Abstract
This study evaluated the relationship between severity of fatty liver and macromineral status in downer dairy cows and determined the usefulness of selected biochemical analytes for assessing prognosis. Blood and liver biopsy specimens were obtained from 36 Holstein downer cows shortly after the cows became recumbent and before they were treated. Liver tissue was examined histologically and serum activity of liver-derived enzymes and concentration of total lipids, triglycerides, bile acids, glucose, β-hydroxybutyrate, acetoacetic acid, total bilirubin, non-esterified fatty acids (NEFA), cholesterol and macrominerals (Ca, Mg, K, Na, P) were determined. Fatty liver infiltration was severe in 44% of the cows and moderate in 44%. Serum activities of ornithine carbamoyltransferase and glutamate dehydrogenase, and NEFA/cholesterol ratio were good indicators of fatty liver. Cows with severe fatty liver had the lowest mean K values. The prognosis is guarded for downer cows with moderate and severe fatty liver and when total bilirubin concentration is high.
Résumé
Évaluation clinicopathologique des vaches laitières couchées atteint de la stéatose hépatique. Cette étude a évalué le lien entre la gravité de la stéatose hépatique et le statut des éléments minéraux chez les vaches laitières couchées et a déterminé l’utilité de certaines substances à analyser pour évaluer le pronostic. Les spécimens de sang et de biopsie du foie ont été obtenus auprès de 36 vaches Holstein couchées après leur décubitus et avant le traitement. Les tissus du foie ont fait l’objet d’un examen histologique et l’activité sérique des enzymes dérivés du foie et la concentration des lipides totaux, des triglycérides, des acides biliaires, du glucose, du β-hydroxybutyrate, des acides acétoacétiques, de la bilirubine totale, des acides gras libres, du cholestérol et des éléments minéraux (Ca, Mg, K, Na, P) ont été déterminés. L’infiltration de la stéatose hépatique était grave chez 44 % des vaches et modérée chez 44 %. Les activités sériques de l’ornithine carbamoyltransférase et de la glutamate déhydrogénase et le ratio des acides gras libres et du cholestérol étaient de bons indicateurs de la stéatose hépatique. Les vaches atteintes de stéatose hépatique grave présentaient les valeurs de K moyennes les plus basses. Le pronostic est réservé pour les vaches couchées atteintes de la stéatose hépatique modérée et grave et lorsque la concentration de la bilirubine totale est élevée.
(Traduit par Isabelle Vallières)
Introduction
Dwner cow syndrome refers to cows that become recumbent and fail to rise; this is a major concern in dairy farms worldwide. The syndrome occurs mainly in the early postparturient period and is caused by several diseases. The most common cause of downer cow syndrome is hypocalcemia (milk fever) (1) but it is also caused by injuries, muscle damage, macromineral deficiencies, toxic mastitis or metritis (2). Fatty liver may also contribute to cows becoming downers (3). Almost all high producing dairy cows are in negative energy balance in early lactation because energy requirements exceed feed consumption capacity (4). The liver plays a central role in metabolism and dairy cows are generally prone to liver disease (5); a high proportion of dairy cows experience fatty liver before and after parturition (6–10).
Although fatty liver is an important risk factor for the occurrence of downer cow syndrome (3), it is often misidentified or overlooked because it is difficult to diagnose. Clinical signs in downer cows usually do not accurately reflect hepatic dysfunction, unless the liver damage is severe. The definitive means of diagnosing fatty liver is determining the fat content of hepatic tissue collected by liver biopsy (11), although there are a variety of biochemical parameters, such as aspartate aminotransferase (AST), total bilirubin (tBIL), gamma glutamyltransferase (GGT), total bile acids (SBA), and glutamate dehydrogenase (GDH), which provide some diagnostic information (12). It was recently reported that ornithine carbamoyltransferase (OCT) may be a very sensitive indicator of various degrees of fatty liver (13). There have been few reports of the relationship between downer cow syndrome and fatty liver and we are unaware of any published investigations on the relationship between fatty liver and serum OCT activity or blood concentrations of macrominerals in downer cows.
The purposes of the study were to evaluate the relationship between severity of fatty liver and macromineral status in downer cows and to assess the usefulness of the determination of OCT, other enzyme activity, or other biochemical analytes in evaluating prognosis.
Materials and methods
Animal selection
Thirty-six Holstein cows (6 of which were first-calf heifers) from 23 dairy farms (fewer than 3 from each farm) in Greece were used. Cows that became recumbent in the first week of lactation were eligible for inclusion in the study and were referred by the local veterinarians who serviced the herds. All cows were sampled as soon as possible after recumbency (time between referred recumbency and sampling ranged from 30 min to 6 h) and before any treatments were administered. Downer cows in the present study had failed to rise within 6 h after the 1st treatment. Samples were collected from March through December 2004. The study protocol was performed in compliance with institutional guidelines and European Union legislation for research on animals. All owners gave informed consent for cattle to be included in the study and undergo the testing procedures.
Data collected when the cows were examined included age, parity, date of calving, recent health and production problems, time (h) from the onset of recumbency, and body condition score (scale of 1 to 5). A thorough physical examination was performed on each cow including rectal temperature, pulse rate, inspection of mucous membranes and examination for mastitis, metritis and bone fractures. Then blood and liver tissue samples were collected. Cows without signs of disease other than hypocalcemia were then treated with 500 mL of 23% calcium borogluconate IV, 250 mL of 23% calcium borogluconate SC, 500 mL of 35% dextrose IV, and 150 mL propylene glycol PO.
The 36 cows that were finally included in the study (from a total of 74 initially referred) fit the definition of “downer cows.” These cows were between 10 and 72 h after calving. None of them had a history of musculoskeletal injury, nor showed evidence of other disease (such as, fever, vaginal discharge, mastitis).
The clinical outcome of these 36 downer cows was determined on the 7th day in milk (DIM) and cases were followed up by telephone. Each cow was assigned 1 of 2 clinical outcomes as follows: 1) cured (able to stand and return to good general health by 7 DIM) or 2) died (dead within the first 7 DIM or still recumbent and euthanized by the 7th DIM).
Sample collection
Blood samples were collected from the jugular vein of each cow using an 18-gauge needle into glass tubes without anticoagulant. After clotting for 20 min, serum was separated at the farm by centrifugation at 1600 × g for 15 min, transferred to plastic vials, and transported at 4°C to the laboratory, where it was stored refrigerated at 4°C or frozen at −20°C. Refrigerated serum was analyzed within 6 h for enzyme activity, acetoacetic acid (AcAc) and beta-hydroxybutyrate (β-HB), glucose (GLU), blood urea nitrogen (BUN), albumin (ALB), cholesterol (CHOL), and tBIL concentrations. Frozen serum was analyzed within 10 d for SBA and non-esterified fatty acids (NEFA) concentrations.
Liver tissue was obtained by transcutaneous biopsy through the 11th right intercostal space using a liver biopsy needle (Berlin Model, 2.5 mm × 25 cm; Eickemeyer Medizintechnik für Tierärzte, Tuttlingen, Germany). Biopsy specimens (150 to 350 mg of liver tissue) were divided into 2 parts; one was fixed in neutral-buffered 10% formalin for histological examination and the other was stored at −20°C for determination of total lipid and triglyceride concentrations.
Serum biochemical analysis
Spectrophotometric kinetic methods were used to determine serum activities of AST (14), alanine aminotransferase (ALT) (14), alkaline phosphatase (ALP) (15), GGT (16), sorbitol dehydrogenase (SDH) (17), GDH (18), creatine kinase (CK) (19) and concentration of β-HB (20) and AcAc (20). All measurements were obtained at a temperature of 30°C. Colorimetric spectrophotometric methods were used for determination of OCT activity (21) and tBIL (22), ALB (23), BUN (24), CHOL (25), and GLU (26) concentrations. Commercial kits were used for determination of SBA (No. 450-A, Trinity Biotech plc; Brau, Co Wicklow, Ireland) and NEFA (Wako Cod 999-75406; Wako Chemicals GmbH, Neuss, Germany) concentrations. All blood samples were analyzed for serum total Ca and Mg concentrations by flame atomic absorption spectophotometry (Perkin-Elmer A analyst 100; Atomic absorption spectroscopy 1996, Analytical methods. Norwalk, Conn: Perkin-Elmer Corp, Norwalk, Connecticut, USA). Total serum concentration of phosphorus (P) was determined using the heteropoly acid-blue method (27). Serum potassium (K) and sodium (Na) concentrations were determined by flame atomic emission spectrophotometry (Sherwood 410 flame photometer; Sherwood Scientific, Cambridge, England).
Liver biochemical analysis
Total lipid concentration in liver tissue was measured using chloroform-methanol-water extraction (28). Liver triglyceride concentration was determined by first saponifying the extracted lipids with 1 mL of 0.5N KOH solution and 1 mL of absolute ethanol for 60 min at 70°C. The resultant triacylglycerols were measured as described previously (29). Both liver lipid and triglyceride content were reported as mg/g of wet liver tissue. A spectrophotometer was used for all measurements (Hitachi U-2000; Hitachi, Tokyo, Japan).
Histological examination
Biopsy specimens fixed in neutral-buffered 10% formalin solution were cut to 3 to 4 μm thickness sections, and stained with hematoxylin and eosin (H&E). Specimens were examined by light microscopy for lipid content. Liver fat content was classified according to a 6-point scale of severity of fatty infiltration (GfL), using a semi-quantitative scoring system (13). Range of GfL scores was from 0 (no fat droplets visible) to 5 (panlobular fatty infiltration).
Grouping by severity of fatty liver
Cows were assigned to 1 of 3 groups of fatty liver infiltration based on GfL and TG liver content, as follows: 1) mild fatty liver (cows with GfL = 2 and liver TG < 20 mg/g), 2) moderate fatty liver (cows with GfL = 3 or 4 and liver TG = 20 to 50 mg/g), and 3) severe fatty liver (cows with GfL = 5 and liver TG > 50 mg/g). No cows were classified as GfL 0 or 1.
Reference healthy fresh cows
The reference range for biochemical variables evaluated in the study was determined using blood and liver tissue samples from 10 healthy Holstein cows from the same 23 farms (maximum 1 per farm) that contributed cows to the study. The selected cows were within the first 7 DIM, had no history of disease for the current lactation, were clinically healthy at the time of sampling, and had no histologically visible fat in the liver (GfL < 2) as determined later by histopathological evaluation. All of the variables were measured using the same methods used for the diseased cattle. The minimum and maximum values obtained from these 10 cows were used as lower and upper ends of the reference range, respectively.
Healthy fresh cows
During a large-scale study held in Greece for metabolic diseases in Holstein cattle (13) a reference population of 70 healthy fresh cows was created. Although these 70 animals were clinically healthy, they did have mild fatty liver (GfL = 2) within the first 30 DIM. All parameters were measured with exactly the same methods as for the downer cows of the present study. The biochemical results of these 70 cows are presented in Table 1 as values for “healthy fresh cows.”
Table 1.
Blood and liver test results, age, and body condition scores from healthy reference cows and downer cows with mild, moderate, or severe fatty liver
| Reference healthy fresh cows < 7 DIM, n = 10 |
Healthy fresh cows GfL 2, n = 70 |
Downer cows mild fatty liver, n = 4 |
Downer cows moderate fatty liver, n = 16 |
Downer cows severe fatty liver, n = 16 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± s | Median | Mean ± s | Median | Mean ± s | Median | Mean ± s | Median | Mean ± s | Median |
| Ca (mmol/L) | 2.1 ± 0.04 | 2.1a | 2.1 ± 0.02 | 2.1a | 1.8 ± 0.22 | 1.8b | 1.9 ± 0.13 | 1.8b | 1.9 ± 0.08 | 1.8b |
| Mg (mmol/L) | 0.9 ± 0.09 | 0.9a | 0.9 ± 0.02 | 0.9a | 0.9 ± 0.06 | 0.9a | 0.9 ± 0.08 | 0.8a | 0.8 ± 0.07 | 0.7a |
| K (mmol/L) | 5.1 ± 0.27 | 5.5a | 5.2 ± 0.5 | 5.1a | 4.1 ± 0.27 | 4.5b | 4.2 ± 0.19 | 4.15b | 3.6 ± 0.21 | 3.25c |
| Na (mmol/L) | 147.9 ± 2.76 | 150a | 149 ± 0.8 | 149a | 148.0 ± 2.7 | 146a | 150.8 ± 3.03 | 151a,b | 137.8 ± 3.99 | 142.5a,c |
| P (mmol/L) | 2.7 ± 0.11 | 2.6a | 2.8 ± 0.05 | 2.9a | 2.0 ± 0.25 | 1.7b | 1.7 ± 0.15 | 1.7b | 1,7 ± 0.15 | 1.5b |
| GGT (U/L) | 19.4 ± 1.29 | 20a | 17 ± 0.53 | 17a | 19.3 ± 2.09 | 18a | 21.4 ± 2.08 | 19.5a | 24.4 ± 1.40 | 21.2a |
| AST (U/L) | 46.8 ± 2.65 | 43a | 42.5 ± 1.56 | 42.5a | 83.9 ± 14.3 | 95b | 241.7 ± 49.31 | 232c | 275.4 ± 66.69 | 248c |
| ALT (U/L) | 34.1 ± 2.22 | 37a | 20 ± 0.76 | 20a | 41.1 ± 8.01 | 44.5a | 74.3 ± 13.02 | 63.3b | 87.2 ± 25.02 | 67.5b |
| ALP (U/L) | 118.4 ± 14.52 | 102a | 127 ± 0.28 | 125a | 223.8 ± 27.56 | 203.5b | 185.2 ± 16.63 | 204b | 226.9 ± 45.30 | 195b |
| SDH (U/L) | 18.8 ± 2.12 | 18.7a | 12.5 ± 0.74 | 12.5a | 14.2 ± 10.53 | 13.35a | 23.2 ± 3.70 | 19.85a | 39.6 ± 14.47 | 18.85a |
| GDH (U/L) | 3.4 ± 0.53 | 3.5a | 3.6 ± 0.52 | 3.7a | 3.9 ± 1.42 | 4.7a | 10.9 ± 2.48 | 7.85b | 8.9 ± 1.14 | 7.8b |
| OCT (U/L) | 10.6 ± 1.99 | 11.2a | 14.5 ± 0.69 | 14.7a | 11.5 ± 1.3 | 13a | 27.2 ± 3.05 | 25.8b | 47.6 ± 11.11 | 41.6c |
| CK (U/L) | 43.7 ± 3.40 | 43a | 46.5 ± 1.90 | 46.5a | 171.8 ± 49.4 | 205b | 1196.6 ± 263.7 | 1380c | 2304.3 ± 406.4 | 2075d |
| ALB (g/L) | 0.04 ± 0.002 | 0.04a | 0.04 ± 0.006 | 3.4a | 0.04 ± 0.005 | 0.03b | 0.03 ± 0.001 | 0.03b | 0.03 ± 0.001 | 0.03b |
| BUN (mmol/L) | 6.21 ± 0.48 | 5,71a | 6.17 ± 0.26 | 5.7a | 6.0 ± 1.4 | 8.57a | 11.03 ± 2.61 | 6.43a | 17.53 ± 3.02 | 13.92b |
| GLU (mmol/L) | 4.64 ± 0.25 | 4.51a | 4.38 ± 0.06 | 4.34a | 5.66 ± 0.99 | 4.52a | 4.45 ± 0.41 | 4.87a | 3.89 ± 0.59 | 3.17a |
| CHOL (mmol/L) | 3.24 ± 0.11 | 3.7a | 3.74 ± 088 | 3.8a | 3.85 ± 1.04 | 3.99a | 1.89 ± 0.15 | 2.94b | 1.77 ± 0.20 | 2.69b |
| tBIL (μmol/L) | 5.5 ± 0.68 | 5.2a | 7.0 ± 0.34 | 6.0a | 18.8 ± 10.9 | 10.1a | 16.8 ± 2.2 | 17.1b | 24.1 ± 6.5 | 22.2b |
| SBA (μmol/L) | 43.8 ± 11.29 | 40.5a | Not available | 36.3 ± 12.21 | 28.4a | 30.4 ± 4.76 | 24.8a | 45.9 ± 8.71 | 35.8a | |
| NEFA (mmol/L) | 0.551 ± 0.07 | 0.54a | Not available | 0.543 ± 1.28 | 0.55a | 0.911 ± 0.06 | 0.89b | 1.087 ± 0.09 | 1.19c | |
| AcAc (mmol/L) | 0.055 ± 0.01 | 0.04a | 0.039 ± 0.01 | 0.033a | 0.062 ± 0.01 | 0.058a | 0.089 ± 0.02 | 0.081b | 0.100 ± 0.02 | 0.1b |
| β-HB (mmol/L) | 0.775 ± 0.02 | 0.74a | 0.690 ± 0.02 | 0.69a | 0.818 ± 0.28 | 0.725a | 0.849 ± 0.10 | 0.75a | 1.041 ± 0.21 | 0.9b |
| tLPD (mg/g) | 136.2 ± 12.6 | 137.5a | 142 ± 4.19 | 139.1a | 127.8 ± 23.3 | 129a | 228.6 ± 10.1 | 224b | 282.2 ± 14.6 | 300c |
| TG (mg/g) | 10.53 ± 0.61 | 10.8a | 16.27 ± 0.48 | 15.9b | 15.94 ± 2.18 | 17.2b | 36.53 ± 1.77 | 37.1c | 77.74 ± 7.49 | 62d |
| Age (years) | 5.4 ± 0.37 | 4.5a | 5.0 ± 0.15 | 5a | 4.4 ± 0.47 | 5a | 5.9 ± 0.39 | 6b | 6.2 ± 0.39 | 5.5b |
| BCS | 3.2 ± 0.18 | 3a | 3.0 ± 0.05 | 3a | 2.8 ± 0.13 | 3a | 3.1 ± 0.17 | 3a | 2.9 ± 0.19 | 3a |
| NEFA/CHOL ratio | 0.17 | 0.15a | Not available | 0.24 | 0.14a | 0.53 | 0.30b | 0.73 | 0.44b | |
s — standard deviation.
Within a row, different superscripts denote significant (P < 0.05) differences among groups.
Triglyceride (TG) and total lipids (tLPD) values are in wet liver tissue.
BUN — blood urea nitrogen, OCT — ornithine carbamoyl transferase, GDH — glutamate dehydrogenase, AST — aspartate aminotranferase, ALT — alanine aminotransferase, ALP — alkaline phosphatase, SDH — sorbitol dehydrogenase, GfL — grades of fatty liver, TG — triglyceride content in hepatic tissue, tLPD — total lipid concentration in hepatic tissue, β-HB — β-hydroxybutyrate, AcAc — acetoacetic acid, GLU — glucose, ALB — albumin, BCS — body condition score, CHOL — cholesterol, Ca — total serum calcium, (P) — inorganic phosphorus, (Mg) — magnesium, NEFA — non-esterified fatty acids, K — potassium, Na — sodium, SBA — serum bile acid, tBIL — total bilirubin, CK — creatine kinase, GGT — γ-glutamyltransferase.
Statistical analysis
Analysis was performed using a commercial software program (SPSS, version 16.0; SPSS, Chicago, Illinois, USA). The Spearman rank bivariate correlation was used to investigate the relationship between variables. Because the assumptions of ANOVA (homogeneity of variances and normality) were not satisfied we compared biochemical parameter measurements among the 3 groupings of fatty liver severity using a Kruskall-Wallis non-parametrical test. When significant differences among groups were observed, the Mann-Whitney test was used for pairwise comparisons, in order to identify which group medians were significantly different. Fisher’s exact chi-squared (X 2) test was used to assess independence between fatty liver group classification and clinical outcome. Finally, a Mann-Whitney test was used to compare medians of biochemical parameter measurements in the 2 different clinical outcome groups (cured — died). For all tests, values of P < 0.05 were considered significant.
Results
Liver evaluation
Liver tissues from all 36 downer cows were classified as GfL 2 or higher, resulting in the classification shown in Table 1, where the results of the liver biochemical analyses are also presented.
Clinical outcome
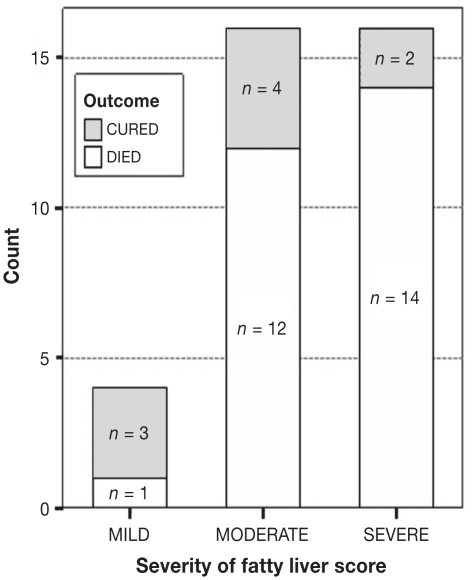
Classification of cows according to the outcome and the severity of fatty liver is shown in Figure 1. The time frame for their recovery was between 1 and 6 d after 1st treatment. Nine of the 36 cows were classified as cured and the remaining 27 cows had died by 7 DIM. There was a significant association between clinical outcome (cured or died) and severity of fatty liver, as revealed by Fisher’s exact X 2 test (P < 0.05).
Figure 1.
Clinical outcome within 7 days in milk for the 36 downer cows based on severity of fatty liver.
For 11 parameters (OCT, GDH, AST, ALT, CK, SDH, GGT, BUN, K, tBIL, and triglycerides) there were significantly different parameter distribution medians in animals that were cured versus animals that died (Table 2).
Table 2.
Median values of the parameters with statistically significantly different medians (P < 0.05) between the two outcome groups
| Parameter | K (mmol/L) Median | BUN (mmol/L) Median | tBIL (μmol/L) Median | CK (U/L) Median | OCT (U/L) Median | GDH (U/L) Median | AST (U/L) Median | ALT (U/L) Median | SDH (U/L) Median | GGT (U/L) Median | TG (mg/g) Median |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Groups | |||||||||||
| Cured n = 9 | 4.4 | 7.14 | 4.62 | 310 | 17.1 | 6.0 | 121 | 37 | 13.3 | 17 | 36 |
| Died n = 27 | 3.6 | 14.28 | 22.2 | 1950 | 35.0 | 9.1 | 230 | 70 | 24.0 | 22 | 51 |
BUN — blood urea nitrogen, OCT — ornithine carbamoyl transferase, GDH — glutamate dehydrogenase, AST — aspartate aminotranferase, ALT — alanine aminotransferase, SDH — sorbitol dehydrogenase, TG — triglyceride content in hepatic tissue, K — potassium, tBIL — total bilirubin, CK — creatine kinase, GGT — γ-glutamyltransferase.
Serum biochemical analyses
Median serum OCT activity increased significantly with the severity of fatty liver (Table 1) and was significantly correlated with parameters such as triglycerides, AST, SDH, ALP, GDH, tBIL, and CHOL (Table 3). Median serum AST activity was significantly higher in downer cows with severe and moderate fatty liver and serum GDH activity increased with increasing liver lipid content (Table 1). Serum CK activity was very high in the downer cows, and median CK activity increased significantly from the mild to severe fatty liver groups (Table 1).
Table 3.
Spearman correlation coefficients (r) for selected blood and liver variables, from downer cows with fatty liver as in Table 1. All correlations presented are significant (P < 0.05)
| AST | OCT | SDH | GDH | ALP | GGT | NEFA | CK | tBIL | CHOL | AcAc | β-HB | GLU | tLPD | TG | BUN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OCT | 0.566 | |||||||||||||||
| SDH | 0.503 | 0.621 | ||||||||||||||
| GDH | 0.554 | 0.425 | ||||||||||||||
| ALP | 0.685 | 0.590 | 0.476 | |||||||||||||
| GGT | 0.367 | 0.513 | ||||||||||||||
| NEFA | 0.683 | |||||||||||||||
| CK | 0.741 | 0.333 | ||||||||||||||
| tBIL | 0.610 | 0.703 | 0.548 | 0.594 | 0.355 | |||||||||||
| CHOL | −0.390 | −0.634 | −0.452 | −0.475 | ||||||||||||
| AcAc | 0.339 | 0.440 | ||||||||||||||
| β-HB | −0.332 | 0.456 | 0.764 | |||||||||||||
| GLU | −0.376 | −0.432 | −0.369 | 0.481 | ||||||||||||
| tLPD | 0.562 | 0.521 | −0.506 | 0.336 | ||||||||||||
| TG | 0.648 | 0.645 | 0.557 | 0.448 | −0.358 | 0.426 | 0.378 | −0.417 | 0.635 | |||||||
| BUN | 0.416 | 0.508 | 0.367 | |||||||||||||
| Ca | 0.370 | −0.425 | −0.427 | |||||||||||||
| K | −0.301 | −0.338 | −0.354 | |||||||||||||
| P | 0.330 | −0.340 | 0.408 |
BUN — blood urea nitrogen, OCT — ornithine carbamoyl transferase, GDH — glutamate dehydrogenase, AST — aspartate aminotranferase, ALP — alkaline phosphatase, SDH — sorbitol dehydrogenase, TG — triglyceride content in hepatic tissue, tLPD — total lipid concentration in hepatic tissue, β-HB — β-hydroxybutyrate, AcAc — acetoacetic acid, GLU — Glucose, CHOL — cholesterol, Ca — total serum calcium, P — inorganic phosphorus, NEFA — non-esterified fatty acids, K — potassium, tBIL — total bilirubin, CK — creatine kinase, GGT — γ-glutamyltransferase.
Median serum tBIL concentration was significantly elevated in moderate and severe fatty liver groups (Table 1). Median serum NEFA concentration was significantly higher in cows with moderate and severe fatty liver compared with those having mild fatty liver (Table 1). The NEFA/cholesterol ratios averaged 0.17 in the reference cows, 0.24 in the mild fatty liver group, rose to 0.53 for the moderate fatty liver group, and up to 0.73 in the severe fatty liver group (Table 1).
Downer cows had significantly lower median serum Ca and P concentrations, but these medians were not significantly different between the fatty liver groups (Table 1). All groups of downer cows had significantly reduced median serum K concentration and the lowest values were recorded in the severe fatty liver group, where some cows were hypokalemic (serum K <3 mmol/L) (Table 1).
Discussion
One of the aims of the study was to investigate the occurrence and severity of fatty liver in downer cows soon after calving. In this group of 36 downer cows, which had all calved within 72 h of sampling, 44% had severe fatty liver and 44% had moderate fatty liver. This finding supports the theory that fatty liver is present prepartum (30) and is likely caused by mobilization of body reserves just before calving (31). About 25% of dairy cows are expected to have some degree of fatty liver during the 1st week after calving (6). In the present study, 88% of the animals had moderate or severe fatty liver soon after calving, suggesting that fatty liver might be more common in downer cows than in the general population of dairy cows that recently calved.
First-lactation animals accounted for 16% of the downer cows enrolled in this study. Five of these 6 animals had severe fatty liver, suggesting that heifers are also vulnerable to this condition. This result is in agreement with some studies (9) but in contrast to others (6).
The liver occupies a central position in the metabolism of the cow and fatty liver is accompanied by disturbances in hepatic structure and function (6). Fatty liver severity is related to age; cows with severe fatty liver being substantially older (mean age 6.2 y) than the reference cows or cows with mild fatty liver. Fatty liver is related to post-parturient disorders like hypocalcemia, ketosis, metritis, which occur more often in older cows. It has been reported to be more common in cows than in heifers (6). Also, the negative energy balance, which typically follows the higher milk production of dairy cows after their 2nd lactation, increases the risk for fatty liver in high producing multiparous cows compared with heifers.
Downer cows usually have reduced appetite which aggravates fatty liver, initiating a vicious cycle of worsening appetite. This may explain why moderate or severe fatty liver often leads to liver failure and death (8,30). None of the cows included in the present study had evidence of other disease; thus, we regarded liver failure as the likely cause of death for the cows that did not recover. There was a significant association (P < 0.05) between clinical outcome (cured or died) and severity of fatty liver. Necropsy was performed in only 3 cases with severe fatty liver and, as expected, the findings were extensive hepatic lipidosis and muscle damage attributed to ischemia from recumbency. The lack of postmortem examinations on the other cows is a limitation of the study, as the cause of death could not be verified.
In dairy cows, lipid accumulates in the liver chiefly in the form of triglycerides (4). In the present study, many of the cows that died had markedly high concentrations of triglyceride, up to almost 10 times the reference value. In the severe fatty liver group the significantly higher concentrations of triglycerides indicate the influence of fatty liver in the prognosis of downer cows, since 14/16 cows died. Furthermore, the median liver triglyceride concentrations were significantly higher in cows that died during the study period compared to cows that were cured during the same period.
Accumulation of triglyceride in the cytoplasm of hepatocytes causes disturbances in hepatic structure and function that are likely to have particular clinical importance (6) when they coexist with aggravating factors such as postpartum stress and recumbency. Enzyme leakage from hepatocytes is one manifestation of these disturbances (32). The present results indicate that OCT increases in parallel with the severity of fatty liver and is a measurement that could potentially be used to differentiate animals with mild, moderate, and severe fatty liver. Most cows with moderate or severe fatty liver had high serum OCT activity, likely as a result of lipid accumulation in hepatocytes that caused dilatation and dysfunction of mitochondria (33). Although not widely studied, this enzyme may be very useful in the diagnosis of fatty liver, and especially in evaluating the severity of the fatty liver (13,33). Ornithine carbamoyltransferase correlated well with AST activity in the present study, as it did in other studies (21,33). In another field study, OCT activity exceeded the reference range more frequently than AST in ketotic cows with fatty liver (34).
In the present study, median OCT was significantly different in animals with moderate and severe fatty liver and, also, both compared to mild fatty liver, to healthy fresh and reference cows. The increase in OCT activity accurately reflected moderate and severe fatty liver, despite some overlap in ranges. Median OCT activity was also significantly elevated in cows that died, compared with cows that were cured. Considering these results, we conclude that OCT is a reliable index of fatty liver severity, which, in turn, is an indicator of poor prognosis of downer cows. Although the relatively small number of downer cases did not enable sensitivity evaluation to verify a cut-off point, the data indicate that OCT values above 40 U/L suggest severe fatty liver and guarded prognosis for downer cows.
Serum AST activity may have value in diagnosing fatty liver (7). Others (8,33) have raised doubt about the value of AST, mainly because it is not liver-specific (35) and is easily elevated in muscle damage (36). In downer cows with increased AST activity, concurrent analysis of serum CK activity helps to identify the origin of AST (muscle or liver). In the present study, increases in AST were likely due to muscle damage, because the correlation between serum CK and AST activity was high. A portion of the AST elevation may have been liver-derived, as evidenced in the weaker correlations of AST with TG, tBIL, OCT and ALT. Consequently, as the liver-derived portion of serum AST activity cannot be distinguished, the diagnostic value of AST in downer cows suspected for liver dysfunction is diminished.
Serum GDH activity increased with increasing hepatic TG content, allowing the differentiation of moderate and severe fatty liver from mild cases and reference cows, but not the differentiation between moderate and severe fatty liver. Glutamate dehydrogenase is not associated with calving (30), so detection of high activity in recently calved cows may be an important indicator of fatty liver and of the poor prognosis of downer cows. Increased GDH activity also indicates acute hepatocellular damage (30). In a previous study, using a GDH cut-off value of 5.7 U/L, 70% of the moderate to severe (GfL = 4) and only 48.5% of severe (GfL = 5) fatty liver cases were diagnosed (13). Although the relatively small number of downer cases did not enable sensitivity evaluation to verify a cut-off point, the data would suggest that GDH values > 16 U/L might indicate guarded prognosis for downer cows due to severe liver damage.
The present study confirmed that determination of serum total bilirubin is valuable for the diagnosis of fatty liver in downer cows. Total bilirubin is often increased after calving (37) and is also increased during periods of anorexia (38). Thus, it is expected to be high in downer cows. Total bilirubin concentration was significantly higher in all downer cows compared to reference and healthy fresh cows. Moreover, it was significantly elevated in moderate and severe fatty liver groups (mean values ≥ 1 mg/dL), possibly providing a means to distinguish between mild and moderate/severe fatty liver. The fact that median total bilirubin concentration was significantly elevated in animals that eventually died during the study, makes high concentrations an indication of guarded prognosis.
In the present study, serum NEFA concentration was increased in cows with moderate and severe fatty liver, an observation which agrees with earlier published information (39). Non-esterified fatty acids are considered useful for detection of fatty liver in downer cows, as a high NEFA concentration is indicative of extended lipid mobilization and is highly correlated with liver lipid content (8). Serum NEFA gradually increases during the last week before parturition and then acutely increases at calving, which triggers even more fatty liver infiltration (40). In downer cows this phenomenon is more intense because the appetite loss (41) and the difficulties in accessing food lead to a higher negative energy balance, which in turn increases NEFA mobilization and blood concentration. The NEFA serum concentration is also stress-sensitive (42), which increases NEFA release, resulting in more rapid lipid accumulation in the liver.
Fatty liver is characterized by abnormal lipid and lipoprotein concentrations (43). In the present study, serum cholesterol concentration was significantly decreased in cattle with moderate and severe fatty liver compared to the healthy cows and cows with mild fatty liver, and was inversely related to NEFA concentrations. These results are in accordance with earlier reports in which fatty liver infiltration was associated with decreased serum cholesterol, higher NEFA, and higher NEFA/cholesterol ratio (44). The NEFA/cholesterol ratios herein were about 3 times higher in cows with moderate fatty liver and more than 4 times higher in cows with severe fatty liver compared with the reference cows. Ratios of NEFA/cholesterol > 0.4 in downer cows suggested that at least moderate fatty liver was present.
The downer cows had significantly lower median serum Ca concentration compared with the reference and healthy fresh cows. This was expected, because hypocalcemia is the most frequent cause of recumbency in fresh cows (1). Some of the downer cows suffered severe hypocalcemia (Ca concentrations as low as 0.7 mmol/L). There was no difference in serum Ca between the fatty liver groups. We speculate that the coexistence of fatty liver with hypocalcemia may have negatively affected the downer cows and worsened their prognosis.
All downer cows had significantly lower median serum K concentrations compared with reference and healthy fresh cows, and cows with the lowest mean K values belonged in the severe fatty liver group. Some of the severe fatty liver cows had hypokalemia. Median K concentration was significantly lower in animals that died compared to animals that were cured. It is generally accepted that cows being off-feed for more than 3 d will finally result in hypokalemia. In the present study, however, the cows were hypokalemic without being off-feed, as the sampling was performed within 6 h after their recumbency. The degree of hypokalemia may be partly attributed to the various degrees of inappetance that these cows had. Nevertheless, we can not draw conclusions about the reason the downer cows were hypokalemic. Given that hypokalemia can lead to muscle weakness and degeneration and recumbency (45), K concentration should always be evaluated in downer cows and considered in the prognosis.
In conclusion, fatty liver was common in the downer cows. Cows with severe fatty liver and cows that died up to 7 DIM had the lowest mean K values. Because no single serum biochemical variable serves as an absolute indicator of fatty liver, a combination of variables may be valuable in making a diagnosis. We suggest that measurements of OCT and GDH might be useful in diagnosing fatty liver in downer cows. When the NEFA/ cholesterol ratio was > 0.4, at least moderate fatty liver was evident. The prognosis was guarded when total bilirubin concentration was high. The prognosis of downer cows with moderate and severe fatty liver was poor. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Barrington GM. Parturient paresis in cows. In: Aiello S, editor. The Merck Veterinary Manual. 8th ed. Whitehouse Station, New Jersey: Merck and Co; 1998. pp. 739–741. [Google Scholar]
- 2.Gerloff BJ. Herd health. In: Radostits O, editor. Food Animal Production Medicine. 3rd ed. Philadelphia: WB Saunders; 2001. p. 465. [Google Scholar]
- 3.Rukkwamsuk T, Kruip TA, Wensing T. Relationship between over-feeding and overconditioning in the dry period and the problems of high producing dairy cows during the postparturient period. Vet Q. 1999;21:71–77. doi: 10.1080/01652176.1999.9694997. [DOI] [PubMed] [Google Scholar]
- 4.Collins RA, Reid IM. A correlated biochemical and stereological study of periparturient fatty liver in the dairy cow. Res Vet Sci. 1980;28:373–376. [PubMed] [Google Scholar]
- 5.Staufenbiel R, Staufenbiel B, Rossow N, Klukas H, Johannsen U. Diagnostik der Leberverfettung bei der Milchkuh. Deut Tierärztl Woch. 1993;100:225–230. [PubMed] [Google Scholar]
- 6.Reid IM. Incidence and severity of fatty liver in dairy cows. Vet Rec. 1980;107:281–284. doi: 10.1136/vr.107.12.281. [DOI] [PubMed] [Google Scholar]
- 7.Reid IM, Rowlands GJ, Dew AM, Collins RA, Roberts CJ, Manston R. The relationship between post-parturient fatty liver and blood composition in dairy cows. J Agric Sci Cam. 1983;101:473–480. [Google Scholar]
- 8.Szanszlo F, Karsai F. Cholsäurespiegel im Blutserum von Milchkühen mit stoffwechselbedingler Lebererkrankung. Deut Tierärztl Woch. 1991;98:79–82. [PubMed] [Google Scholar]
- 9.Cebra CK, Garry FB, Getzy DM, Fettman MJ. Hepatic lipidosis in anorectic, lactating Holstein cattle: A retrospective study of serum biochemical abnormalities. J Vet Intern Med. 1997;4:231–237. doi: 10.1111/j.1939-1676.1997.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 10.Rehage J, Qualmann K, Meier C, Stockhofe-Zurwieden N, Hoeltershinken M, Pohlenz J. Total serum bile acid concentrations in dairy cows with fatty liver and liver failure. Deut Tierärztl Woch. 1999;106:26–29. [PubMed] [Google Scholar]
- 11.Woitow G, Staufenbiel R, Langhans J. Proc Symposium Energie-u. Fettstoffweschel der milchkuh. Humboldt-Univ; Berlin: 1991. Vergleich der aussage des histologisch und biochemisch bestimmten LeberFettgehaltes; pp. 327–351. [Google Scholar]
- 12.Bobe G, Young JW, Beitz DC. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J Dairy Sci. 2004;87:3105–3124. doi: 10.3168/jds.S0022-0302(04)73446-3. [DOI] [PubMed] [Google Scholar]
- 13.Kalaitzakis E, Roubies N, Panousis N, Pourliotis K, Kaldrymidou E, Karatzias H. Clinicopathologic evaluation of hepatic lipidosis in peri-parturient dairy cattle. J Vet Intern Med. 2007;21:835–845. doi: 10.1892/0891-6640(2007)21[835:ceohli]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Thefeld W, Hoftmeister H, Bush EW, Koller PU, Vollmar J. Referenzwerte for die bestimmung der transaminasen GOT und GPT sowie der alkalischen phosphatase in serum mit oprimierten standard methoden. Deut Med Woch. 1974;99:343–351. doi: 10.1055/s-0028-1107760. [DOI] [PubMed] [Google Scholar]
- 15.Schlebusch H, Rick N, Lang H, Knedel M. Normalbereiche der activ-itäten klinish wichtiger enzyme. Deut Med Woch. 1974;99:765–766. doi: 10.1055/s-0028-1107840. [DOI] [PubMed] [Google Scholar]
- 16.Szasz G. Reaction-rate method for γ-glutamyl transferase activity in serum. Clin Chem. 1976;22:2051–2055. [PubMed] [Google Scholar]
- 17.Gerlach U, Hiby W. Sorbitol dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 2. New York: Acad Pr; 1974. pp. 512–523. [Google Scholar]
- 18.Bringaud F, Stripecke R, Frech GC, et al. Mitochondrial glutamate dehydrogenase from Leishmania tarentolae is a guide RNA-binding protein. Mol Cell Biol. 1997;17:3915–3923. doi: 10.1128/mcb.17.7.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horder M, Elser RC, Gerhardt W, Mathieu M, Sampson EJ. International Federation of Clinical Chemistry, Scientific Division Committee on Enzymes: Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes. Part 7. IFCC method for creatine kinase (ATP: creatine N-phosphotransferase, EC 2.7.3.2) Eur J Clin Chem Clin Biochem. 1991;29:435–456. [PubMed] [Google Scholar]
- 20.Gau N. Acetoacetic acid. In: Pesce AJ, Kaplan LA, editors. Methods in Clinical Chemistry. St. Louis, Missouri: Mosby; 1987. pp. 97–100.pp. 101–104. [Google Scholar]
- 21.Tsuchiya R, Fujise H, Nishizono K, Ashida Y, Yamada T, Kobayashi K. Assay of ornithine carbamoyl transferase activity: Modification for application to bovine serum. J Vet Med Sci. 1994;56:21–26. doi: 10.1292/jvms.56.21. [DOI] [PubMed] [Google Scholar]
- 22.Jendrassik L, Grof P. Vereinfachte photometrische methoden zur bestimmung des billirubins. Biochem Z. 1938;297:81–89. [Google Scholar]
- 23.Doumas BT, Watson WA, Biggs HG. Albumin standards the measurement of serum albumin with bromocresol green. Clin Chem. 1971;31:37–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 24.Fawcett JK, Scott JE. A rapid and precise method for determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974;12:226–227. [PubMed] [Google Scholar]
- 26.Barham D, Trinder P. Improved colour reagent for determination of blood glucose by oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 27.Boltz DF, Lueck CH. Phosphorus. In: Boltz DF, editor. Colorimetric Determination of Nonmetals. New York: Wiley Interscience; 1958. pp. 41–46. [Google Scholar]
- 28.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipid from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 29.Eggstein M, Kuhlmann E. Triglycerides and glycerol: Determination after alkaline hydrolysis. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2nd ed. Vol. 3. New York: Acad Pr; 1974. pp. 1825–1831. [Google Scholar]
- 30.West HJ. Liver function of dairy cows in late pregnancy and early lactation. Res Vet Sci. 1989;46:231–237. [PubMed] [Google Scholar]
- 31.Top Van den AM, Tol Van A, Jansen H, Geelen MJ, Beynen AC. Fatty liver in dairy cows post partum is associated with decreased concentration of plasma triacylglycerols and decreased activity of lipoprotein lipase in adipocytes. J Dairy Res. 2005;72:129–137. doi: 10.1017/s0022029905000774. [DOI] [PubMed] [Google Scholar]
- 32.Kauppinen K. ALAT, AP, ASAT, GGT, OCT activities and urea and total bilirubin concentrations in plasma of normal and ketotic dairy cows. Zbl Vet Med A. 1984;8:567–576. doi: 10.1111/j.1439-0442.1984.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 33.Johannsen U, Menger S, Staufenbiel R, Klukas H. Untersuchungen zur morphologie und function der leber von hochleistungskühen 2 wochen post partum. Deut Tierärztl Woch. 1993;100:177–181. [PubMed] [Google Scholar]
- 34.Mehnert E. Anwendung der Infrarotspektroskopie zur Bestimmung des Lipidgehaltes im Leberbioptat des Rindes. Monatsh Veterinärmed. 1987;42:809–811. [Google Scholar]
- 35.Garry FB, Fettman MJ, Curtis CR, Smith JA. Serum bile acid concentrations in dairy cattle with hepatic lipidosis. J Vet Intern Med. 1994;8:432–438. doi: 10.1111/j.1939-1676.1994.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 36.Gül Y, Gründer HD. Gallensäurenbestimmung im blutserum und ihre bedeutung für die leberdiagnostik bei rindern. Deut Tierärztl Woch. 1988;95:140–146. [PubMed] [Google Scholar]
- 37.Rohn M, Tenhagen BA, Hofmann W. Survival of dairy cows after surgery to correct abomasal displacement: 1. Clinical and laboratory parameters and overall survival. J Vet Med A. 2004;51:294–299. doi: 10.1111/j.1439-0442.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 38.Fürll M. Stoffwechselkontrollen und Stoffechelüberwachung bei rindern. Nutztierpraxis Actuell. 2004;9:8–17. [Google Scholar]
- 39.Zerbe H, Schneider N, Leibold W, Wensing T, Kruip TAM, Schuberth HJ. Altered functional and immunophenotypical properties of neutrophilic granulocytes in post partum cows associated with fatty liver. Theriogenology. 2000;54:771–786. doi: 10.1016/S0093-691X(00)00389-7. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Añon M, Bertics J, Luck M, Grummer RR. Peripartum liver triglycerides and plasma metabolites in dairy cows. J Dairy Sci. 1994;77:1521–1528. doi: 10.3168/jds.S0022-0302(94)77092-2. [DOI] [PubMed] [Google Scholar]
- 41.Dale H, Vik-Mo L, Fjellheim P. A field survey of fat mobilization and liver function of dairy cows during early lactation. Relationship to energy balance, appetite and ketosis. Nord Vet med. 1979;3:97–105. [PubMed] [Google Scholar]
- 42.Kauppinen K. Correlation of whole blood concentrations of acetoac-etate, beta-hydroxybutyrate, glucose and milk yield in dairy cows as studied under field conditions. Acta Vet Scand. 1983;24:337–348. doi: 10.1186/BF03546708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayssiguier Y, Mazur A, Gueux E, Roberts CJ. Plasma lipoproteins and fatty liver in dairy cows. Res Vet Sci. 1988;45:389–393. [PubMed] [Google Scholar]
- 44.Holtenius P. Plasma lipids in normal cows around partus and in cows with metabolic disorders with and without fatty liver. Acta Vet Scand. 1989;30:441–445. doi: 10.1186/BF03548021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peek SF, Divers TJ, Guard C, Rath A, Rebhun WC. Hypokalemia, muscle weakness and recumbency in dairy cattle (17 Cases 1991–1998) in Proceedings. Preconvention Seminar 7: Dairy Herd Problem Investigation Strategies American Association of Bovine Practitioners. 36th Annual Conference; Columbus, Ohio. 2003. [Google Scholar]