Abstract
This is the second of a 2-part review of spinal cord injury. The focus herein is to highlight recent findings regarding prognostic indicators used for spinal cord injury (SCI) in dogs, promote an awareness of the current recommendations of standard of care for traumatic spinal cord injury in veterinary medicine, and highlight the findings of clinical trials of therapies for spinal cord injury in dogs. This 2-part review provides information that will assist general and specialty veterinary practitioners in evidence-based veterinary medical practice in an area that has become particularly specialized.
Résumé
Lésions de la moelle épinière II : Indicateurs de pronostic, normes de soins et essais cliniques. Le présent document représente la deuxième partie d’une revue en 2 parties sur les lésions de la moelle épinière (LME). L’objet du présent article consiste à souligner les constatations récentes concernant les indicateurs de pronostic utilisés pour les LME chez les chiens, à promouvoir la sensibilisation à l’égard des recommandations actuelles pour les normes de soins en médecine vétérinaire relativement aux traumatismes de la moelle épinière et à mettre en lumière les constatations d’essais cliniques réalisés pour traiter les blessures de LME chez les chiens. Cette revue en 2 parties fournit des renseignements qui aideront les omnipraticiens et les spécialistes vétérinaires dans la pratique factuelle de la médecine vétérinaire pour un domaine qui est devenu particulièrement spécialisé.
(Traduit par Isabelle Vallières)
Introduction
Traumatic spinal cord injury (SCI) is a disease with devastating effects in dogs, including paresis or paralysis and/or urinary and fecal incontinence. The most common causes of spinal cord injury in dogs include trauma induced by prolapsed intervertebral discs and exogenous sources of trauma such as motor vehicle accidents (1–3). The pathophysiological consequences of SCI are brought about by primary and secondary injury mechanisms. Primary SCI results from the direct mechanical injury to the spinal cord. For example, extruded intervertebral disc material can cause both contusion and compression causing mechanical injury to the vasculature and neural and supporting tissue of the spinal cord. Primary injury may also take the form of laceration, shearing, and traction. Secondary SCI causes physical expansion of the primary injury and results from a variety of biochemical and vascular events. Regardless of the mechanism of injury, spinal cord tissue [neurons and supporting cells (glia)] is destroyed and connections between brain-spinal cord and spinal cord-spinal cord neurons are lost.
The way in which the central nervous system heals is unique and the local environment within the injured spinal cord is inhibitory to neuronal regeneration. Basic, translational, and clinical research aim to treat SCI using a variety of strategies.
Current therapies for SCI, experimentally and/or clinically, include: 1) preventing secondary injury, 2) promoting regeneration and/or sprouting of remaining axons, 3) enhancing the purposeful function of remaining neural circuitry, 4) replacing destroyed spinal cord tissue, and 5) a combination of these approaches.
Prognostic indicators for traumatic spinal cord injury
One of the common questions owners of SCI dogs have is “Will my dog be able to walk again?” or “What is the likelihood that my dog will not have any more back pain?” Several attempts to describe surrogate markers or prognostic indicators of recovery following SCI have been made. Herein, we review the clinical signs and advanced imaging that have been studied as prognostic indicators in animals with naturally occurring SCI.
Clinical signs
Temporal aspects of onset and duration of clinical signs have been investigated as prognostic indicators, with variable results, for dogs with SCI resulting from intervertebral disc herniation. The rapidity of development of clinical signs does not seem to affect outcome for dogs with intact pain perception (4). One study, examining the rates of recovery for dogs without perception of pain caudal to the level of the SCI, and undergoing surgical decompression, found that animals that were unable to locomote within 1 h from the onset of clinical signs were less likely to satisfactorily recover (regain pain perception, locomotor ability and voluntary micturition) compared with animals that took more than 1 h to lose the ability to locomote (5). This is likely because of the more peracute severity of the concussive force by which the spinal cord was impacted by the intervertebral disc material. In another study, however, the rapidity of developing clinical signs could not be used to predict whether or not an animal would regain locomotor ability (6). In both studies, duration of paraplegia prior to a dog receiving decompressive surgery was not important in predicting whether or not the animal would be able to recover locomotor ability.
Traditionally, loss of pain perception caudal to the level of the SCI has been used as a poor prognostic indicator for recovery from SCI. A relatively recent retrospective study of dogs with severe SCI identified that pain perception as a prognostic indicator depends on 1) the type of traumatic event causing the SCI, and 2) the rapidity with which pain perception returns (6). In particular, 2 of 9 paraplegic dogs without pain perception and having SCI resulting from exogenous trauma and vertebral fractures and/or spinal luxations, treated medically or surgically, were eventually able to locomote. None of these 9 dogs regained pain perception and the 2 that regained the ability to locomote had periodic urinary and fecal incontinence (6). In the same study, dogs with severe SCI, resulting from intervertebral disc herniation, and loss of pain perception and having undergone decompressive surgery were investigated. The authors found that 68% of paraplegic dogs without pain perception caudal to the level of the SCI, and having undergone decompressive surgery, regained the ability to walk. Interestingly, all dogs that regained pain perception were eventually able to locomote. Seventy-eight percent of the dogs regaining pain perception did so within 2 wk after decompressive surgery, and 97% had regained pain perception by 1 mo after decompressive surgery. Interestingly, 7 of 18 dogs that did not regain pain perception were able to locomote (range of time to walk 16–72 wk after decompressive surgery), though they never regained fecal or urinary continence. Consequently, waiting for 1 mo in animals lacking pain perception caudal to the level of the SCI, will aid in prognosticating whether or not the animal will regain the ability to locomote. Importantly, approximately 40% of animals that regained pain perception caudal to the level of the SCI, and the ability to locomote had mild intermittent urinary and fecal incontinence. Another interesting finding from this study was that for paraplegic dogs lacking pain perception, and resulting from intervertebral disc herniation, recovery of locomotor ability occured more quickly for younger and small-breed dogs. This is interesting given that recent studies have shown that elderly rats have greater spinal cord demyelination and impaired re-myelinating abilities following SCI (7,8). Importantly, however, a dog’s physical stature does not appear to matter for prognostication for dogs with thoracolumbar SCI resulting from intervertebral disc herniation (type I disc disease) and intact pain perception (9–11).
Advanced imaging
The rationale behind using advanced imaging as a prognostic tool for SCI stems from pathophysiological processes that ensue after injury to the spinal cord. As mentioned in part I of this review, contusion, concussion, and compression of the spinal cord and vascular tissue cause inflammation, hemorrhage, ischemia, edema, and direct mechanical damage to the spinal cord. These pathological changes can be visualized with advanced imaging techniques, especially magnetic resonance (MR) imaging.
Several retrospective studies using advanced imaging findings as prognostic indicators for recovery have recently been published. From these studies, it appears that the degree of spinal cord compression, based upon MR imaging, is not predictive of recovery (12,13). However, it appears that dogs with an area of hyperintensity of the spinal cord (on T2-weighted images) at least as long as the L2 vertebral body, preoperatively, have a poor prognosis for recovery after thoracolumber disc herniation (14). It should be noted, however, that successful recovery was defined as regaining the ability to locomote unaided and with or without mild neurologic deficits, including absent or mild urinary and fecal incontinence (12,14).
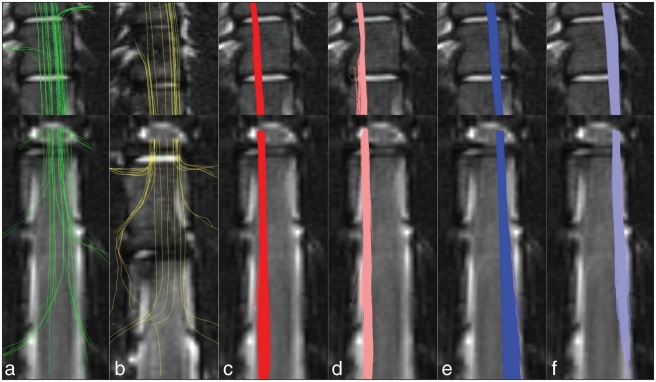
At a very intuitive level, animals having undergone complete or nearly complete transection of their spinal cords, albeit rare, have a poor prognosis for satisfactory recovery. This is evident from the findings of Olby et al (6), where only 2 of 9 dogs with severe traumatic SCIs regained the ability to walk in the absence of regaining pain perception. Although it appears that only 10% of descending axons are required for successful locomotion (15), we know that not all descending spinal tracts contribute equally to locomotion and that there is redundancy in some of these pathways (16–20). Consequently, it is likely that the answer to the question “what is spared?” is highly relevant to predicting recovery after SCI. At first, identifying what particular pathways are spared may seem an impossible task; however, recent developments in diffusion MR imaging make these tasks possible. Diffusion MR imaging methods are based upon measuring the diffusion of water within the microenvironment of living tissue. A particular diffusion MR imaging method, known as diffusion tensor imaging, measures the restricted diffusion of water along, for example, neural tracts. When diffusion tensor imaging is used to specifically image white matter tracts, this is termed tractography. Given that tractography (Figure 1) is possible, even after spinal cord injury, in the future we will undoubtedly be able to better identify what particular white matter tracts are affected and therefore prognosticate the likelihood of recovery (21).
Figure 1.
Selective tractography showing dorsal column (a), ventral column (b) and lateral tracts (c–f). Orientation is coronal, top-left is rostral-left. [Reprinted from Cohen-Adad, Benali, and Rossignol, 2008 (21), with permission from Elsevier].
Standards of care for spinal cord-injured dogs
The phrase “standards of care” for diseases refers to the generally accepted method of diagnosing and/or treating and/or managing a particular disease. This section focuses on the controversy of corticosteroid use for SCI, the importance of surgical treatment, the importance of an appropriate urinary bladder management program for dogs with SCI, and reviews current viewpoints regarding standards of care for SCI animals.
To give or not to give corticosteroids?
At the time of completion of a large multicenter human trial investigating high dose methylprednisolone therapy for SCI humans [the 2nd National Acute Spinal Cord Injury Study (NASCIS)] the medical treatment standard of care for humans with SCI occurring within 8 h of injury was to administer 30 mg/kg intravenous bolus of methylprednisolone sodium succinate (methylprednisolone) followed by an additional 23 h of 5.4 mg/kg constant rate infusion of methylprednisolone (22). Soon after the publication of the 2nd NASCIS trial, the veterinary community adopted high dose methylprednisolone as a standard of medical therapy for acute SCI dogs and cats.
A 3rd NASCIS trial, conducted in the late 1990’s, compared high-dose methylprednisolone therapy with the 21-aminosteroid, tyrilizad mesylate (tyrilizad), and found no beneficial effect of tyrilizad over methylprednisolone, but reported a beneficial effect of methylprednisolone if given within 3 h following SCI compared to the standard 8-hour window (23). Relatively recently, scrutiny over statistical analyses and interpretation of the data obtained from the NASCIS trials have been published (24,25). When physicians and human neurosurgeons have been asked why they prescribe methylprednisolone for acute SCI, it has been made clear that many do so for fear of litigation and not because they believe it has a beneficial effect on patient outcome (26). Furthermore, 76% of physicians who deal with SCI patients in Canada now do not prescribe methylprednisolone, a finding that is reversed from a survey conducted 5 y previously (27). Methylprednisolone is not without untoward side-effects. These deleterious effects include melena, diarrhea, vomiting, hematemesis, hematochezia, anorexia, urinary tract infection (UTI), pneumonia, and sepsis (22,23,28,29).
A recent study examining recovery in non-ambulatory small-breed dogs that had intact pain perception caudal to the level of the SCI, and were treated by hemilaminectomy and without methylprednisolone for acute intervertbral disc herniation, demonstrated that 100% of these animals were ambulatory by 6 wk following surgical intervention (29). This study demonstrates that even without methylprednisolone, animals will recover following decompressive surgery. This study did not, however, evaluate any potential beneficial effect in large breed dogs and in dogs having no pain perception caudal to the level of the SCI, at presentation.
As a consequence of the questionable benefits of high-dose methylprednisolone therapy in humans in the face of untoward side-effects, high dose methylprednisolone therapy has fallen out of favor as a standard of medical care for human (27) and veterinary patients with acute SCI. Choosing to not use high-dose methylprednisolone for dogs with acute SCI may, however, be premature and is not based upon sound scientific evaluation, given that methylprednisolone has not been rigorously evaluated following naturally occurring SCI in dogs. Consequently, a blinded multi-center trial is being conducted that will, in part, evaluate methylprednisolone in dogs with acute SCI resulting from type I intervertebral disc herniation (30).
Based on the same rationale for using high dose methylprednisolone for acute traumatic SCI, veterinarians have also used dexamethasone for traumatic SCI (31). Evidence regarding the positive benefits of using dexamethasone for SCI is lacking, however. A recent, relatively large, retrospective study examining the adverse effects of dexamethasone for acute thoracolumbar intervertebral disc herniation revealed that animals that were administered dexamethasone were more likely to have complications (such as, vomiting, diarrhea, urinary tract and wound infections, pneumonia, anemia) compared to untreated animals (31). In fact, dexamethsone-treated dogs were 3.4 times more likely to develop untoward side-effects compared to animals not treated with dexamethasone (31). Given these results, and the fact that there is no clear evidence that dexamethasone is helpful in promoting recovery following SCI, one must weigh the potential risks to the patient against the likely benefits of administering dexamethasone. Clients should also be made aware of the potential side-effects of dexamethasone therapy prior to their providing informed consent.
Is surgery helpful for IVDD?
With respect to non-medical care of SCI patients (surgical options), the evidence is clear that decompressive surgery is beneficial at improving the likelihood of successful sensorimotor recovery following acute prolapse of the intervertebral disc. Although medical management alone may result in significant improvement in neurological signs and without recurrence in approximately 54% of non-ambulatory/positive pain perceiving dogs with intervertebral disc disease (IVDD) (32), up to 100% of similar presenting dogs will have successful recovery following surgical decompression alone. For paraplegic dogs lacking pain perception resulting from acute intervertebral disc herniation and having undergone surgical decompression, it is expected that 78% of these animals will regain pain perception within 2 wk of injury and all of these animals will regain the ability to locomote (6). Furthermore, 7 of 18 dogs with prolapsed intervertebral disc herniation, paraplegia, and loss of pain perception that did not recover pain perception regained locomotor ability, but did not regain fecal or urinary continence. In comparison, only 0% to 7% of dogs lacking pain perception caudal to the level of the SCI, that are treated medically will have a successful outcome (be normal or substantially improved in their sensorimotor abilities), although studies evaluating outcome of medically treated dogs lacking pain perception, caudal to the level of the SCI, are relatively sparse (33).
Are bladder management programs important?
With regard to other aspects of standards of care for complications associated with dogs with SCI, there have been 2 recent studies examining urinary tract infections in dogs being treated for SCI due to IVDD (34,35). Results from these studies reveal a number of factors that should be considered in the urinary management of SCI dogs. One study demonstrated that female dogs, dogs that could not ambulate or voluntarily void urine, dogs that had not been administered antimicrobials during surgery, and dogs whose body temperature fell below 35°C during surgical decompression of the spinal cord all have an increased likelihood of developing a UTI (35). The other study revealed that the frequency of UTIs in dogs with IVDD and having an indwelling urinary catheter (42%) was not different from dogs with indwelling urinary catheters for other reasons (55%) (34). This study did, however, reveal that the likelihood of urinary tract infection increased [odds ratio (OR) = 1.27; 95% confidence interval (CI) = 1.05–1.55] for each day that the urinary catheter was left in place, and the odds of developing a urinary tract infection were higher if antimicrobials were administered during the time that the indwelling urinary catheter was left in place [OR = 4.54; 95% CI = 1.83–11.27] (34). Results from these studies indicate that indiscriminate antimicrobial use for animals having an indwelling catheter should be avoided, though intraoperative antimicrobials should be administered at the time of surgical decompression of the spinal cord. Furthermore, the risk factors for developing a UTI should be avoided where possible, and duration of having an indwelling urinary catheter should be minimized.
What are the current views regarding standards of care for SCI dogs and cats?
The standards of care for SCI currently recommended by some veterinary surgeons and neurologists (36,37) include: 1) appropriate treatment of concomitant disease or injuries; 2) maintaining adequate neural tissue oxygenation and perfusion; 3) ameliorating any further mechanical damage to the spinal cord via fracture stabilization and/or decompressive surgery; 4) rational exercise restriction and physical therapy program; and 5) appropriate bladder management program. Rigorous prospective, blinded clinical trials, evaluating various uni- and multi-modal approaches in dogs with naturally occurring SCI are essential and will undoubtedly alter current diagnosis, treatment, and management practices for companion animals affected by acute SCI.
Aims of recent and current clinical trials
Several clinical trials are aimed at promoting conduction of electrical signals, replacing lost tissue, promoting neural regeneration, and rescuing tissue from secondary injury. Since the early 1990s, a variety of therapies have been investigated in dogs with naturally occurring SCI resulting from acute intervertebral disc herniation. Several of these therapies are being examined in human clinical trials.
Pathological consequences of SCI are demyelination of the remaining axons, alterations in local ionic concentrations, and mechanical disruption of axonal membranes, all of which result in conduction block of neural signals traveling within the spinal cord. Since 1991, there have been 4 canine clinical trials aimed at improving behavioral recovery by different means of improving the conduction or repairing the axonal membranes so as to improve electrical conduction within the spinal cord. The 4 strategies used include blockade of potassium channels with 4-aminopyridine, application of oscillating electrical fields, use of intravenous surfactants, and the transplantation of olfactory ensheathing glia. In addition, a recent clinical trial attempting to rescue damaged spinal cord tissue by combating secondary injury mechanisms has been reported (38).
The most thoroughly investigated therapy in chronic severe spinal cord-injured dogs is 4-aminopyridine (4-AP) (39). The rationale for using this drug is that by blocking voltage-gated potassium channels, that normally would have been “blocked” by the myelin sheath, an improvement in action potential conduction within the spinal cord would occur and sensorimotor behavior would be improved. In this phase-I trial, it was found that administration of short-acting 4-AP to dogs affected by IVDD and that were completely paralyzed, caused improvement in hind limb placing, perception of pain, and partial recovery of the cutaneous trunci reflex. These improvements were noticed in only some of the dogs and for only a few hours after administering the drug. Administration of 4-AP was associated with elevation in body temperature, increased anxiety, and/or seizures that were controlled with diazepam. Nevertheless, subsequent clinical trials in humans were conducted and recently, the results of a sustained release 4-AP correlate (Fampridine) have demonstrated a possible benefit in improving spasticity (40). Recently, pharmacokinetics and safety of 4-AP derivatives, with presumed increased safety and untoward side-effects compared with 4-AP, were examined in dogs (41). This study showed improved pharmacokinetic and safety profiles of 4-AP derivatives compared with 4-AP; therefore, 4-AP derivatives may prove clinically useful in dogs with SCI.
The application of oscillating electrical fields is another mode of therapy that has been examined in completely paralyzed dogs that lack pain perception due to intervertebral disc extrusion or exogenous trauma (42). The premises behind this trial were the experimental evidence that electrical fields applied to neurons caused the neurons to sprout and regenerate and that this regeneration was associated with recovery of the cutaneous trunci reflex in SCI guinea pigs (43,44). Results from this trial revealed that dogs treated with the electrical stimulator had an overall “better” combined recovery score at 6 mo post-surgery compared with control animals (42). Much of this recovery was in the form of improved sensory ability. Nevertheless, this method of improving sensorimotor recovery has gone on to be evaluated for use in humans (45). So far, the electrical device appears safe for use in humans (45).
Another SCI therapy, having been preliminarily examined in dogs with naturally occurring SCI (IVDD only), is the use of intravenously administered surfactants (46). The premise behind this work is that certain surfactants can fuse severed axons or repair damaged axons, thereby potentially improving sensorimotor recovery after SCI. Results of this study demonstrated that the administration of polyethylene glycol or P-188 is safe and without untoward side-effects.(46) This study lacked a concurrently run control group and instead compared the effects of the polymers against previously published historical controls. Furthermore, all dogs in this study had been administered methylprednisolone. Nevertheless, statistical comparisons revealed that both P-188 and polyethylene glycol-treated animals had improved recovery compared to the historical control animals. Improvement in sensory ability, proprioception, and locomotion accounted for the significant difference in total neurological score between polymer-treated and historical control groups. Given that the main purpose of this study was to demonstrate safety and to be a pilot study in nature, there is a need to conduct a blinded controlled study in which the polymer is administered alone and not in conjunction with methylprednisolone. Such a study is presently underway at North Carolina State University (30).
Transplantation of olfactory ensheathing glia is another approach currently being investigated for use in dogs with SCI (47). The rationale for this approach stems from the unique ability of these cells to promote regeneration in experimentally induced injured spinal cord and foster a more hospitable environment for axonal growth, increase tissue sparing after SCI, increase tissue bridging and reduce cavity formation following experimental SCI, and improve angiogenesis within the injured spinal cord [for review see (48)]. Olfactory ensheathing glia can be harvested from the olfactory epithelium and transplanted in the same animal thereby minimizing the likelihood of rejection (47,49). Thus far, transplantation of olfactory ensheathing glia into dogs spinal cords appears to be possible and is safe (47). Trials of transplantation of olfactory ensheathing glia for human SCI are also in progress, though many aspects of 1 of these trials have been criticized (50) and formal results are pending from others (51).
One final recent clinical trial for canine SCI has investigated the use of the antioxidant, N-acetylcysteine, a precursor to glutathione and a key antioxidant in mammalian systems. Consequently, it is thought that N-acetylcysteine can minimize secondary injury that occurs after SCI by scavenging free radicals itself or by maintaining therapeutic levels of glutathione, hence this premise forms the basis for investigating N-acetylcysteine clinically in SCI dogs. The results of the study, however, did not reveal a beneficial effect of preoperatively administered N-acetylcysteine on clinical recovery in dogs having undergone decompressive surgery for intervertebral disc herniation. In part, this negative result may have occurred because the dogs used were not completely paralyzed and without pain perception caudal to the level of the SCI, and because the method of evaluating sensorimotor recovery may not have been sensitive enough to detect differences between N-acetylcysteine-treated and saline-treated groups. It is well known that dogs with intact pain sensation caudal to the level of the SCI will go on to recover substantial sensorimotor behavior without any intervention other than surgical decompression, thus therapeutic benefit may go unnoticed if a sensitive measure of behavioral recovery is not employed (52–54). Furthermore, it is well known that animals that have appropriate uninjured descending spinal pathways remaining following SCI will undergo a variety of neuroplastic changes to bring about near-normal sensorimotor capabilities (16,55,56). Consequently, animals with intact pain perception caudal to the level of the SCI (neurologically incomplete injury) may not benefit as much from medical therapeutic intervention as those lacking pain perception caudal to the level of the SCI. Another possible reason for this study to have not found an effect of N-acetylcysteine in SCI dogs is that only 1 dosage was investigated, and this dosage failed to reduce a urinary marker of oxidative stress. Although, at first glance, it appears as though N-acetylcysteine is not beneficial for SCI in dogs, it is important that further studies investigating its use in animals lacking pain perception caudal to the level of the SCI and using sensitive and objective measures be performed, and that the pharmacokinetics of N-acetylcysteine in SCI dogs be performed so as to determine a rational therapeutic dosing regime.
In conclusion, it is generally agreed amongst those in the spinal cord injury research community that there will not be 1 solely effective therapy (a magic bullet) for SCI. Instead, effective therapy for SCI will likely involve a tailor-made multimodal approach that takes into consideration the signalment and history of the patient, as well as the etiology of the SCI. In particular, as was discussed in part I of this review, there is a need for developing robust clinical trials investigating physical therapy (especially treadmill assisted therapy) solely and in combination with other therapies for dogs and cats with SCI; afterall, it is well known that cats with experimental SCI benefit substantially from treadmill training (57). CVJ
Footnotes
Dr. Webb’s research is supported by a Discovery Grant through the Natural Sciences and Engineering Research Council of Canada (NSERC); S. Ngan is supported, in part, by a Queen Elizabeth II Graduate Scholarship through the Government of Alberta.
Reprints will not be available from the authors.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bruce CW, Brisson BA, Gyselinck K. Spinal fracture and luxation in dogs and cats: A retrospective evaluation of 95 cases. Vet Comp Orthop Traumatol. 2008;21:280–284. [PubMed] [Google Scholar]
- 2.Coates JR. Intervertebral disk disease. Vet Clin North Am Small Anim Pract. 2000;30:77–110. doi: 10.1016/s0195-5616(00)50004-7. [DOI] [PubMed] [Google Scholar]
- 3.McKee WM. Spinal trauma in dogs and cats: A review of 51 cases. Vet Rec. 1990;126:285–289. [PubMed] [Google Scholar]
- 4.Scott HW. Hemilaminectomy for the treatment of thoracolumbar disc disease in the dog: A follow-up study of 40 cases. J Small Anim Pract. 1997;38:488–494. doi: 10.1111/j.1748-5827.1997.tb03303.x. [DOI] [PubMed] [Google Scholar]
- 5.Scott HW, McKee WM. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J Small Anim Pract. 1999;40:417–422. doi: 10.1111/j.1748-5827.1999.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 6.Olby N, Levine J, Harris T, et al. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001) J Am Vet Med Assoc. 2003;222:762–769. doi: 10.2460/javma.2003.222.762. [DOI] [PubMed] [Google Scholar]
- 7.Siegenthaler MM, Berchtold NC, Cotman CW, Keirstead HS. Voluntary running attenuates age-related deficits following SCI. Exp Neurol. 2008;210:207–216. doi: 10.1016/j.expneurol.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cudia SP, Duval JM. Thoracolumbar intervertebral disk disease in large, nonchondrodystrophic dogs: A retrospective study. J Am Anim Hosp Assoc. 1997;33:456–460. doi: 10.5326/15473317-33-5-456. [DOI] [PubMed] [Google Scholar]
- 10.Macias C, McKee WM, May C, Innes JF. Thoracolumbar disc disease in large dogs: A study of 99 cases. J Small Anim Pract. 2002;43:439–446. doi: 10.1111/j.1748-5827.2002.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 11.Yovich JC, Read R, Eger C. Modified lateral spinal decompression in 61 dogs with thoracolumbar disc protrusion. J Small Anim Pract. 1994;35:351–356. [Google Scholar]
- 12.Penning V, Platt SR, Dennis R, Cappello R, Adams V. Association of spinal cord compression seen on magnetic resonance imaging with clinical outcome in 67 dogs with thoracolumbar intervertebral disc extrusion. J Small Anim Pract. 2006;47:644–650. doi: 10.1111/j.1748-5827.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Ryan TM, Platt SR, Llabres-Diaz FJ, McConnell JF, Adams VJ. Detection of spinal cord compression in dogs with cervical intervertebral disc disease by magnetic resonance imaging. Vet Rec. 2008;163:11–15. doi: 10.1136/vr.163.1.11. [DOI] [PubMed] [Google Scholar]
- 14.Ito D, Matsunaga S, Jeffery ND, et al. Prognostic value of magnetic resonance imaging in dogs with paraplegia caused by thoracolumbar intervertebral disk extrusion: 77 cases (2000–2003) J Am Vet Med Assoc. 2005;227:1454–1460. doi: 10.2460/javma.2005.227.1454. [DOI] [PubMed] [Google Scholar]
- 15.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 16.Webb AA, Muir GD. Sensorimotor behaviour following incomplete cervical spinal cord injury in the rat. Behav Brain Res. 2005;165:147–159. doi: 10.1016/j.bbr.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Webb AA, Muir GD. Course of motor recovery following ventrolateral spinal cord injury in the rat. Behav Brain Res. 2004;155:55–65. doi: 10.1016/j.bbr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Webb AA, Muir GD. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur J Neurosci. 2003;18:412–422. doi: 10.1046/j.1460-9568.2003.02768.x. [DOI] [PubMed] [Google Scholar]
- 19.Webb AA, Muir GD. Compensatory locomotor adjustments of rats with cervical or thoracic spinal cord hemisections. J Neurotrauma. 2002;19:239–256. doi: 10.1089/08977150252806983. [DOI] [PubMed] [Google Scholar]
- 20.Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Adad J, Benali H, Hoge RD, Rossignol S. In vivo DTI of the healthy and injured cat spinal cord at high spatial and angular resolution. Neuroimage. 2008;40:685–697. doi: 10.1016/j.neuroimage.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 23.Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 24.Hurlbert RJ. The role of steroids in acute spinal cord injury: An evidence-based analysis. Spine. 2001;26:S39–S46. doi: 10.1097/00007632-200112151-00009. [DOI] [PubMed] [Google Scholar]
- 25.Hurlbert RJ. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 26.Hurlbert RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci. 2002;29:236–239. doi: 10.1017/s0317167100002006. [DOI] [PubMed] [Google Scholar]
- 27.Hurlbert RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41–45. doi: 10.1017/s031716710000754x. [DOI] [PubMed] [Google Scholar]
- 28.Culbert LA, Marino DJ, Baule RM, Knox VW., III Complications associated with high-dose prednisolone sodium succinate therapy in dogs with neurological injury. J Am Anim Hosp Assoc. 1998;34:129–134. doi: 10.5326/15473317-34-2-129. [DOI] [PubMed] [Google Scholar]
- 29.Bush WW, Tiches DM, Kamprad C, Murtaugh RJ, Barr CS. Functional outcome following hemilaminectomy without methylprednisolone sodium succinate for acute thoracolumbar disk disease in 51 non- ambulatory dogs. J Vet Emerg Crit Care. 2007;17:72–76. [Google Scholar]
- 30.Olby NJ. Methylprednisolone sodium succinate and polyethylene glycol in canine spinal cord injury. Morris Animal Foundation Sponsorship Booklet. 2008 [Google Scholar]
- 31.Levine JM, Levine GJ, Boozer L, et al. Adverse effects and outcome associated with dexamethasone administration in dogs with acute thoracolumbar intervertebral disk herniation: 161 cases (2000–2006) J Am Vet Med Assoc. 2008;232:411–417. doi: 10.2460/javma.232.3.411. [DOI] [PubMed] [Google Scholar]
- 32.Levine JM, Levine GJ, Johnson SI, et al. Evaluation of the success of medical management for presumptive thoracolumbar intervertebral disk herniation in dogs. Vet Surg. 2007;36:482–491. doi: 10.1111/j.1532-950X.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 33.Davies JV, Sharp NJH. A comparison of conservative treatment and fenestration for thoracolumbar intervertebral-disk disease in the dog. J Small Anim Pract. 1983;24:721–729. [Google Scholar]
- 34.Bubenik LJ, Hosgood GL, Waldron DR, Snow LA. Frequency of urinary tract infection in catheterized dogs and comparison of bacterial culture and susceptibility testing results for catheterized and non-catheterized dogs with urinary tract infections. J Am Vet Med Assoc. 2007;231:893–899. doi: 10.2460/javma.231.6.893. [DOI] [PubMed] [Google Scholar]
- 35.Stiffler KS, Stevenson MA, Sanchez S, et al. Prevalence and characterization of urinary tract infections in dogs with surgically treated type 1 thoracolumbar intervertebral disc extrusion. Vet Surg. 2006;35:330–336. doi: 10.1111/j.1532-950X.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 36.Vitale CL, Coates JR. Acute spinal cord injury. Standards of Care: Emergency and critical care medicine. 2007;9:1–11. [Google Scholar]
- 37.Griffin JF, Levine JM, Kerwin SC. Thoracolumbar intervertebral disc disease in dogs. Standards of Care: Emergency and critical care medicine. 2007;9:1–8. [Google Scholar]
- 38.Baltzer WI, McMichael MA, Hosgood GL, et al. Randomized, blinded, placebo-controlled clinical trial of N-acetylcysteine in dogs with spinal cord trauma from acute intervertebral disc disease. Spine. 2008;33:1397–1402. doi: 10.1097/BRS.0b013e3181753c37. [DOI] [PubMed] [Google Scholar]
- 39.Blight AR, Toombs JP, Bauer MS, Widmer WR. The effects of 4-aminopyridine on neurological deficits in chronic cases of traumatic spinal cord injury in dogs: A phase I clinical trial. J Neurotrauma. 1991;8:103–119. doi: 10.1089/neu.1991.8.103. [DOI] [PubMed] [Google Scholar]
- 40.Cardenas DD, Ditunno J, Graziani V, et al. Phase 2 trial of sustained- release fampridine in chronic spinal cord injury. Spinal Cord. 2007;45:158–168. doi: 10.1038/sj.sc.3101947. [DOI] [PubMed] [Google Scholar]
- 41.Olby NJ, Smith DT, Humphrey J, et al. Pharmacokinetics of 4-aminopyridine derivatives in dogs. J Vet Pharmacol Ther. 2009;32:485–491. doi: 10.1111/j.1365-2885.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 42.Borgens RB, Toombs JP, Breur G, et al. An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J Neurotrauma. 1999;16:639–657. doi: 10.1089/neu.1999.16.639. [DOI] [PubMed] [Google Scholar]
- 43.Borgens RB, Blight AR, McGinnis ME. Functional recovery after spinal cord hemisection in guinea pigs: The effects of applied electric fields. J Comp Neurol. 1990;296:634–653. doi: 10.1002/cne.902960409. [DOI] [PubMed] [Google Scholar]
- 44.Borgens RB, Blight AR, McGinnis ME. Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science. 1987;238:366–369. doi: 10.1126/science.3659920. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro S, Borgens R, Pascuzzi R, et al. Oscillating field stimulation for complete spinal cord injury in humans: A phase 1 trial. J Neurosurg Spine. 2005;2:3–10. doi: 10.3171/spi.2005.2.1.0003. [DOI] [PubMed] [Google Scholar]
- 46.Laverty PH, Leskovar A, Breur GJ, et al. A preliminary study of intravenous surfactants in paraplegic dogs: Polymer therapy in canine clinical SCI. J Neurotrauma. 2004;21:1767–1777. doi: 10.1089/neu.2004.21.1767. [DOI] [PubMed] [Google Scholar]
- 47.Jeffery ND, Lakatos A, Franklin RJ. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma. 2005;22:1282–1293. doi: 10.1089/neu.2005.22.1282. [DOI] [PubMed] [Google Scholar]
- 48.Richter MW, Roskams AJ. Olfactory ensheathing cell transplantation following spinal cord injury: Hype or hope? Exp Neurol. 2008;209:353–367. doi: 10.1016/j.expneurol.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 49.Skinner AP, Pachnicke S, Lakatos A, Franklin RJ, Jeffery ND. Nasal and frontal sinus mucosa of the adult dog contain numerous olfactory sensory neurons and ensheathing glia. Res Vet Sci. 2005;78:9–15. doi: 10.1016/j.rvsc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Dobkin BH, Curt A, Guest J. Cellular transplants in China: Observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tator CH. Review of treatment trials in human spinal cord injury: Issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- 52.Olby N, Harris T, Burr J, et al. Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J Neurotrauma. 2004;21:49–59. doi: 10.1089/089771504772695940. [DOI] [PubMed] [Google Scholar]
- 53.Muir GD, Webb AA. Mini-review: Assessment of behavioural recovery following spinal cord injury in rats. Eur J Neurosci. 2000;12:3079–3086. doi: 10.1046/j.1460-9568.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 54.Webb AA, Jeffery ND, Olby NJ, Muir GD. Behavioural analysis of the efficacy of treatments for injuries to the spinal cord in animals. Vet Rec. 2004;155:225–230. doi: 10.1136/vr.155.8.225. [DOI] [PubMed] [Google Scholar]
- 55.Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- 56.Muir GD, Steeves JD. Sensorimotor stimulation to improve locomotor recovery after spinal cord injury. Trends Neurosci. 1997;20:72–77. doi: 10.1016/s0166-2236(96)10068-0. [DOI] [PubMed] [Google Scholar]
- 57.Barriere G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]