Abstract
We designed and validated a novel knee joint fixation/distraction system to study tendon–to-bone healing in an in vivo rat model of anterior cruciate ligament (ACL) reconstruction. The system uses an external fixator to apply a cyclic distraction of the knee joint while monitoring the resultant force developed across the joint, thus providing a temporal indication of structural changes during the healing process of the bone-tendon-bone reconstruction. The validation was performed using an optical kinematic tracking system to determine the local displacement of the knee. The average system compliance was determined to be 42.4 ± 8.8 μm/N with a coefficient of variation of 20.7%. The compliance was used to obtain a best fit correction factor which brought the total root mean square error of knee joint distraction to within 179 μm (16.1%) of the applied distraction. We performed a pilot study using 15 rats that had ACL reconstructions using a flexor digitorum longus tendon autograft and found that the animals tolerated the indwelling fixator and daily anesthesia over a 10 day loading protocol. Our knee joint fixation/distraction system provides a valuable tool to study how mechanical stimuli affect in vivo bone-tendon-bone healing.
Keywords: tendon, bone, healing, ACL, in vivo, cyclic strain, mechanical load
1 Introduction
A traumatic rupture of the anterior cruciate ligament (ACL) of the knee is a common athletic injury with as many as 100,000 occurring each year in the United States [1]. Loss of ACL function results in knee instability with the potential for further knee damage, and may predispose the individual to the early onset of osteoarthritis. Surgical treatment consists of ligament reconstruction using a tendon graft, placed in bone tunnels drilled into the femur and tibia, approximating the position of the native ACL and reestablishing its constraining link (Fig. 1). This procedure requires secure tendon-to-bone healing to be successful [2].
Fig. 1.
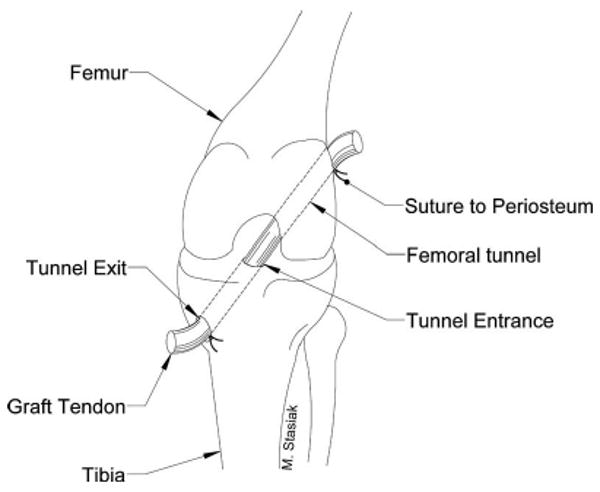
Illustration of an ACL reconstruction using a tendon graft pulled through drilled bone tunnels in the femur and tibia. The graft is secured with sutures to the periosteum at the tunnel exits outside of the joint in our animal model.
The resumption of mechanical load on the reconstructed ACL normally begins during postoperative rehabilitation. Typically this rehabilitation involves some degree of passive and active joint motion while avoiding excessive loading of the tendon graft as it heals to the bone. This joint motion places the tendon graft in tension, and produces shear stress at the tendon-to-bone tunnel interface [3]. Although it is well established that tensile strain has a positive effect on tendon and ligament physiology, its effect on the healing tendon-bone or ligament-bone junction is poorly understood. As a result, there is little consensus on the optimum time of onset of postoperative rehabilitation and the subsequent mechanical loading of the ligament graft [4,5].
The insertion site or enthesis of the native ACL is a highly specialized tissue consisting of a progression through four distinct zones: ligament, fibrocartilage, mineralized fibrocartilage, and bone, which serves to resist stress concentrations and securely anchor the ligament to bone [6,7]. Mechanical loading is thought to play a role in the post-natal development of these four zones [8,9]. Animal models of ligament reconstruction via tendon graft demonstrate that the four zone morphology and composition of the native enthesis is not restored during the healing process. Rather, a tendon graft heals to bone via formation of a fibrovascular scar tissue interface with material properties inferior to the native enthesis [10,11].
Our long-term goal is to study the effects of mechanical loading on the healing tendon-to-bone tunnel interface, and hopefully identify a mechanical load regime and time of application for optimal rehabilitation of post-surgical ACL reconstruction. We developed a novel mechanical system using a previously developed rat model of ACL reconstruction [12,13]. A custom designed external joint fixation device (ex-fix) is surgically placed during ACL reconstruction on the femur and tibia of each animal. This ex-fix is interfaced to our mechanical loading system to apply controlled cycles of distraction to the reconstructed knee joint, reproducing the relative graft motion of the tendon in the bone tunnel, or interface shear described above. Our novel system allows for repetitive, controlled loading of a healing tendon graft reconstruction an in vivo animal model.
To effectively allow comparison of subject groups undergoing different loading protocols and to measure temporal changes in force as a function of joint distraction during healing, the system was designed to satisfy the following requirements.
Allow for cyclic distraction of the knee joint space in an anesthetized animal that has undergone ACL reconstruction using a tendon graft placed in bone tunnels in the femur and tibia.
Allow the experimenter to vary the amount of distraction and number of cycles.
Record the force generated across the soft tissue spanning the knee joint space as a function of distraction, for each cycle of each loading session.
Constrain knee joint motion between loading sessions to eliminate or minimize uncontrolled distraction during cage activity.
Avoid undue discomfort and allow normal cage activity and feeding.
2 Methods
2.1 System Overview
The system consists of two components: a metal, external fixator that spans the knee of each animal and a motorized cyclic distraction mechanism (CDM). During daily loading sessions the ex-fix is attached to the CDM. The bar linking the femoral and tibial parts of the ex-fix is removed, and controlled cyclic distraction of the knee joint is performed by the CDM, resulting in a cyclic elongation of the healing tendon graft.
2.2 Fixator Design
The ex-fix provides for the controlled distraction of the knee joint and the elimination or minimization of knee motion when locked with a rigid bar between loading sessions (Fig. 2). It consists of identical femoral and tibial “pin-clamps,” suspended by universal joints from the removable locking bar. The universal joints allow positioning of each pin-clamp so its jaws can be tightened onto two threaded 0.9 mm diameter pins (MicroAire 1600-635T Surgical Instruments, Charlottesville, VA) drilled into each bone, securing the ex-fix to the animal. This pin diameter was the maximum allowable without inducing an unacceptable incidence of fractures. After final adjustment, the screws on the universal joints and pin-clamps are tightened to maintain their relative orientation.
Fig. 2.
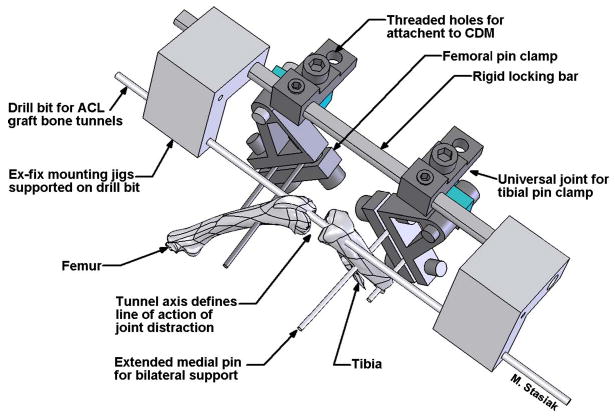
Illustration of ex-fix placement immediately before ACL reconstruction. Mounting jigs are temporarily supported on the bone tunnel drill bit. These jigs hold the ex-fix parallel to the tunnel axes while two pins each, (0.9 mm threaded k-wires) are drilled into the femur and tibia. After the ex-fix pin clamps are tightened down onto these pins, the drill bit and mounting jigs are removed. The graft tendon can now be pulled through the tunnels and secured with sutures to the periosteum at the tunnel exits, completing the ACL reconstruction.
Initial prototypes of the ex-fix demonstrated excessive compliance caused by cantilever bending of the 0.9 mm diameter pins, which limited knee distraction. As a remedy a bilateral support of one pin from each bone was implemented (Fig. 3). Tubular clamps supported from the CDM secured the free end of the threaded pin protruding through the skin on the medial side of the limb. This reduced pin deflection and compliance of the ex-fix to an acceptable amount relative to the distraction distance, as shown in the results.
Fig. 3.
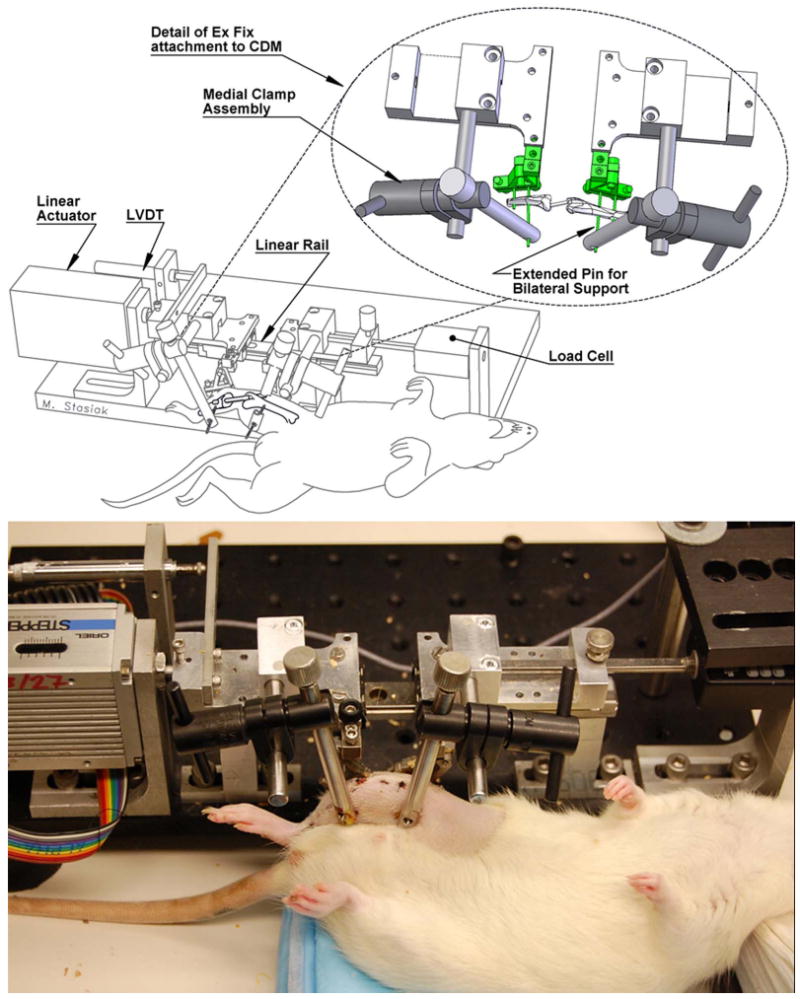
Anesthetized rat during loading in the CDM with bilateral support of bone pins (schematic on top with photo on bottom)
2.3 Cyclic Distraction Mechanism
The CDM used a linear bearing (Nippon Bearing No. SEBS 12A2-145, Wood Dale, IL) consisting of two roller bearing blocks riding on a rigid linear rail (Fig. 3). A stepper motor-driven linear actuator (Oriel No. 18503 via Oriel controller No 20010, Oriel Corp., Stratford, CT; accuracy is ±5 μm, repeatability is <2 μm) moves one block while the other is held stationary by a load cell (Transducer Techniques MDB-5, Temecula, CA; nonlinearity is <0.05% of rated output, nonrepeatability is <0.05% of rated output) fixed to the frame of the CDM. A linear variable differential transformer (LVDT) measures the displacement of the moving block (Schaevitz LBB315 PA-100, Measurement Specialties, Inc., Hampton, VA; linearity < ±0.20% of full range output, repeatability is 0.10 μm).
During animal loading, the ex-fix is attached to the linear bearing with its femoral part secured to the stationary block and the tibial part secured to the motor-driven block. The ex-fix locking bar is removed to allow the actuator to displace the tibia from the femur. The load cell simultaneously reads the force developed through all soft tissues across the distracted joint space, including the graft tendon.
A custom program was developed in LABVIEW (National Instruments, Austin, TX) to control the CDM and record the load-distraction data. The program operated in closed loop mode and used displacement feedback from the LVDT to control the position of the stepper motor actuator. The force/distraction data were recorded to a file through a data acquisition card (No. 6036E, National Instruments, Austin, TX). The control program also provided for continuous correction of the displacement due to the mechanical compliance of the system.
3 Characterization of the System
3.1 Compliance
We determined the compliance of the entire system (CDM with ex-fix), rather than listing the specifications of its individual components, keeping in mind that mechanical stimulation of the graft depends on the accuracy and repeatability of joint space distraction. We also characterized the variability in the compliance since numerous intraoperative factors may affect system compliance between animals.
System compliance was calculated indirectly in five animals with bilateral fixation in vitro by taking the difference between the displacement-load response of the ACL-reconstructed knee and the displacement-load response of the knee and system. The linear slope of the resulting displacement-load curve represented the compliance of the system alone. Displacement of the knee joint was measured by a kinematic tracking system as described in Sec. 3.3 while displacement of the knee and system was measured by the LVDT mounted on the CDM.
System compliance was also directly measured in three animals with bilateral fixation in vitro by securing a 1.2 mm diameter metal wire across the knee joint. After the wire became taught, it allowed no further distraction of the knee joint; therefore, the resulting joint distraction curve was the deformation in the system. Slope of the linear portion of the displacement-load curve was the compliance.
In both experiments, device compliance was calculated using the data from the sixth loading cycle. Maximum load for these experiments was limited to 39.2 N (4000 g) because higher levels caused increased incidence of bone fracture.
We also indirectly characterized the compliance of the system with unilateral fixation. This provided a basis for comparing the effect of adding bilateral support of the bones on compliance. The characterization was conducted using ten animals in vitro. Maximum load in these experiments was limited to 12 N to ensure that the excursion range of our stepper motor or LVDT was not exceeded when using this lower compliance ex-fix.
The outcome measures for the data collected from the tests of each fixation method were: average device compliance, standard deviation and coefficient of variation (COV) to assess interanimal variability, and the average R2 value and its COV as a measure of the goodness of fit of a linear model for the compliance of each individual ex-fix. Finally, a least squares linear approximation for all of the compliance data collected from both the bilateral and the unilateral groups and the associated R2 value was calculated to determine the single compliance coefficient that most closely fit all of the experimental displacement-load responses in each group.
3.2 Accuracy of Knee Distraction
For future experiments our primary independent variable will be distraction of the knee joint. Therefore, we characterized how accurately the device distracted the joint using five cadaveric animals. The knee distraction experiments were conducted using bilateral fixation since this approach will be used in future studies.
Knee distraction was calculated using both the fixator compliance for each specimen and the least squares compliance for all specimens, and compared to an independent measurement of knee distraction as obtained using the kinematic tracking system. The load across the joint was then plotted against distraction as measured by: the kinematic system (our “gold standard”), the LVDT, and for both the animal-specific and the least squares compliance correction factors. We calculated the root mean square (RMS) error between knee distraction as measured by the kinematic system, and the displacements recorded by the LVDT, and the displacements predicted using the animal-specific and the least squares compliance correction factors.
3.3 Measurement of Knee Distraction
Measurements for knee distraction were obtained using a three-dimensional (3D) kinematic tracking system (ProReflex MCU, Qualisys Inc., Gothenburg, Sweden). This system recorded knee distraction by monitoring the 3D spatial position of reflective markers that were glued to the femoral and tibial tunnel exits of the reconstructed cadaveric knees (Fig. 4). The tracking system was accurate to 53 μm RMS error (2.6%) with a COV of 3.2% for displacements of 2.00 mm using a digital caliper (Fowler Inc., Newton, MA) as our reference standard. The manufacturer-provided specifications stated a caliper resolution of 10 μm, an accuracy of 20 μm, and a repeatability of 10 μm.
Fig. 4.
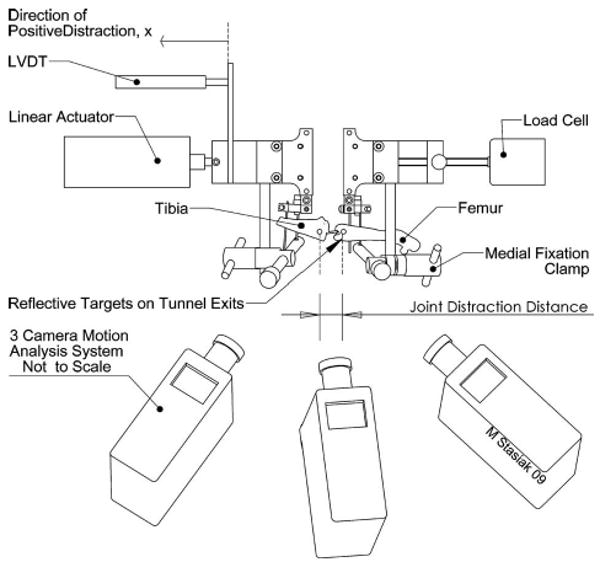
The kinematic tracking system measured the 3D spatial position of reflective markers glued to the femur and tibia, subsequently, displacement across the joint space was calculated. These data were used to develop a correction factor to account for the compliance of the ex-fix.
Three-dimensional position data of each marker were recorded for 6 distraction cycles at 10 Hz. The 3D spatial coordinates of each marker were post-processed using a fourth-order Butterworth low pass filter with a 0.01 Hz cut-off frequency. The filter was implemented using the signal processing toolbox available in MAT-LAB (Mathworks, Natick, MA). Subsequently, the magnitude of the distance between markers on the tibia and femur was calculated over the six distraction cycles. Accuracy calculations were made using the data of the sixth loading cycle.
4 Pilot Study
The purpose of the in vivo pilot study was to determine if the animals could tolerate the surgical procedure, continuous wearing of the ex-fix, and daily loading regimen under anesthesia. With the approval of our Institutional Animal Care and Use Committee, 15 male Sprague Dawley rats underwent surgical placement of the ex-fix, along with ACL reconstruction of the right knee using a flexor digitorum longus tendon autograft. On postoperative day 4, the animals began a ten day long loading regime, consisting of a daily 50 cycles of knee distraction to 2.5 mm within a load limit of 23.5 N. Distraction was performed in the CDM at 0.17 Hz, under 2% isoflurane anesthesia, resulting in total anesthesia time of approximately 20 min, allowing for set up and loading.
Since femoral or tibial fracture is a concern in this small animal model, we evaluated for fracture using high resolution anterior-posterior and oblique radiographs (Faxitron Model No. MX-20 DC4, Faxitron X-ray Corporation, Wheeling, IL). Images of each rat were obtained prior to the first loading session to identify any perioperative fractures, and then every 2 days over the course of the loading regimen. We also monitored the daily load-displacement curves as an indicator of slip in the mechanical components of the device, loosening at the pin-bone interface, or catastrophic failure of any of the tissues.
5 Results
The average system compliance for all specimens using bilateral fixation was 42.4±8.8 μm/N (Fig. 5). This yielded a COV of 20.7%. Average system compliance using unilateral fixation was 145.2±44.9 μm/N (Fig. 5) yielding a COV of 30.9%. The average R2 values for fitting a linear equation to the compliance data for the bilateral and unilateral fixation were 0.989 ± 0.005 and 0.988 ± 0.007, respectively. This yielded a COV of 0.5% and 0.7% for bilateral and unilateral fixation, respectively.
Fig. 5.
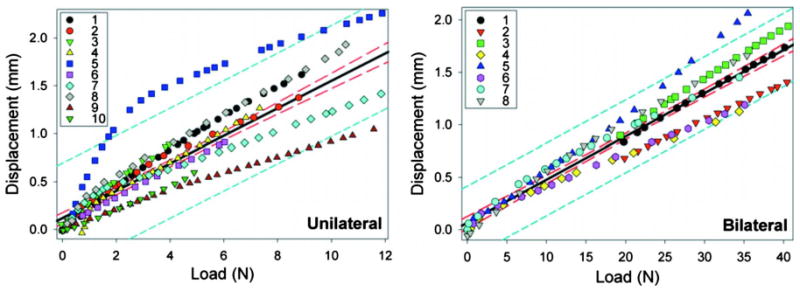
Displacement-load responses of ten unilateral (left) and eight bilateral (right) external fixators obtained from our in vitro loading experiments. The least squares linear approximation (solid lines) for unilateral and bilateral fixation yielded a best fit compliance of 143.4 μm/N and 41.3 μm/N with R2 values of 0.72 and 0.92, respectively. The 95% confidence bands and the 95% prediction bands of the linear approximation are designated with long dashes (inner lines) and short dashes (outer lines), respectively.
The least squares compliance for all the specimens with bilateral fixation was 41.3 μm/N with an R2 value of 0.92. The least squares compliance for all the specimens with unilateral fixation was 143.4 μm/N with an R2 value of 0.72.
The total RMS error in knee distraction with bilateral fixation using the animal-specific compliance correction factor, the best fit correction factor, and compared to the LVDT were 43 μm, 179 μm, and 746 μm, respectively (Fig. 6). The maximum knee distraction in the experiments using bilateral fixation averaged 1.11 ± 0.32 mm. Therefore RMS error of the animal-specific compliance correction factor, the best fit correction factor, and compared to the LVDT represents 3.9%, 16.1%, and 67.2% of the average distraction of the knee, respectively.
Fig. 6.
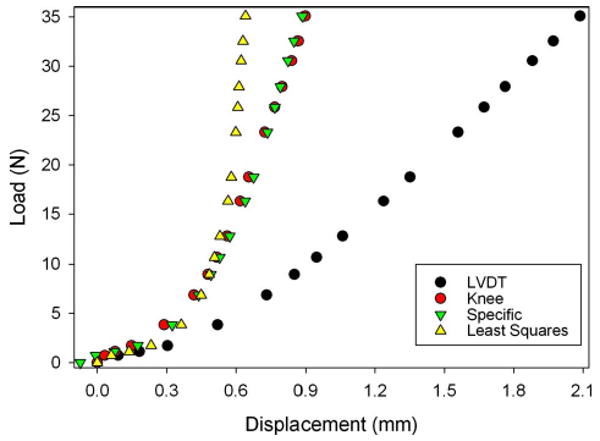
Sample data (specimen 6) comparing the knee displacements obtained from motion analysis (knee) to: (1) displacements measured by the LVDT, (2) predictions of knee displacement obtained using the specific compliance correction factor for an individual animal (specific), and (3) predictions of knee displacement obtained using a least squares fit to all the data
The in vivo pilot study revealed that all animals survived surgical implantation of the external fixation device. The 15 rats in the pilot study were immobilized until post-operative day 4. Prior to the first loading session, three fractures (two femoral, one tibial) were detected with faxitron imaging. Over the test period, two of the remaining 12 animals experienced fracture of the femur. No tibial fractures were detected. One fracture occurred during the first loading session, and the other occurred during the second day of loading. Ten animals survived the duration of ten days of loading and were sacrificed on post-operative day 14. No fractures were detected on post-sacrifice images.
Examination of each animal's load-displacement responses indicate that no catastrophic failure of soft tissue across the joint space occurred during the course of testing (Fig. 7). Major slipping in the mechanical components or loosening at the pin-bone interface was also not evidenced by large drops in load over the course of loading.
Fig. 7.
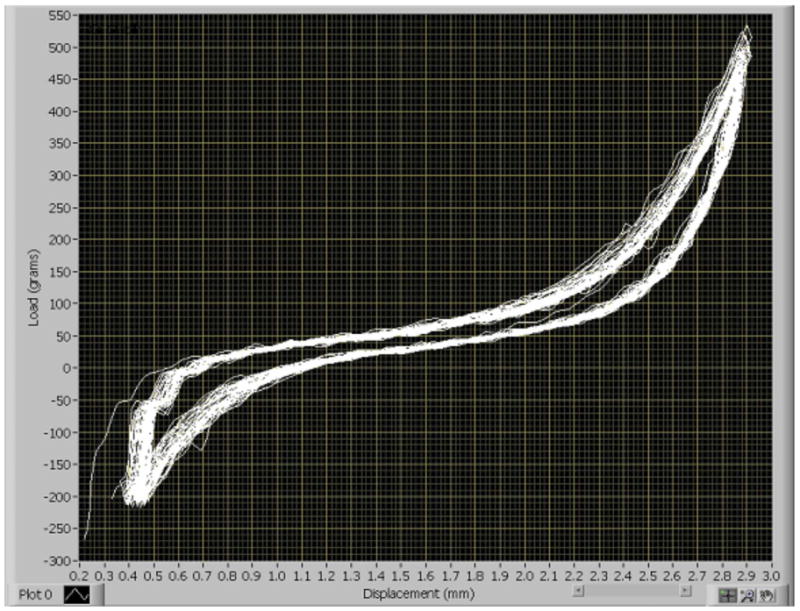
Load displacement curves from 50 cycles of knee joint distraction in the CDM as recorded by the custom program from a single representative animal on day 9 of the pilot study. The curves show no evidence of loosening of the mechanical components or failure of the soft tissue, which would be indicated by sudden drops in load.
6 Discussion
Injuries to ligaments and tendons compromise the stability and function of joints, which can ultimately lead to osteoarthritis. Ligament reconstruction procedures using tendon grafts to repair these injuries often require secure tendon-to-bone healing, yet little is known about the effect of mechanical load on healing of these repairs. To investigate this question we have designed and characterized a device that applies cyclic distraction to a reconstructed ACL in an in vivo animal model. Unlike previous devices, which only allow for load deprivation or static loading of tendons and ligaments, our system allows for the modulation of magnitude, frequency and onset of loading [14–16]. Our long-term goal is to identify the optimal rehabilitation protocol for patients who have undergone knee ligament reconstruction.
The CDM with bilateral fixation exhibited linear deformation characteristics under the loading conditions that it will experience in future experiments. This characteristic enables use of a first order correction factor to account for deformation of the device. Furthermore, the external fixator showed some variability in compliance (COV=21%) across animals. We are not surprised in this variability, however, since numerous intraoperative parameters must be controlled by the surgeon when placing the ligament graft and installing the fixator on the animal. These include location, length and angle of the intracortical pins used to secure the rat to the fixator [17].
We designed the ex-fix to maximize stiffness incorporating use of bilateral pin fixation to decrease fixator compliance relative to unilateral fixation. The results of this study revealed that bilateral fixation decreased compliance by a factor of 3.5, decreased variability in compliance by a factor of 1.5, and improved linearity of our best fit correction term by a factor of 1.3. Furthermore, the average compliance of our bilateral fixator (42.4 μm/N) was about 1.4 times more than a similar external fixator (29.5 μm/N) employing unilateral fixation and 1.1 mm diameter bone pins; however, the variability in the compliance of our device was about 1.8 times less than this design (COV=37%) [18]. Although use of larger diameter pins would further decrease compliance, this proved difficult in our small animal model because of increased risk of fracture. Previous studies employing 1.1 mm pins in a rat femur loading model reported about a 30% complication rate [18].
The least squares linear approximation of fixator compliance decreased RMS error of predicted knee displacements by a factor of 4.2 compared to the LVDT-based measurements (decrease in RMS error from 67.2% to 16.1% of total knee distraction). Although the least squares approximation provided less accurate predictions of knee displacement compared to the animal-specific compliance correction factor (3.9% error), it is impractical to include a specific correction for each individual animal since measuring knee motions with a kinematic system in each animal is too time-consuming. Fortunately, using the least squares linear approximation of fixator compliance still yields acceptable error levels because target knee displacements in subsequent experiments (1–2 mm) will be 6–11 times greater than the error in knee displacement using the least squares correction factor (179 μm). For example, the ratio of maximum knee displacement as measured via motion analysis (1.1 mm) in our evaluation of the bilateral fixator to the error levels in predicted knee displacement using the best fit compliance correction factor (179 μm) is 6.2.
Overall, both the characterization of system compliance and the study of knee displacement accuracy provide us with important guidelines for designing studies based on our primary independent experimental variable, knee distraction. Specifically, these data provide us with basic information to choose discrete levels of knee distraction that are both statistically greater than zero and different from each other. For example, since use of our least squares correction factor yielded 16.1% error in achieving target displacements, we must ensure that target displacements in future test groups differ by at least this much.
The novelty of the device stems from its ability to apply controlled distraction to a healing ACL reconstruction in a convenient and cost-effective rodent model. The device allows the investigator to vary the time of onset of loading (immediately after surgery versus delayed), as well as the duration, frequency and magnitude of knee distraction. There are a wide variety of readily available biologic assays to examine the cellular and molecular response to different loading regimens. Additionally, the investigator can combine these mechanical effects with cytokines believed to modulate tendon-to-bone healing [19,20].
At the time of this writing, we have successfully performed ACL reconstructions and placed the ex-fix on almost 200 animals with an overall fracture rate of approximately 15% and minimal other complications during loading in the CDM [21,22]. Our overall experience with this pilot study and other subject groups shows that rodents tolerate the ex-fix and daily loading under anesthesia well with no discernible adverse effects, and their cage activity is not unduly encumbered by the ex-fix. Further study may be required to evaluate the physiological effects of daily anesthesia on tendon-to-bone healing but is not the subject of this paper.
There are several limitations to our animal model and device. First, there is some slack length in the graft despite fixation under tension at the time of surgery, and there is likely variability in slack length between animals. Although determining the slack length of each individual rat would be ideal, it is impractical since the load contribution of other structures across the joint space masks the point at which the graft begins to take up load. Alternatively, we characterized the average slack length and its variability at time zero in vitro by dissecting down to the bone-tendon-bone construct. For subsequent studies, we can then use this to choose appropriate knee displacement levels that exceed this amount. This would ensure that most of the grafts experience mechanical stimulus at post-operative day zero. Since slack length may change beyond day zero due to both healing between graft and bone, and changes in graft mechanical properties, we focused on ensuring that our device could deliver known amounts of knee distraction. Our ultimate goal is to reproducibly distract the knee to a target displacement.
We also do not know the contribution of other soft tissues to the load-displacement response obtained from the daily loading regimens. To gain a better idea of the force through the graft tendon, we released the anterior joint capsule and all other major ligaments. However, the skin, the neurovascular structures, posterior capsule and several muscle groups (hamstrings, adductors, and gastrocnemius) cannot be transected because their elimination would compromise the viability of the limb. Variation in the load-displacement response may be due to healing or adaptation of these surrounding structures. The unknown and changing contribution of the other soft tissues to the load is the reason we decided to use displacement control instead of load control. These limitations, however, are not relevant to the primary purpose of our loading system, which is to determine the impact of known levels of mechanical stimulus (i.e., knee distraction) on the biology of tendon-bone healing.
In conclusion, we have developed a new procedure and device for applying controlled mechanical stimulus to a healing ACL graft over time in a small animal model. Our evaluation of the device compliance and accuracy of knee distraction yielded important information that will guide us in defining groups with statistically different levels of knee distraction. Our in vivo assessment revealed that the animals tolerated the external fixator and daily anesthesia. Overall, this test system provides an important tool in achieving our ultimate goal of studying the effect of the timing and magnitude of motion on tendon-to-bone healing, and to identify and evaluate the mechanisms by which mechanical stimuli affects tendon-to-bone healing.
Acknowledgments
This work was supported by a grant from the National Institute of Health (Contract No. R01 AR053689-01). The authors gratefully acknowledge Luis Hernandez of the City College of New York, CUNY for his help in prototype manufacturing.
Contributor Information
Mark Stasiak, Email: stasiakm@hss.edu, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021; Department of Biomedical Engineering, City College of New York, New York, NY.
Carl Imhauser, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Jonathan Packer, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Asheesh Bedi, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Robert Brophy, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
David Kovacevic, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Kent Jackson, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Xiang-Hua Deng, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Scott Rodeo, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
Peter Torzilli, Laboratory for Soft Tissue Research, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021; Department of Biomedical Engineering, City College of New York, New York, NY 10031.
References
- 1.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE, Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C, Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and Preventing Noncontact Anterior Cruciate Ligament Injuries: A Review of the Hunt Valley II Meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DC, Posner M, Curl WW, Feagin JA. Isolated Tears of the Anterior Cruciate Ligament: Over 30-Year Follow-Up of Patients Treated With Arthrotomy and Primary Repair. Am J Sports Med. 2008;37(1):65–71. doi: 10.1177/0363546508325660. [DOI] [PubMed] [Google Scholar]
- 3.Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon Healing in a Bone Tunnel Differs at the Tunnel Entrance Versus the Tunnel Exit: An Effect of Graft-Tunnel Motion? Am J Sports Med. 2006;34(11):1790–1800. doi: 10.1177/0363546506290059. [DOI] [PubMed] [Google Scholar]
- 4.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of Biomechanical, Structural, and Compositional Properties Along the Tendon to Bone Insertion Site. J Orthop Res. 2003;21(3):413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 5.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to Bone Healing: Differences in Biomechanical, Structural, and Compositional Properties due to a Range of Activity Levels. ASME J Biomech Eng. 2003;125(1):106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RR, Misol S. Tendon and Ligament Insertion. A Light and Electron Microscopic Study. J Bone Jt Surg Am. 1970;52(1):1–20. [PubMed] [Google Scholar]
- 7.Moira O'Brien M. Tendon Injuries: Basic Science and Clinical Medicine. Springer-Verlag; London: 2005. pp. 3–21. Chap. 1. [Google Scholar]
- 8.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased Muscle Loading Delays Maturation of the Tendon Enthesis During Postnatal Development. J Orthop Res. 2007;25(9):1154–1163. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 9.Fujioka H, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Changes in the Expression of Type-X Collagen in the Fibrocartilage of Rat Achilles Tendon Attachment During Development. J Orthop Res. 1997;15(5):675–681. doi: 10.1002/jor.1100150508. [DOI] [PubMed] [Google Scholar]
- 10.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-Healing in a Bone Tunnel. A Biomechanical and Histological Study in the Dog. J Bone Jt Surg, Am. 1993;75(12):1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Renstrom P. Tendon Injuries: Basic Science and Clinical Medicine. Springer Verlag; London: 2005. pp. 70–85. Chap. 9. [Google Scholar]
- 12.Dagher E, Hays PL, Kawamura S, Godin J, Deng XH, Rodeo SA. Immobilization Modulates Macrophage Accumulation in Tendon-Bone Healing. Clin Orthop Relat Res. 2009;467(1):281–287. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, Rodeo SA. The Role of Macrophages in Early Healing of a Tendon Graft in a Bone Tunnel. J Bone Jt Surg, Am. 2008;90(3):565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K, Kaneda K. Effects of Stress Shielding on the Mechanical Properties of Rabbit Patellar Tendon. ASME J Biomech Eng. 1993;115(1):23–28. doi: 10.1115/1.2895466. [DOI] [PubMed] [Google Scholar]
- 15.King GJ, Damson EL, Frank CB, Shrive NG. A New Device and Method for Controlling the Load in Rabbit Medial Collateral Ligament Reconstructions. ASME J Biomech Eng. 1995;117(1):41–47. doi: 10.1115/1.2792268. [DOI] [PubMed] [Google Scholar]
- 16.Rumian AP, Draper ER, Wallace AL, Goodship AE. The Influence of the Mechanical Environment on Remodelling of the Patellar Tendon. J Bone Jt Surg, Br. 2009;91-B(4):557–564. doi: 10.1302/0301-620X.91B4.21580. [DOI] [PubMed] [Google Scholar]
- 17.Willie B, Adkins K, Zheng X, Simon U, Claes L. Mechanical Characterization of External Fixator Stiffness for a Rat Femoral Fracture Model. J Orthop Res. 2009;27(5):687–693. doi: 10.1002/jor.20792. [DOI] [PubMed] [Google Scholar]
- 18.Palomares KT, Gleason RE, Mason ZD, Cullinane DM, Einhorn TA, Gerstenfeld LC, Morgan EF. Mechanical Stimulation Alters Tissue Differentiation and Molecular Expression During Bone Healing. J Orthop Res. 2009;27(9):1123–32. doi: 10.1002/jor.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of Recombinant Human Bone Morphogenetic Protein-2 to Enhance Tendon Healing in a Bone Tunnel. Am J Sports Med. 1999;27(4):476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 20.Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA. Bone Morphogenetic Proteins-Signaling Plays a Role in Tendon-to-Bone Healing: A Study of rhBMP-2 and Noggin. Am J Sports Med. 2007;35(4):597–604. doi: 10.1177/0363546506296312. [DOI] [PubMed] [Google Scholar]
- 21.Brophy R, Kovacevic D, Imhauser C, Stasiak M, Jackson K, Deng XH, Rodeo S. Effect of Applied Strain Versus Immobilization on Tendon-to-Bone Healing in a Rat Model of ACL Reconstruction. Transactions of the 54th Annual Meeting, Orthopaedic Research Society; San Francisco, CA. 2008. Paper No. 258. [Google Scholar]
- 22.Bedi A, Kovacevic D, Fox A, Imhauser C, Brophy R, Stasiak M, Deng XH, Rodeo S. The Effect of Early and Delayed Mechanical Loading on Tendon-to-Bone Healing After ACL Reconstruction. Transactions of the 55th annual meeting, Orthopaedic Research Society; Las Vegas, NV. 2009. Paper No. 235. [Google Scholar]


