJ, Waddell TK, Liu M, Arenovich T, Sato M, et al. Impact of human donor lung gene expression profiles on survival after lung transplantation: a case-control study. Am J Transplant. 2008;8(10):2140–2148. doi: 10.1111/j.1600-6143.2008.02354.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaneda H, Waddell TK, de Perrot M, Bai XH, Gutierrez C, Arenovich T, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6(3):544–551. doi: 10.1111/j.1600-6143.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 33.Ray M, Dharmarajan S, Freudenberg J, Zhang W, Patterson GA. Expression profiling of human donor lungs to understand primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(10):2396–2405. doi: 10.1111/j.1600-6143.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- 34.McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J. 2004;24(4):664–673. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- 35.Newman V, Gonzalez RF, Matthay MA, Dobbs LG. A novel alveolar type I cell-specific biochemical marker of human acute lung injury. Am J Respir Crit Care Med. 2000;161(3 Pt 1):990–995. doi: 10.1164/ajrccm.161.3.9901042. [DOI] [PubMed] [Google Scholar]
- 36.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]



