Signalment is an important component of any neurological examination. In veterinary neurology there are several instances of coat color and coat color patterns that are linked with neurologic and ophthalmic manifestations of disease. In this overview, we describe some of these conditions, in a variety of species with respect to signalment, history, clinical examination findings, and pathophysiology.
Inherited sensorineural deafness related to coat color
Deafness is defined as a loss of hearing. Deafness can be partial or complete, congenital or acquired (1). Deafness can arise from a failure of conduction of sound waves to the inner ear (conduction deafness) or it may result from disease affecting the neural structures of the hearing pathway (sensorineural deafness) (1). In the case of sensorineural deafness, the disease can be congenital or acquired (1). Congenital sensorineural deafness may be related to coat color or coat color pattern (1).
Non-coat color-related inherited sensorineural deafness has been observed as an autosomal-recessive condition associated with congenital vestibular disease in the doberman pinscher (2). Coat color-related sensorineural deafness has been described in numerous species including cats (3–6), dogs (7–17), horses (18), llamas (19), and alpacas (19). A common phenotype for animals with coat color-related sensorineural deafness, regardless of the species, is that they have substantial white patterning or merle- or dappled-colored coats. Furthermore, we know that animals with these predisposing coat color patterns are more likely to be deaf if they have heterochromia irides or blue eyes (3,18–20).
In animals with coat color-related sensorineural deafness, deafness develops soon after birth and may be related to a failure of migration of neural crest cell progeny (melanoblasts) and/or maturation of melanocytes, and/or premature death or dysfunction of melanocytes within the inner ear within a region of the cochlea known as the stria vascularis (21,22). The deafness is complete and may affect both or only 1 ear. The exact mode of inheritance is variable depending on the species and breed of animal. For instance, deafness in white cats or cats with significant white patterning is commonly associated with inheritance of the “white” gene (23), albeit this trait is polygenic; similarly, in paint horses, deafness is likely polygenic though many affected animals are heterozygous for the endothelin receptor type B (EDNRB) gene which is responsible for lethal white foal syndrome (18). In dogs with coat color-related deafness, the disease is polygenic though it is associated with the piebald and merle genes (7,9,10). The mode of inheritance in camelids is unknown though it has been reported only in solid white llamas and alpacas with pale blue irides (19). In humans, inherited deafness associated with pigmentation is reported as a feature in people afflicted with Waardenburg’s syndrome (24) or Tietz syndrome (25). In both Waardenburg and Tietz syndromes, mutations in various genes responsible for melanocyte development and migration, and inherited as autosomal recessive or dominant traits, have been reported (24,25).
Sensorineural deafness, regardless of the cause, can be objectively diagnosed with electrophysiologic testing (1). For animals with coat color-related sensorineural deafness, there is no treatment. The condition, however, is painless and non-progressive. Animals with this form of deafness may, however, have other abnormalities making up a more generalized syndrome seen with particular coat color patterns. For example, dogs homozygous for the merle gene may be deaf and have microphthalmia with other associated ocular conditions and be sterile (26,27).
Lethal white foal syndrome (LWFS) (a.k.a. overo lethal white syndrome)
Foals born with lethal white foal syndrome (LWFS) are typically entirely white, have blue irides, typically are offspring of overo parents (Figure 1), and are usually clinically normal at birth (28–30). Within hours of being born, however, affected foals will show clinical signs of colic and all affected foals will ultimately succumb to the condition. There has been no success reported for any therapy for foals affected with this condition. Postmortem examination of affected foals reveals a poorly developed intestinal tract (31). Histologic examination reveals, further, an absence and/or maldevelopment of segments of the enteric nervous system (29,31).
Figure 1.
Photograph of a frame overo Paint horse. Frame overos have sharply defined, irregular, horizontally oriented white patches. As here, they are often bald-faced and white patches seldom cross the topline, creating a “frame” of non-white coat. [Reprinted with permission under the GNU free documentation licence from Wikipedia (http://en.wikipedia.org/wiki/Overo; image from www.horsevet.co.uk)].
Lethal white foal syndrome is inherited as an autosomal recessive trait. Specifically, foals born with 2 copies of a mutated EDNRB gene are affected (28–30). Endothelin receptors are important in neural crest cell migration (28–30). Neural crest cells are precursor cells to a variety of cell types including melanocytes, neural and glial cells of the peripheral, including the enteric, nervous systems. A consequence of a foal carrying 2 copies of the mutated EDNRB gene is that neural crest cells do not migrate to the skin or the gut appropriately, thus resulting in foals being born with a white hair coat and aganglionic colon. A similar condition, Hirschsprung’s disease (32), exists in humans. In children with Hirschsprung’s disease, surgical resection of the affected colon is performed with variable success (33). Given the high reliance on the colon for digestion in horses, surgical resection is likely not practical and affected foals are humanely euthanized. Importantly, however, affected foals could potentially serve as a translational model of Hirschsprung’s disease with respect to evaluating the efficacy of stem cell therapy. Stem cell therapy has been proposed as a potential therapy in humans (33).
Given that horses can be genotyped for the gene of interest, horses of breeds with paint coat colors, or with Paint horse lineage, should be evaluated prior to breeding, thereby reducing the likelihood of foals being born with this condition and possibly eliminating the mutated EDNRB gene from the population.
Congenital stationary night blindness (CSNB) in Appaloosa horses
Congenital stationary night blindness (CSNB) has been reported in a variety of species including dogs, horses, mice, and humans. As the name indicates, CSNB is a congenital condition. This condition has been described in various breeds of horses (34–36), though we concentrate on the condition described in Appaloosa horses. Regardless of the breed of horse, CSNB is nonprogressive. Affected animals may appear to have cautious behavior in dim-light conditions and may be difficult to train (34–36). Animals may present with neuro-ophthalmic signs including bilateral dorsomedial strabismus and spontaneous nystagmus (34).
The condition that is described in Appaloosa horses is related to their coat color (34). In particular, it has recently been CSNB is made based upon performing a scotopic (dark-adapted) electroretinogram (ERG) (36). Affected animals will have an ERG with an absent b-wave (34,36).
Recently, it has been found that there is differential expression of the transient receptor potential cation channel member 1 (TRMP-1) gene in the skin and retina (37) and that a mutation in the TRMP-1 gene is responsible for both night blindness and leopard complex phenotypes (38). TRPM-1 protein is important in cellular calcium homeostasis, and thus cellular signaling within various cells including neurons and melanocytes, albeit likely body region specific, is likely altered in affected animals.
Coat color dilution lethal (a.k.a. lavender foal syndrome)
Coat color dilution lethal, or lavender foal syndrome, is a coat color-related neurological condition seen in Arabian foals (39,40). As the name implies, foals are born with dilute coat color. The coat color may appear silver, pewter, “lavender,” or “pink” (39,40). Affected foals display a variety of neurological abnormalities upon birth, including intermittent or consistent opisthotonus, intermittent paddling movements of the limbs, limb extensor muscle rigidity, ocular strabismus, and spontaneous nystagmus (39,40). Spinal reflexes may be exaggerated and animals have exaggerated responses to tactile stimulation (39,40). Foals are unable to right themselves from a laterally recumbent position (39,40). Foals typically have a suck reflex, though the reflex may be weak in some instances (39,40). Foals have seemingly appropriate mentation (40). Given the severity and permanence of clinical signs, and that there is no cure or treatment for this condition, foals are euthanized soon after birth (39,40). Differential diagnoses for newborn foals with neurological signs similar, though not identical to those with lavender foal syndrome, include hypoxic ischemic encephalopathy or neonatal septicemia (40). The obvious difference between these differential diagnoses and lavender foal syndrome is the dilute coat color in foals affected with lavender foal syndrome, albeit other clinical and laboratory findings are important in differentiating these conditions.
A single base deletion mutation in the myosin 5A (MYO5A) gene has recently been demonstrated in affected animals. Specifically, the disease is transmitted as an autosomal recessive trait (41). It was shown, using a relatively small sample size, that 6/58 Egyptian Arabians (10.3%) and 1/56 non-Egyptian Arabians (1.8%) were carriers of this mutation (41). The MYO5A gene is responsible for producing myosin-5a which is important in vesicle trafficking. Vesicle trafficking is important in melanocytes and neurons, thus interference accounting for the phenotype of dilute coat color in combination with neurologic abnormalities. Mutations in the MYO5A gene have been reported to cause Griscelli syndrome in humans – a disease characterized by hypopigmentation, neurologic abnormalities, and immunodeficiency (42–44). Submission of blood for testing is possible and animals can be screened for the mutation prior to breeding, thus preventing occurrence of this disease and ideally affording the ability to eradicate this disease from Arabian horses, and presumably part-bred Arabian horses also.
Inherited strabismus and nystagmus in cats
Albinism may be complete (complete lack of pigmentation) or partial [a reduction in the degree of pigmentation (may be regional)]. Albinism may result from the failure of migration of neural crest cells (precursor of melanocytes) and hence result in reduced or absent melanocytes in a nonpigmented area, or may result from reduced melanin production [for example, gene mutation for enzymes involved in melanin production (such as tyrosinase)] (45).
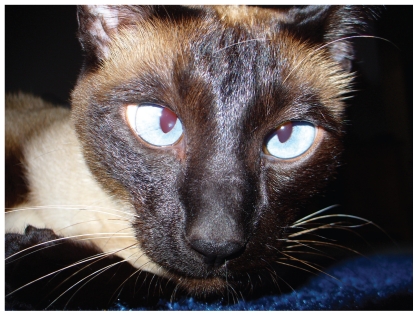
Congenital inherited strabismus and spontaneous nystagmus (CISSN) is commonly thought of as occurring in Siamese cats (Figure 3). Siamese coat color patterning is a form of albinism where mutation in the gene encoding tyrosinase (TYR) results in a temperature sensitive expression of TYR (46). In Siamese cats, cooler extremities have normal expression of TYR and hence are pigmented (mask of face, tips of ears, paws, and tail) (46). Meanwhile, warmer areas of the animal’s body have reduced TYR expression and hence are poorly pigmented (for example, body). For the purposes of our discussion, it is important to recognize, however, that various neuro-ophthalmic and visual abnormalities have been linked with various forms of albinism in other mammalian species (such as, white tigers, albino primates, and albino cats) (47–50).
Figure 3.
Photograph of a siamese cat with convergent strabismus. Note that the convergent strabismus in this Siamese cat (reprinted with permission under the Creative Commons Attribution – Share Alike 2.5 generic licence). (http://commons.wikimedia.org/wiki/File:Siamese_Cat_Cross-Eyed.jpg).
These neuro-ophthalmic and visual disturbances result from abnormal projections of axons from the temporal retinal ganglion cells to the lateral geniculate nucleus. Specifically, while in unaffected cats axons arise from the temporal retina terminate in the ipsilateral lateral geniculate nucleus, in Siamese and albino cats these axons terminate in the contralateral lateral geniculate nucleus (51). In the Siamese cat, the ultimate effect of this retinal ganglion cell axon misdirection is neuroanatomic alteration in the projections to the visual cortex and hence deficits in stereoscopic vision (depth perception) and the existence of nasal visual field blindness (51). Additionally, Siamese cats, as with other albinotic animals, also have misguided projections of axons to other brain structures important in visuomotor reflexes and the integration and coordination of eye and head movements (51). Interestingly, retinal ganglion cell axon misrouting has been corrected by the insertion of a functional tyrosinase (responsible for pigment production) gene in mice and rabbits (51). Consequently, it can be surmised that congenital inherited strabismus and spontaneous nystagmus (CISSN) is a result of functional mutations in the tyrosinase gene.
Multiple congenital ocular anomaly (MCOA) syndrome in horses
A decade ago, a condition affecting American Rocky Mountain horses was described (52). Since this time, the condition has also been reported in purebred and cross-bred Rocky and Kentucky Mountain horses in Canada (53). Other breeds affected by this syndrome include Mountain Pleasure horse, Morgans, Belgians, and American miniature horses (52,54). Animals can be affected by one of 2 different phenotypes. In the 1st phenotype (cyst phenotype) animals have cysts arising from the ciliary body, peripheral retina and/or iris (54). These animals may also have concomitant retinal dysplasia and/or retinal detachment. In the 2nd phenotype [multiple congenital ocular anomaly (MCOA) phenotype], these animals have all of the ocular anomalies seen in the cyst phenotype (54). Multiple congenital ocular anomaly phenotype horses may also have any combination or permutation of congenital cataracts, cornea globosa, iris hypoplasia, iridocorneal angle abnormalities, among others (52). As a consequence of these abnormalities horses may have varying degrees of vision loss and internal ophthalmoparesis (weakness of the iris muscles) (52,53). Interestingly, intraocular pressures of affected horses are not different from those of unaffected horses (52). This condition is common in horses that have a silver coat color (52). It is not definitively known whether the mutation responsible for the silver coat color is the same as for MCOA; additional studies are underway to examine this hypothesis more carefully (54). Nevertheless, MCOA is seen more commonly in horses with silver coat coloration (Figure 4) (52). Multiple congenital ocular anomaly has been mapped to a specific region on horse chromosome 6 and has been confirmed as having a codominant mode of inheritance (54). Once a definitive causative mutation is identified, animals will be able to be genetically screened thereby permitting prevention of this disease in future offspring.
Figure 4.
A silver-colored Rocky Mountain Horse. The typical shiny white mane and tail as well as a slightly diluted body color with dapples is seen in this genetically black silver-colored horse. The phenotype is caused by dilution of eumelanin in the hair to white or grey. The dilution is most visible in the long hairs of the mane and tail. The horse has also been diagnosed with MCOA. [Reprinted with permission under the Creative Commons Attribution Licence; from (54)].
In conclusion, neurologic and neuro-ophthalmic diseases associated with coat color are not uncommon in our domesticated species. Being cognizant of the clinical manifestations of these diseases and the coat color phenotype will hasten reaching a diagnosis and being able to offer advice to clients owning affected animals.
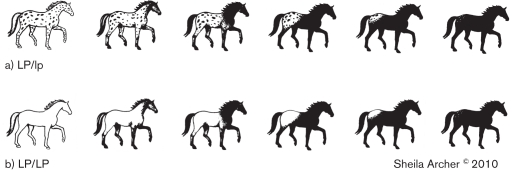
Figure 2.
White pattern continuum for heterozygote (LP/lp) and homozygote (LP/LP) leopard complex gene. Note that there is a continuum of white pattern in both heterozygote and homozygote horses. Horses homozygous for Lp, however, have little to no pigmented spotting within the white patterned areas. (Reproduced with permission from Sheila Archer).
Table 1.
Useful weblinks related to coat color-related neurologic and neuro-ophthalmic conditions
| Disease | Useful links |
|---|---|
| Deafness (all forms) | Deafness in Dogs and Cats© George Strain www.lsu.edu/deafness/deaf.htm |
| Lethal white foal syndrome | Animal Genetics Inc www.animalgenetics.us/ UC Davis Veterinary Genetics Laboratory www.vgl.ucdavis.edu |
| Equine silver dilution gene | UC Davis Veterinary Genetics Laboratory |
| Lavender foal syndrome | Brooks Equine Genetics Laboratory www.ansci.cornell.edu/brooks/index.html The Onderstepoort Veterinary Genetics Laboratory web.up.ac.za/default.asp?ipkCategoryID=11671 |
| Feline coat color (Siamese mutation, etc) | UC Davis Veterinary Genetics Laboratory |
| Canine merle gene | Idexx Laboratories idexx.ca/diseasesinseason_profile.cfm |
| Information on canine coat color genetics | Genetics of coat color and type in dogs© Sheila Schmutz homepage.usask.ca/~schmutz/dogcolors.html |
| Information pertaining to stationary night blindness and other projects involving Appaloosa horses | The Appaloosa Project www.appaloosaproject.info/ |
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Webb AA. Brainstem auditory evoked response (BAER) testing in animals. Can Vet J. 2009;50:313–318. [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkes MK, Palmer AC. Congenital deafness and vestibular deficit in the Doberman. J Small Anim Pract. 1992;33:218–224. [Google Scholar]
- 3.Cvejic D, Steinberg TA, Kent MS, Fischer A. Unilateral and bilateral congenital sensorineural deafness in client-owned pure-breed white cats. J Vet Intern Med. 2009;23:392–395. doi: 10.1111/j.1939-1676.2008.0262.x. [DOI] [PubMed] [Google Scholar]
- 4.Ryugo DK, Cahill HB, Rose LS, et al. Separate forms of pathology in the cochlea of congenitally deaf white cats. Hear Res. 2003;181:73–84. doi: 10.1016/s0378-5955(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 5.Mair IW. Hereditary deafness in the white cat. Acta Otolaryngol Suppl. 1973;314:1–48. [PubMed] [Google Scholar]
- 6.Bergsma DR, Brown KS. White fur, blue eyes, and deafness in the domestic cat. J Hered. 1971;62:171–185. doi: 10.1093/jhered/62.3.171. [DOI] [PubMed] [Google Scholar]
- 7.Strain GM, Clark LA, Wahl JM, Turner AE, Murphy KE. Prevalence of deafness in dogs heterozygous or homozygous for the merle allele. J Vet Intern Med. 2009;23:282–286. doi: 10.1111/j.1939-1676.2008.0257.x. [DOI] [PubMed] [Google Scholar]
- 8.Famula TR, Cargill EJ, Strain GM. Heritability and complex segregation analysis of deafness in Jack Russell terriers. BMC Vet Res. 2007;3:31. doi: 10.1186/1746-6148-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platt S, Freeman J, di Stefani A, Wieczorek L, Henley W. Prevalence of unilateral and bilateral deafness in border collies and association with phenotype. J Vet Intern Med. 2006;20:1355–1362. doi: 10.1892/0891-6640(2006)20[1355:pouabd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Strain GM. Deafness prevalence and pigmentation and gender associations in dog breeds at risk. Vet J. 2004;167:23–32. doi: 10.1016/s1090-0233(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 11.Coppens AG, Resibois A, Poncelet L. Bilateral deafness in a maltese terrier and a great pyrenean puppy: Inner ear morphology. J Comp Pathol. 2000;122:223–228. doi: 10.1053/jcpa.1999.0360. [DOI] [PubMed] [Google Scholar]
- 12.Strain GM. Aetiology, prevalence and diagnosis of deafness in dogs and cats. Br Vet J. 1996;152:17–36. doi: 10.1016/s0007-1935(96)80083-2. [DOI] [PubMed] [Google Scholar]
- 13.Holliday TA, Nelson HJ, Williams DC, Willits N. Unilateral and bilateral brainstem auditory-evoked response abnormalities in 900 Dalmatian dogs. J Vet Intern Med. 1992;6:166–174. doi: 10.1111/j.1939-1676.1992.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 14.Sims MH, Shull-Selcer E. Electrodiagnostic evaluation of deafness in two English setter littermates. J Am Vet Med Assoc. 1985;187:398–404. [PubMed] [Google Scholar]
- 15.Anniko M, Fabiansson E, Nilsson O. Deafness in an old English sheep-dog. A case report. Arch Otorhinolaryngol. 1977;218:1–7. doi: 10.1007/BF00469728. [DOI] [PubMed] [Google Scholar]
- 16.Anderson H, Henricson B, Lundquist PG, Wedenberg E, Wersall J. Genetic hearing impairment in the Dalmatian dog. An audiometric, genetic and morphologic study in 53 dogs. Acta Otolaryngol. 1968;(Suppl–34) [PubMed] [Google Scholar]
- 17.Roberts SR. Color dilution and hereditary defects in collie dogs. Am J Ophthalmol. 1967;63:1762–1775. doi: 10.1016/0002-9394(67)93663-x. [DOI] [PubMed] [Google Scholar]
- 18.Magdesian KG, Williams DC, Aleman M, Lecouteur RA, Madigan JE. Evaluation of deafness in American Paint horses by phenotype, brainstem auditory-evoked responses, and endothelin receptor B genotype. J Am Vet Med Assoc. 2009;235:1204–1211. doi: 10.2460/javma.235.10.1204. [DOI] [PubMed] [Google Scholar]
- 19.Gauly M, Vaughan J, Hogreve SK, Erhardt G. Brainstem auditory-evoked potential assessment of auditory function and congenital deafness in llamas (Lama glama) and alpacas (L pacos) J Vet Int Med. 2005;19:756–760. doi: 10.1892/0891-6640(2005)19[756:bapaoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Strain GM, Kearney MT, Gignac IJ, et al. Brainstem auditory-evoked potential assessment of congenital deafness in Dalmatians: Associations with phenotypic markers. J Vet Intern Med. 1992;6:175–182. doi: 10.1111/j.1939-1676.1992.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 21.Steel KP, Barkway C. Another role for melanocytes: Their importance for normal stria vascularis development in the mammalian inner ear. Development. 1989;107:453–463. doi: 10.1242/dev.107.3.453. [DOI] [PubMed] [Google Scholar]
- 22.Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geigy CA, Heid S, Steffen F, et al. Does a pleiotropic gene explain deafness and blue irises in white cats? Vet J. 2007;173:548–553. doi: 10.1016/j.tvjl.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Pingault V, Ente D, stot-Le MF, et al. Review and update of mutations causing Waardenburg syndrome. Hum Mutat. 2010;31:391–406. doi: 10.1002/humu.21211. [DOI] [PubMed] [Google Scholar]
- 25.Smith SD, Kelley PM, Kenyon JB, Hoover D. Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J Med Genet. 2000;37:446–448. doi: 10.1136/jmg.37.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelatt KN, Powell NG, Huston K. Inheritance of microphthalmia with coloboma in the Australian shepherd dog. Am J Vet Res. 1981;42:1686–1690. [PubMed] [Google Scholar]
- 27.Gelatt KN, McGill LD. Clinical characteristics of microphthalmia with colobomas of the Australian shepherd dog. J Am Vet Med Assoc. 1973;162:393–396. [PubMed] [Google Scholar]
- 28.Yang GC, Croaker D, Zhang AL, et al. A dinucleotide mutation in the endothelin-B receptor gene is associated with lethal white foal syndrome (LWFS); a horse variant of Hirschsprung disease. Hum Mol Genet. 1998;7:1047–1052. doi: 10.1093/hmg/7.6.1047. [DOI] [PubMed] [Google Scholar]
- 29.Metallinos DL, Bowling AT, Rine J. A missense mutation in the endothelin-B receptor gene is associated with Lethal White Foal Syndrome: An equine version of Hirschsprung disease. Mamm Genome. 1998;9:426–431. doi: 10.1007/s003359900790. [DOI] [PubMed] [Google Scholar]
- 30.Santschi EM, Purdy AK, Valberg SJ, et al. Endothelin receptor B polymorphism associated with lethal white foal syndrome in horses. Mamm Genome. 1998;9:306–309. doi: 10.1007/s003359900754. [DOI] [PubMed] [Google Scholar]
- 31.McCabe L, Griffin LD, Kinzer A, et al. Overo lethal white foal syndrome: Equine model of aganglionic megacolon (Hirschsprung disease) Am J Med Genet. 1990;36:336–340. doi: 10.1002/ajmg.1320360319. [DOI] [PubMed] [Google Scholar]
- 32.Tam PK, Garcia-Barcelo M. Genetic basis of Hirschsprung’s disease. Pediatr Surg Int. 2009;25:543–558. doi: 10.1007/s00383-009-2402-2. [DOI] [PubMed] [Google Scholar]
- 33.Thapar N. New frontiers in the treatment of Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2009;48 (Suppl 2):S92–S94. doi: 10.1097/MPG.0b013e3181a15d62. [DOI] [PubMed] [Google Scholar]
- 34.Sandmeyer LS, Breaux CB, Archer S, Grahn BH. Clinical and electroretinographic characteristics of congenital stationary night blindness in the Appaloosa and the association with the leopard complex. Vet Ophthalmol. 2007;10:368–375. doi: 10.1111/j.1463-5224.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 35.Nunnery C, Pickett JP, Zimmerman KL. Congenital stationary night blindness in a Thoroughbred and a Paso Fino. Vet Ophthalmol. 2005;8:415–419. doi: 10.1111/j.1463-5224.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 36.Witzel DA, Smith EL, Wilson RD, Aguirre GD. Congenital stationary night blindness: An animal model. Invest Ophthalmol Vis Sci. 1978;17:788–795. [PubMed] [Google Scholar]
- 37.Bellone RR, Brooks SA, Sandmeyer L, et al. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellone RR, Forsyth G, Leeb T, et al. Fine-mapping and mutation analysis of TRPM1: A candidate gene for leopard complex (LP) spotting and congenital stationary night blindness in horses. Brief Funct Genomic Proteomic. 2010 doi: 10.1093/bfgp/elq002. [DOI] [PubMed] [Google Scholar]
- 39.Page P, Parker R, Harper C, Guthrie A, Neser J. Clinical, clinicopathologic, postmortem examination findings and familial history of 3 Arabians with lavender foal syndrome. J Vet Intern Med. 2006;20:1491–1494. doi: 10.1892/0891-6640(2006)20[1491:ccpefa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Fanelli HH. Coat color dilution lethal (‘lavender foal syndrome’): A tetany syndrome of Arabian foals. Equine Vet Educ. 2005;17:260–263. [Google Scholar]
- 41.Brooks SA, Gabreski N, Miller D, Brisbin A, Brown HE, Streeter C, et al. Whole genome SNP association in the horse: Identification of a deletion in myosin Va responsible for lavender foal syndrome. PLoS Genetic. 2010 doi: 10.1371/journal.pgen.1000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takagishi Y, Murata Y. Myosin Va mutation in rats is an animal model for the human hereditary neurological disease, Griscelli syndrome type 1. Ann N Y Acad Sci. 2006;1086:66–80. doi: 10.1196/annals.1377.006. [DOI] [PubMed] [Google Scholar]
- 43.Menasche G, Ho CH, Sanal O, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1) J Clin Invest. 2003;112:450–456. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastural E, Barrat FJ, Dufourcq-Lagelouse R, et al. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genet. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- 45.Dessinioti C, Stratigos AJ, Rigopoulos D, Katsambas AD. A review of genetic disorders of hypopigmentation: Lessons learned from the biology of melanocytes. Exp Dermatol. 2009;18:741–749. doi: 10.1111/j.1600-0625.2009.00896.x. [DOI] [PubMed] [Google Scholar]
- 46.Lyons LA, Imes DL, Rah HC, Grahn RA. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus) Anim Genet. 2005;36:119–126. doi: 10.1111/j.1365-2052.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 47.Bernays ME, Smith RI. Convergent strabismus in a white Bengal tiger. Aust Vet J. 1999;77:152–155. doi: 10.1111/j.1751-0813.1999.tb11220.x. [DOI] [PubMed] [Google Scholar]
- 48.Guillery RW, Kaas JH. Genetic abnormality of the visual pathways in a “white” tiger. Science. 1973;180:1287–1289. doi: 10.1126/science.180.4092.1287. [DOI] [PubMed] [Google Scholar]
- 49.Creel D, Hendrickson AE, Leventhal AG. Retinal projections in tyrosinase-negative albino cats. J Neurosci. 1982;2:907–911. doi: 10.1523/JNEUROSCI.02-07-00907.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gross KJ, Hickey TL. Abnormal laminar patterns in the lateral geniculate nucleus of an albino monkey. Brain Res. 1980;190:231–237. doi: 10.1016/0006-8993(80)91172-5. [DOI] [PubMed] [Google Scholar]
- 51.Kaas JH. Serendipity and the Siamese cat: The discovery that genes for coat and eye pigment affect the brain. ILAR J. 2005;46:357–363. doi: 10.1093/ilar.46.4.357. [DOI] [PubMed] [Google Scholar]
- 52.Ramsey DT, Ewart SL, Render JA, Cook CS, Latimer CA. Congenital ocular abnormalities of Rocky Mountain Horses. Vet Ophthalmol. 1999;2:47–59. doi: 10.1046/j.1463-5224.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- 53.Grahn BH, Pinard C, Archer S, et al. Congenital ocular anomalies in purebred and crossbred Rocky and Kentucky Mountain horses in Canada. Can Vet J. 2008;49:675–681. [PMC free article] [PubMed] [Google Scholar]
- 54.Andersson LS, Juras R, Ramsey DT, et al. Equine Multiple Congenital Ocular Anomalies maps to a 4.9 megabase interval on horse chromosome 6. BMC Genet. 2008;9:88. doi: 10.1186/1471-2156-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]