Abstract
Organophosphate pesticide (OPPs) concentrations in artesian wells located in Thai agricultural and non-agricultural communities were studied during both wet and dry seasons. A total of 100 water samples were collected and subjects were asked to complete a survey. Gas chromatography flame photometric detector was used for OPP analysis. The average OPP concentration in the agricultural communities (0.085 and 0.418 µg/l in dry and wet season) was higher than in the non-agricultural communities (0.004 µg/l in both seasons). Ingestion of OPPs in contaminated water in the agricultural communities were estimated to be 0.187 and 0.919 µg/day during the dry and wet seasons, respectively, and 0.008 µg/day during both seasons in the non-agricultural communities. Agricultural communities were exposed to pesticide residues under the oral chronic reference dose. This study suggests that people in agricultural communities may be exposed to significantly greater levels of pesticides than non-agricultural populations during the dry and wet seasons (p < .001, .001).
Keywords: organophosphate pesticides residues, drinking water, health risk assessment, agricultural community
INTRODUCTION
Access to clean water is a fundamental human right that is vital for a healthy population. The availability of safe and reliable water has remarkably increased the quality of life for the people of Thailand. This is reflected in Thailand’s continuous upward trend in the Human Development Index (UNDP 2006; Thai Health Department 2002). The water supply in Thailand now reaches 98% of the population in urban areas and 70% of the population in rural areas. There remain, however, many water quality problems, particularly pesticide contamination in both ground and surface water sources (Thai Health Department 2002).
Thailand grows and exports a large volume of agricultural products such as rice, fruit, cassava, and para-rubber. Approximately 55.7% of the Thai population works in the agricultural sector (Agricultural Extension Department 2007). Cropping agriculture is the primary source of agriculture, and pesticides and fertilizers are used extensively to minimize crop loss and increase productivity. Pesticide residues in both ground and surface waters may affect environmental quality and human health (Purdue University Cooperative Extension Service 2006; Siriwong 2007).
The Bang Rieng sub-district of Songkhla Province, Thailand, is primarily an agricultural area and is divided into two regions based on the pattern of agricultural practices: conventional and integrated pest management (IPM). Conventional agriculture refers to a commercial agriculture system where farmers mainly use pesticides for pest control. IPM agricultural, on the other hand, focuses on reducing pesticide use through alternative techniques such as biological control, crop rotation, or netted crop growing. This study focuses on the conventional agricultural areas due to high pesticide usage associated with these areas. The conventional agricultural region within the Bang Reing sub-district consists of approximately 891 rai (143 km2) of vegetable farm area, including 92 households (Prince of Songkhla University 2004; Office of Bang Reing sub-district 2004). The vegetable farmers are living in, or around, the vegetable farm. The non-vegetable areas (reference areas) are rubber plantations and are 7–8 km from the farm areas, within the same sub-district. The reference areas consist of 1,056 rai (169 km2) including 96 households (Office of Bang Reing sub-district 2004).
According to previous studies, organophosphate pesticides (OPPs) are the most commonly used pesticides in conventional farming areas (Visuthismajarn et al. 2005). Through personal interviews it was found that chlorpyrifos, dicrotofos, and profenofos were highly used on conventional farms. Chlopyrifos is used as an insecticide on farms to kill worms and is used in the household to kill cockroaches. Dicrotophos and profenophos are often used as an acaricide to kill mites. Previous studies have also found that farm children living in or near the vegetable farming areas in the Bang Reing agriculture sub-district were found to excrete higher levels of dialkylphosphate metabolite (biomarker of organophosphate exposure) in their urine than reference groups living outside the farm area (Petchuay et al. 2006).
Organophosphate pesticides (OPPs) are commonly applied on Bang Rieng farms for crop protection. Organophosphates are classified as non-persistent pesticides. The half-life of chlopyrifos in soil is less than 60 days with little or no leaching observed (USEPA 1999a). Dicrotophos degradation is not induced by exposure to light. The half-life of dicrotophos in sandy loam was recorded at 2.7 days and 7 days under aerobic and anaerobic conditions, respectively (USEPA 1999b). Profenofos dissipates from neutral to alkaline soil with a half-life of several days and under normal use it is not highly mobile and will not leach into groundwater (USEPA 1998). OPPs can be easily broken down by environmental factors and are rapidly decomposed by microbes. However, repeated human exposure to even low levels of OPPs for long periods of time can result in accumulation of OPPs in the body. Long-term exposure to OPPs can lead to illness and cholinesterase inhibition causing weakness or paralysis of the muscles (USEPA 1999c). According to the Thai Epidemiology Division, more than 2000 Thai farmers experienced adverse health effects attributed to OPP exposure in 2002 (Thai Epidemiology 2006). Vegetable growers can be exposed to OPPs both directly and indirectly via inhalation, dermal contact, and ingestion, due to OPPs residues in air, soil, ground and surface waters, and food.
Vegetable growers in the Bang Rieng area consume a large amount of water from artesian wells located on the farms. Artesian wells are the only source of water for households in the study and reference groups. The water in these wells has multiple uses including drinking, cooking, and bathing, and mixing pesticides. The artesian wells are also contaminated with pesticides as a result of leaching when pesticides are sprayed, spilled during mixing, or disposed of in the ground. Consumption of contaminated water is suspected to be a primary source of indirect exposure. This study aims to assess vegetable growers’ exposure to OPPs resulting from ingestion of contaminated drinking water from artesian wells.
MATERIALS AND METHODS
Sample Collection and Analyses
Thirty-three vegetable growers were selected as the study group. Vegetable growers were selected on the basis of the extent of organophosphate pesticide usage on their farm. Information on pesticide use was obtained from a year 2004 population database on vegetable farmers (Office of Bang Reing sub-district 2004). All vegetable growers had their own artesian well for household use. The control group was comprised of seventeen people from the reference area who also had artesian wells for household use. The primary householder working on the farm was selected to answer the survey questions. The 33 vegetable growers and 17 non-vegetable growers, whose wells were sampled, were interviewed in person. Questions focused on frequency and quantity of water consumption, bodyweight, and pesticide usage.
This study was approved by the Ethical Review Committee for Research Involving Human Subjects and/or Use of Animals in Research, Health Science Group of Faculties, Colleges and Institutes, Chulalongkorn University, Thailand under document No. 097/2006. All participants signed a consent form prior to participation in this study.
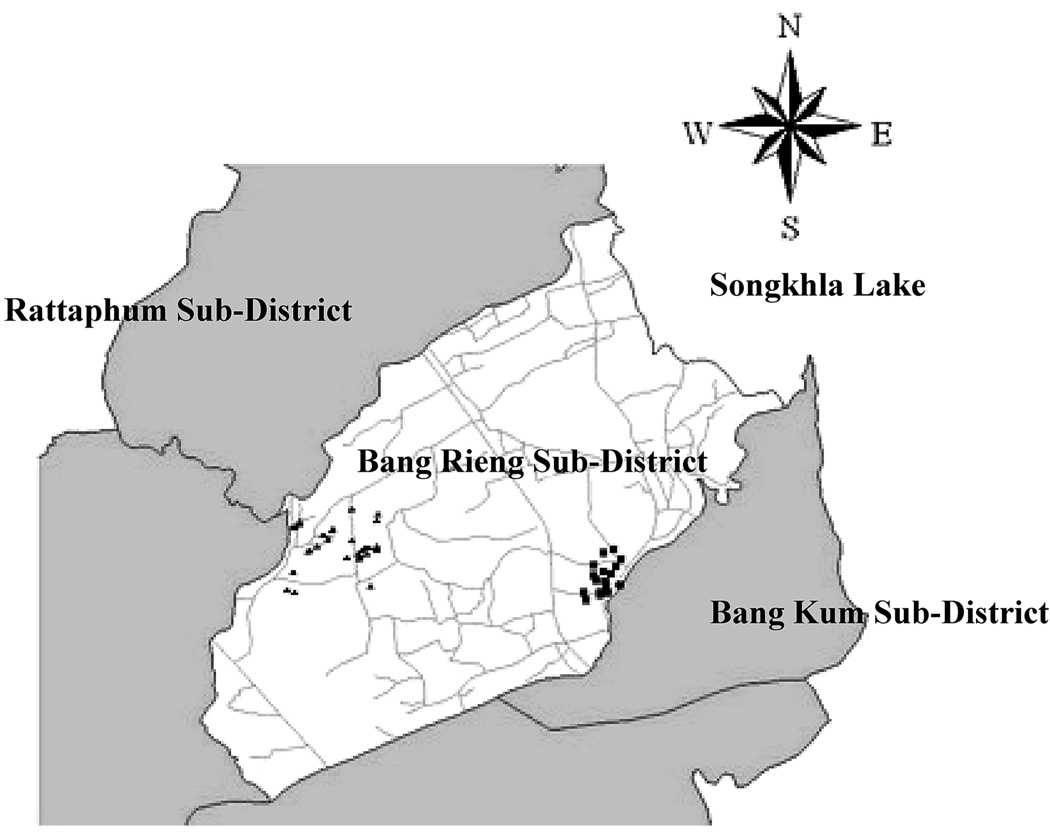
Altogether, 100 water samples were collected from artesian wells. Fifty water samples, 33 from vegetable farm areas and 17 from non-vegetable farm areas, were collected during the dry season (April–June 2006). Fifty water samples, 33 from vegetable farm areas and 17 from non-vegetable farm areas, were collected during the wet season (September–October 2006) (Figure 1).
Figure 1.
Locations of the artesian wells located in the Bang Rieng sub-district, Khuan Neing district, Songkhla Province [Farm areas (▲), n = 33; Reference areas (■), n = 17].
Water samples were collected using 2.5-liter amber glass bottles with screw-top, Teflon-lined covers. The bottles were prewashed with a non-phosphate detergent and then rinsed with distilled water and methanol. Samples were collected from taps supplied by artesian wells. Prior to each sample’s collection, the tap was kept open for 5 minutes to wash out any contaminants in the pipes and to rinse out the bottles. Water samples were maintained below 4°C during transportation and storage, and were analyzed within 7 days, following sample collection methods (Lawrence 1996).Water samples were then analyzed for chlorpyrifos, dicrotofos, and profenofos.
A solid phase extraction (SPE) method was used to extract the water samples. We used 500 mg Carbograph SPE packing, a homogenous, non-porous graphite carbon black with a surface area of 100 m2/g and a particle size range of 38–125 µm. Prior to sample extraction, the SPE cartridge was activated by passing 5 ml of dichloromethane: methanol (80:20), 2 ml of methanol, and 5 ml of an acidic water, (pH 2.0) through the cartridge. After activation of the SPE cartridge, a 1-liter water sample was passed through the cartridge at a flow rate of approximately 20–100 ml/min. The SPE cartridge was then washed with 5 ml of deionized water and dried for 5 min to remove interstitial water. The sorbed OPP compounds in the SPE cartridge were eluted with 1 ml of methanol and 5 ml of dichloromethane: methanol (80:20). The elute was evaporated with nitrogen gas and reconstituted with 20 µl of toluene for analysis using gas chromatography (Jha and Wydoski 2003; Alltech 2001).
An Agilent 6890 Gas Chromatograph (GC) equipped with a flame photometric detector was used for quantification. OP compounds were completely separated using a Zebron 1701 fused silica capillary column (30 m × 0.32 mm I.D. × 0.25 µm film thickness) coated with 14% cyanopropyl phenyl and 86% dimethyl polysiloxane. We injected 1.0 µl of analyte into the GC on splitless mode with a 0.75 min vent delay. The injector temperature was maintained at 250°C. The initial oven temperature was set at 80°C for 2 min and then increased at a rate of 15°C/min until it reached 180°C, where it remained for 2 min. The temperature was then increased at a rate of 6°C/min. until it reached 260°C, where it remained for 2 min using this temperature program. The retention times of dicrotofos, chlorpyrifos, and profenofos were 13.924, 16.917, and 20.255 min, respectively.
Exposure Assessment and Risk Characterization
The average daily intake of OPPs was determined using Eq. (1), following the U.S. Environmental Protection Agency (USEPA) (1999c).
| (1) |
where C [µg/l] = pesticide concentration, IR [l/day] = intake rate of water
This was converted to an average daily dose by dividing by the bodyweight (Eq. (2)).
| (2) |
where BW = bodyweight (average bodyweight of an individual from each group)
Bodyweight and intake rate (IR) averages were calculated from the information collected from the personal interviews. In addition, the bodyweight and water IR of the two females were in the male bodyweight and IR range. Therefore, males and females were not separated in estimating dose and ADD.
Finally, hazard quotients were used to assess the risk of detrimental health effects occurring from the exposure to OPPs arising from ingestion of artesian water. The hazard quotient (HQ) is the ratio of the average daily dose (ADDs) and the reference dose (RfD). This value indicates the degree to which exposure is greater or less than the RfD. When the ratio is equal to or greater than 1, exposure exceeds the RfD and the exposed population may be at risk of detrimental health effects occurring.
The RfD is a daily oral dosage by bodyweight. The RfD is a benchmark dose derived from the No Observable Adverse Effect Level (NOAEL) by consistent application of generally order-of-magnitude uncertainty factors (UFs) that reflect various types of data sets used to estimate RfD (IRIS 1994).
Statistical Analysis
Statistical analysis was performed using SPSS software (Version 16.0). The mean comparison of OPPs in water between vegetable farm areas and non-vegetable farm areas were determined by using independent sample t-test.
RESULTS
This study consisted of 33 vegetable growers (study group), 32 males and 1 female. The average weight of this group was 65 ± 11 kg (range 45–103 kg). The reference group consisted of 17 subjects, 16 males and 1 female. The average weight of this group was 65 ± 6 kg (range 55–75 kg). The average water consumption for both groups was 2.0 ± 0.5 liters/day. The exposure frequency and duration of water intake was the same for both groups; 365 days/year and 45 years, respectively (Table 1).
Table 1.
Characteristics of survey population and pesticide usage.
| Vegetable growers (n = 33) |
Non-vegetable growers (n = 17) |
|||
|---|---|---|---|---|
| Gender | n | % | n | % |
| Male | 32 | 64 | 16 | 32 |
| Female | 1 | 2 | 1 | 2 |
| Weight (kg) | ||||
| 65 ± 11 | 65 ± 6 | |||
| Average water consumption (liters/day) | ||||
| 2.2 (±0.5) | 2.2 (±0.5) | |||
| Exposure frequency—water (days/year) | ||||
| 365 | 365 | |||
| Exposure duration—water (years) | ||||
| 45 | 45 | |||
| Pesticide usage | n | % | n | % |
| Chlopyrifos | 2 | 6 | — | — |
| Dicrotophos | 5 | 15 | — | — |
| Profenofos | 4 | 12 | — | — |
| Chlopyrifos & Dicrotophos | 11 | 33 | — | — |
| Chlopyrifos & Profenofos | 7 | 21 | — | — |
| Dicrotophos & Profenofos | 2 | 6 | — | — |
| Chlopyrifos & Dicrotophos & Profenofos | 2 | 6 | — | — |
OPPs in Water
Water samples were found to contain chlorpyrifos, dicrotofos, and profenofos. The mean OPPs concentration in water (µg/l) with the maximum and minimum values are shown in Table 2. The concentrations of chlorpyrifos, dicrotofos, and profenofos were significantly higher in the vegetable farm areas compared to the reference areas, during both the wet and dry seasons (p ≤ .05). In the vegetable farm areas, concentrations of all three pesticides were highest during the wet season. Mean combined OPPs concentrations in the vegetable farm areas (0.085 and 0.418 µg/l in the dry and wet season) were significantly greater than the mean levels in the reference areas (0.004 µg/l in both seasons) (p ≤ .05).
Table 2.
Organophosphate pesticides in water from artesian wells in vegetable farm areas and reference areas at Bang Rieng sub-district.
| Concentration of organophosphate pesticide in water samples (µg/l) | ||||||
|---|---|---|---|---|---|---|
| Organo-phosphate pesticides |
n | Sampling site |
Dry season | Wet season | ||
| Mean ± S.E. | Range | Mean ± S.E. | Range | |||
| Chlorpyrifos | 33 | Farm | 0.035± 0.006 | 0.001–0.111 | 0.069 ± 0.030 | < 0.0001–0.511 |
| 17 | Reference | 0.003 ± 0.0009 | < 0.0001–0.011 | 0.003 ± 0.001 | < 0.0001–0.011 | |
| Dicrotophos | 33 | Farm | 0.011± 0.003 | < 0.001–0.067 | 0.132 ± 0.070 | < 0.001–1.738 |
| 17 | Reference | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Profenofos | 33 | Farm | 0.039± 0.014 | < 0.001–0.461 | 0.217 ± 0.062 | 0.004–1.323 |
| 17 | Reference | 0.001 ± <0.001 | < 0.001–0.008 | 0.001 ± < 0.001 | < 0.001–0.008 | |
| ΣOPPs | 33 | Farm | 0.085 ± 0.014 | 0.001–0.639 | 0.418 ± 0.137 | 0.004–3.572 |
| 17 | Reference | 0.004 ± 0.001 | < 0.001–0.019 | 0.004 ± 0.001 | < 0.001–0.019 | |
MDL chlorpyrifos = 0.0001 µg/l, dicrotophos = 0.001 µg/l, profenofos = 0.001 µg/l.
Statistical comparison between farm area and reference area using independent sample t-test, significance at p ≤ .05. 14 samples were below the detection limit.
Mean chlorpyrifos concentrations did not exceed U.S. National Drinking Water Standards during the wet or dry seasons (USEPA 2006). Similarly, the mean wet and dry season values for profenofos concentrations did not exceed the Australia Drinking Water Guidelines for Pesticides (Hamilton et al. 2003). However, during the dry and wet seasons, individual profenofos samples from the vegetable farm areas contained up to 1.537 and 4.410 times the Australian Drinking Water Guidelines for Pesticides, respectively. Dicrotofos is a highly toxic pesticide, which has been banned and is not registered for use in developed countries (PAN 2008). However, the use of dicrotofos is allowed in Thailand. There are no drinking water standards or guidelines for dicrotofos from the World Health Organization (WHO), the USEPA, Australia, New Zealand, Japan or other sources to make comparisons (Hamilton et al. 2003). There are also no drinking water standards or guidelines for chlorpyrifos, dicrotophos, or profenofos in Thailand.
Exposure Assessment and Risk Characterization
To calculate exposure dose (Dpot) and average daily intake (ADD), samples with an OPP concentration less than the detection limit were assigned a zero. As shown in Table 3, during the dry season the vegetable growers were exposed to 0.187 µg/day of OPPs in drinking water. During the wet season, the mean OPPs exposure from drinking water by vegetable growers was 0.919 µg/day. Intake of OPPs during the wet season was five times higher than the dry season for vegetable growers. The reference group (living in non-vegetable farm areas) was exposed to approximately 0.008 µg/day of OPPs in the water both during the dry and wet seasons. Mean intake dose of OPPs for the vegetable growers (1.87 and 0.919 µg/day in dry and wet season) were significantly higher than the mean intake of the reference group (p ≤ .05).
Table 3.
The dose estimation of organophosphate pesticide (µg/day) in artesian wells that were collected from vegetable farm areas and reference areas in Bang Reing sub-district, Khuan Neing district, Thailand.
| Potential Dose (µg/day) | ||||||
|---|---|---|---|---|---|---|
| Organo-phosphate pesticides |
n | Sampling site |
Dry season | Wet season | ||
| Mean | Range | Mean | Range | |||
| Chlorpyrifos | 33 | Farm | 0.077 | 0.002–0.244 | 0.152 | 0*–1.124 |
| 17 | Reference | 0.006 | 0*–0.022 | 0.006 | 0*–0.022 | |
| Dicrotophos | 33 | Farm | 0.024 | 0*–0.147 | 0.290 | 0*–3.824 |
| 17 | Reference | 0* | 0* | 0* | 0* | |
| Profenofos | 33 | Farm | 0.086 | 0*–1.014 | 0.477 | 0.009–2.910 |
| 17 | Reference | 0.002 | 0*–0.016 | 0.002 | 0*–0.016 | |
| ΣOPPs | 33 | Farm | 0.187 | 0.0021–1.406 | 0.919 | 0.009–7.859 |
| 17 | Reference | 0.008 | 0*–0.038 | 0.008 | 0*–0.038 | |
The value MDL of chlorpyrifos, dicrotofos, and profenofos was assumed to be zero for calculation.
The average daily dose (ADD) and hazard quotients (HQ) for vegetable farmers and controls are illustrated in Table 4 and Table 5, respectively, for the dry and wet seasons. An HQ value greater than 1 indicates that the exposure has exceeded the RfD. The RfDs determined by the USEPA Integrated Risk Information System (IRIS) for chlorpyrifos, dicrotofos, and profenofos are 0.003, 0.0001, and 0.0001 mg/kg-day, respectively (IRIS 1994; Harvard Center for Risk Analyses 2004).
Table 4.
Average daily dose (ADD) of organophosphate pesticide residues due to water ingestion for the people in Bang Rieng sub-district, Khuan Neing district, Thailand.
| Average Daily Dose (ADD) (× 10−6 mg/kg-d) | ||||||
|---|---|---|---|---|---|---|
| Organo-phosphate pesticides |
n | Sampling site |
Dry season | Wet season | ||
| Mean | Range | Mean | Range | |||
| Chlorpyrifos | 33 | Farm | 1.185 | 0.034–3.757 | 2.335 | 0–17.295 |
| 17 | Reference | 0.092 | 0–0.339 | 0.092 | 0–0.339 | |
| Dicrotophos | 33 | Farm | 0.372 | 0–2.268 | 4.468 | 0–58.824 |
| 17 | Reference | 0 | 0 | 0 | 0 | |
| Profenofos | 33 | Farm | 1.32 | 0–15.603 | 7.345 | 0.135–44.779 |
| 17 | Reference | 0.031 | 0–0.246 | 0.031 | 0–0.246 | |
| ΣOPPs | 33 | Farm | 2.877 | 0.034–21.628 | 14.148 | 0.135–120.899 |
| 17 | Reference | 0.123 | 0–0.585 | 0.123 | 0–0.585 | |
Statistical comparison between farm area and reference area using independent sample t-test, significance at p ≤ .05.
Table 5.
Hazard quotient (HQ) of organophosphate pesticide residues due to water ingestion for the people in Bang Rieng sub-district, Khuan Neing district, Thailand.
| Hazard Quotient (HQ)b | |||||||
|---|---|---|---|---|---|---|---|
| Organo- phosphate pesticides |
Oral reference dosea (mg/kg.day) |
n | Sampling site |
Dry season | Wet season | ||
| Mean | Range | Mean | Range | ||||
| Chlorpyrifos | 0.003 | 33 | Farm | 0.0004 | 0.00001–0.0013 | 0.0008 | 0–0.0058 |
| 17 | Reference | 0.00003 | 0–0.0001 | 0.00003 | 0–0.0001 | ||
| Dicrotophos | 0.0001 | 33 | Farm | 0.0037 | 0–0.0227 | 0.0447 | 0–0.5883 |
| 17 | Reference | 0 | 0 | 0 | 0 | ||
| Profenofos | 0.0001 | 33 | Farm | 0.0132 | 0–0.1560 | 0.0735 | 0.0014–0.4478 |
| 17 | Reference | 0.0003 | 0–0.0025 | 0.0003 | 0–0.0025 | ||
| ΣOPPs | — | 33 | Farm | 0.0173 | 0–0.18 | 0.119 | 0–1.0419 |
| 17 | Reference | 0.00033 | 0–0.0038 | 0.00033 | 0–0.0025 | ||
USEPA IRIS database. Available at: http://risk.lsd.ornl.gov/cig (accessed 6 June 2006); http://www.hcra.hardvard.edu/rip/risk_in_persp_january (accessed 17 August 2008).
The mean ADDs for total OPPs for vegetable growers during both wet and dry seasons were significantly higher than the mean ADDs for the reference group (p = <.001, .001) (Table 4). Further, ADD was significantly higher during the wet season (14.148 × 10−6 mg/kg-day) compared to the dry season (2.877 × 10−6 mg/kg-day). Mean ADDs for both the vegetable growers and the reference groups during the dry and wet season were below the mentioned oral RfDs (Table 5). HQs calculated using the mean ADDs for the vegetable growers and the reference groups during both the dry and wet seasons were less than 1, suggesting that the vegetable growers and the reference group may not be at higher risk from consuming OPPs in contaminated well water.
In addition, we used the cumulative risk assessment guidelines to estimate the vegetable grower’s cumulative exposure through ingestion of contaminated water. The findings of this study shows that OPPs (chlorpyrrifos, dicrotofos, and profenofos) share a common mechanism of toxicity (i.e ., the same toxic effect can occur at the same organ), the inhibition of cholinesterase activity (USEPA 2002). The sum of the highest three OPPs (chlorpyrrifos, dicrotofos, and profenofos) HQ values during the wet season was 1.04, which may suggest that vegetable growers who intake one or more OPPs may be at higher risk during the rainy season.
DISCUSSION
This study is a preliminary risk assessment of ingestion of OPPs through groundwater among farmers in Thailand. As previously mentioned, detectable levels of OPPs did not exceed U.S. drinking water standards or Australian drinking water guidelines. This may be due to the clay and silt soil in the Bang Reing sub-district, which tends to hold water and dissolved chemicals longer and also has a greater surface area to increase adsorption (Ohio State University 1992). In addition, chlorpyrifos, dicrotofos, and profenofos have short half lives (non- persistence pesticides).
Mean OPPs concentrations in vegetable farm areas were higher during the wet season than the dry season. This may be a result of agricultural runoff and leaching during the wet season. Typically, the closer pesticide application is to a heavy or sustained rainfall, the greater likelihood some pesticide leaching will occur. During the dry season there is probably high photodegredation (breakdown of pesticides by light) due to a higher intensity of sunlight. Through personal interviews it was found that pesticide application and timing and pesticide characteristics were similar among vegetable growers. Therefore this information did not influence the differences in OPP concentrations during the wet or dry seasons.
This research has shown that the OPPs residues (chlorpyrifos, dicrotofos, and profenofos) were detected in most of the water samples from the Bang Rieng agricultural area. The people who lived in the agricultural communities were exposed to OPPs by ingestion of contaminated water. The risk characterization provided an indication of the significance of exposure to these pesticides, which was indicated by total intake. The results show that people in the vegetable farm areas have a greater intake (ADDs) of OPPs than people in the reference area because OPPs such as chlorpyrifos, dicrotofos, and profenofos were highly used on the farms. Chlorpyrifos was sometimes used as an insecticide in households of both vegetable farm areas and reference areas. The applied pesticides can penetrate into and through the soil during discharge of groundwater into the aquifer (National Pesticide Telecommunication Network 2008). This resulted in the contamination of artesian wells by OPPs in agricultural areas being higher than non-agricultural areas.
The Hazard Quotients for chlorpyrifos, dicrotofos, and profenofos were less than one in the agricultural communities and non-agricultural communities. The risk characterization indicated that there was a low risk of detrimental health effect arising from exposure to OPPs in artesian (drinking water) for people in both the agricultural area and reference area. However, environmental monitoring and health surveillance should still be applied to this area to prevent possible future risks associated with these OPPs. The Thai government’s mitigation plan suggests providing technical support, tax benefits, and other incentives to help reduce and control the use of pesticides and also recommends efficient and safe methods on pesticide use in the agricultural sectors to effectively reduce pesticide contamination of drinking water (Thai Pollution Control Department 2009).
There is no historical data on water ingestion exposure; therefore, this is the first study on ingestion exposure in Thailand. In addition, there are also no drinking water standards or guidelines for chlorpyrifos, dicrotophos, or profenofos in Thailand. There have been studies on inhalation exposure, which have suggested that there is a low risk for detrimental health effects from inhalation exposure of chlorpyrifos by vegetable growers in the Bang Reing sub-district (Jirachaiyabus 2002). It is important that future pesticide studies cover other exposure routes such as dermal absorption in addition to ingestion and inhalation. Pesticide residues in soil, vegetables, and air should also be analyzed.
Finally, this study provides an understanding of organophosphate exposure via water ingestion in agricultural communities in Bang Reing sub-district, Khuan Neing district, Thailand. The risk assessment approach used in this study should also be applied when data on environmental concentrations of different contaminants become available to provide an indication of the significance of any exposure in agricultural communities.
ACKNOWLEDGMENTS
This study was supported by the National Center of Excellence for Environmental and Hazardous Waste Management (NCE-EHWM), Chulalongkorn University, and partially funded by the 90th Anniversary of the Chulalongkorn University Fund (Ratchadaphiseksompot Endowment Fund). Support also came from the Thai Fogarty ITREOH Center D43 TW007849-01 Fogarty International Center—National Institutes of Health (NIEHS) and NIEHS Center of Excellence, University of Medicine and Dentistry of New Jersey and Rutgers University NIEHS P30 ES05022.
REFERENCES
- Agricultural Extension Department. Agriculture data of Thailand. 2007 Available at http://www.doea.go.th/stat/statt.htm.
- Alltech. Deerfield, IL, USA: IL: Alltech Associates Inc; Data Sheet 210142 U: Carbograph SPE II. 2001
- Hamilton DJ, Ambrus A, Dieterle RM, et al. Regulatory limits for pesticide residues in water. Pure Appl Chem. 2003;75:1123–1155. [Google Scholar]
- Harvard Center for Risk Analysis. Risk in Perspective: The Precautionary Principle in Practice: Comparing US EPA and WHO Pesticide Risk Assessment. 2004 Available at http://www.hcra.hardvard.edu/rip/risk_in_persp_january.
- IRIS (Integrated Risk Information System) Oral chronic reference dose Integrate Risk Information System Database; Toxicity and chemical specific factor database. 1994. 1994 Available at http://risk.lsd.ornl.gov/cig.
- Jha VK, Wydoski DS. USGS Water–Resources Investigations Report 03-4139. Denver, CO, USA: US Department of the Interior, US Geological Survey; 2003. Method of Analysis by the U.S. Geological Survey National Quality Laboratory: Determination of Organophosphate Pesticides in Filtered Water by Gas Chromatography with Flame Photometric Detection. [Google Scholar]
- Jirachaiyabus V. [Thesis] Thailand: Graduate School Chulalongleorn University; 2002. Exposure Assessment of Traditional and IPM Farmers on Using Pesticides: A Case Study at Bang Reing Sub-district. [Google Scholar]
- Lawrence HK. Compilation of EPA’s Sampling and Analysis Methods. 2nd ed. New York, NY, USA: Lewis Publishers; 1996. [Google Scholar]
- National Pesticide Telecommunication Network. Pesticides in drinking water. 2008 Available at http://npic.orst.edu/factsheet/drinkingwater.pdf.
- Office of Bang Rieng sub-district. A survey report on population and housing in Bang Rieng sub-district. Songkla, Thailand: Office of Bang Rieng sub-district; Songkhla Province. 2004
- Ohio State University. Bulletin: Pesticide and Groundwater Contamination. 1992 Available at http://ohioline.osu.edu/b802_7/index.html.
- PAN (Pesticide Action Network) Pesticide registration status dicrotophos—Registration, import consent and bans. 2008 Available at http://pesticideinfo.org/Detail_ChemReg.jsp?Rec_Id=PC35045.
- Petchuay V, Visuthismajarn P, Vitayavirasak B, et al. Monitoring of organophosphate pesticides in preschool children in an agricultural community in Thailand. Int J Occup Environ Health. 2006;12:133–141. doi: 10.1179/oeh.2006.12.2.134. [DOI] [PubMed] [Google Scholar]
- Prince of Songkla University. Information obtained from the GIS survey by the Faculty of Natural Resources. Songkla, Thailand: Sngkhla Province: Prince of Songkla University; 2004. (Unpublished Manuscript) [Google Scholar]
- Purdue University Cooperative Extension Service. Purdue Pesticide Program. 2006 Available at http://btny.purdue.edu/
- Siriwong W. Organochlorine Pesticide Residues in Aquatic Ecosystem and Health Risk Assessment of a Local Agricultural Community [dissertation] Bangkok, Thailand: Graduate School Chulalongkorn University; 2007. [Google Scholar]
- Thai Epidemiology Division. Annual Epidemiology Surveillance Report 2003. Nonthaburi, Thailand: Thai Epidemiology Division; 2006. [Google Scholar]
- Thai Health Department. Nonthaburi, Thailand: Thai Health Department; Water Supply and Sanitation Assessment in Thailand 2000. 2002
- Thai Pollution Control Department. Pollution and Mitigation Policy 1997–2016. 2009 Available at http://www.pcd.go.th/info_serve/en_reg_polhaze.html.
- UNDP (United Nations Development Programme) Human Development Report 2006, Beyond Scarcity: Power, Poverty and the Global Water Crisis. New York, NY, USA: Palgrave Macmillan; 2006. [Google Scholar]
- USEPA (US Environmental Protection Agency) Washington, DC, USA: USEPA, Office of Pesticide Programs Special Docket for Pesticide Reregistration Risk Assessments; The Environmental Fate and Effect Division (EFED) Environmental Risk Assessment for Profenofos. 1998 May; 2007. Available from (DVD Set)
- USEPA. Reregistration Eligibility Science Chapter for Chlopyrifos: Fate and Environmental Risk Assessment Chapter. Washington. DC, USA: USEPA, Office of Pesticide Programs Special Docket for Pesticide Reregistration Risk Assessments; 1999a May; 2007 Available from (DVD Set)
- USEPA. The Environmental Fate and Effect Division (EFED) Reregistration Eligibility Decision (RED) Chapter for Dicrotophos. Washington. DC, USA: USEPA, Office of Pesticide Programs Special Docket for Pesticide Reregistration Risk Assessments; 1999b May; 2007 Available from (DVD Set)
- USEPA. Definitions and General Principles for Exposure Assessment: Guidelines for Exposure Assessment. Washington, DC, USA: Office of Pesticide Programs; 1999c. p. 3. [Google Scholar]
- USEPA. Office of Pesticide Programs; Status of Cumulative Risk Assessment for Organophosphate Pesticides. 2002 Available at: http://epa.gov/pesticides/cumulative/files/guidefinal__4-new.pdf.
- USEPA. Drinking Water Health Advisory. Office of Water, EPA 822-R-06-013. 2006 Available at http://www.epa.gov/waterscience/criteria/drinking/standards/dwstandards.pdf.
- Visuthismajarn P, Jatuphot W, Khamnalrat A, et al. Status and Health Impact for Chemical Use in the Agricultural Sector: Case Study of Bang Rieng Sub-District, Khuan Neing District, Songkhla Province. Songkhla, Thailand: Nonthaburi: Health System Research Institute; 2005. [Google Scholar]