Abstract
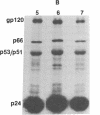
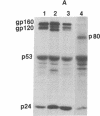
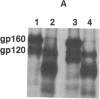
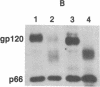
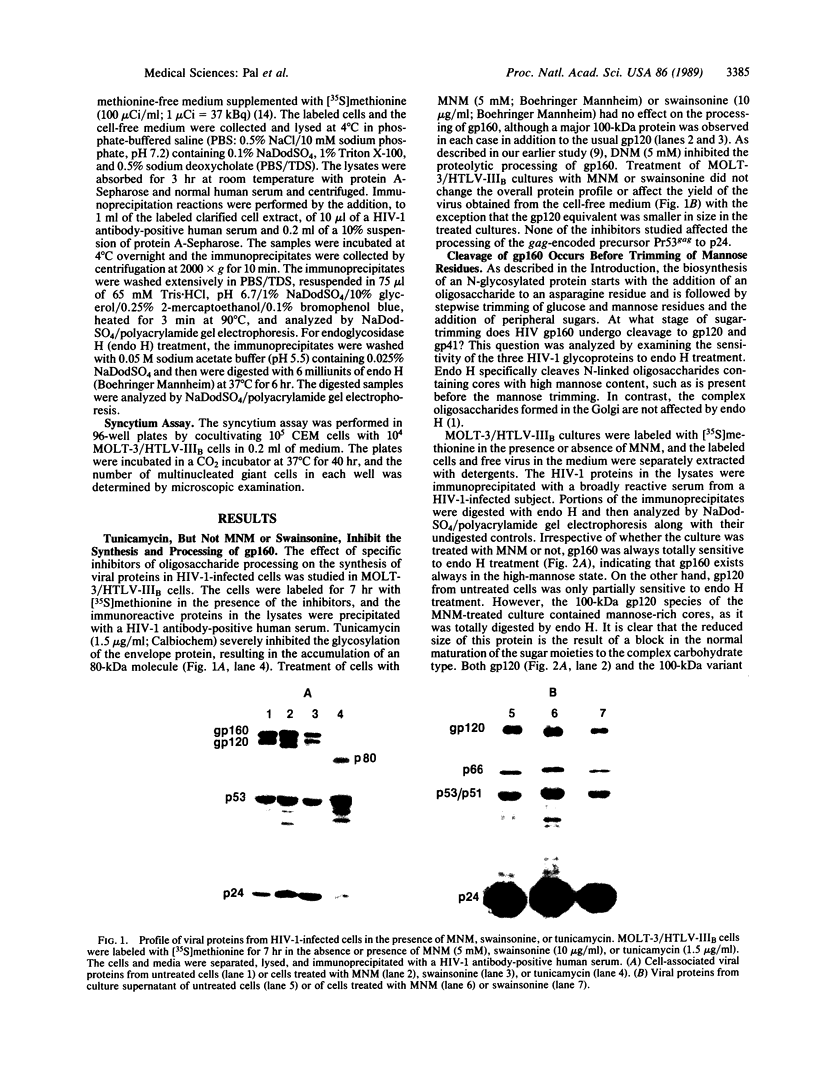
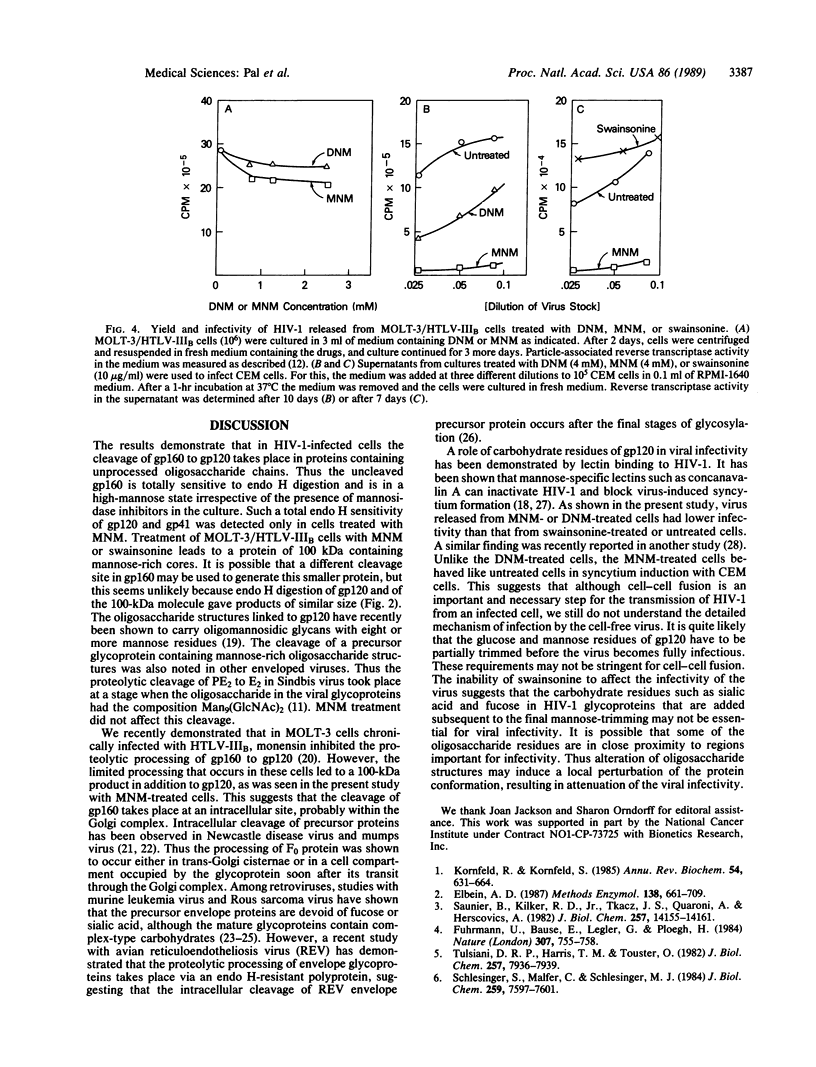
The processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) were studied in infected cells treated with inhibitors of oligosaccharide processing. In MOLT-3 cells chronically infected with HIV-1 (strain HTLV-IIIB), tunicamycin severely inhibited the glycosylation of envelope proteins. Deoxynojirimycin, an inhibitor of glucosidase I in the rough endoplasmic reticulum, inhibited the proteolytic processing of gp160, whereas no such effect was noted with either deoxymannojirimycin or swainsonine, inhibitors of mannosidase I and II, respectively, in the Golgi complex. The processed gp120 and gp41 synthesized in the presence of deoxymannojirimycin were found to contain mannose-rich oligosaccharide cores as evidenced by their susceptibility to endoglycosidase H digestion. The formation of syncytia normally observed when CEM cells are cocultured with HIV-1-infected cells was markedly inhibited in the presence of deoxynojirimycin, but such inhibition was not observed in cells treated with deoxymannojirimycin or swainsonine. The infectivity of virions released from MOLT-3/HTLV-IIIB cells treated with deoxynojirimycin or deoxymannojirimycin was significantly lower than the infectivity of virions released from untreated cells. On the other hand, treatment with swainsonine did not affect the infectivity of the progeny virus. These results suggest that the proteolytic processing of gp160 takes place in infected cells when the glycoprotein has mannose-rich oligosaccharide structures. Trimming of glucose residues and the primary trimming of mannose residues are necessary for the release of infectious virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Bosch J. V., Schwarz R. T., Ziemiecki A., Friis R. R. Oligosaccharide modifications and the site of processing of gPr92env, the precursor for the viral glycoproteins of Rous sarcoma virus. Virology. 1982 May;119(1):122–132. doi: 10.1016/0042-6822(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Glycosylation inhibitors for N-linked glycoproteins. Methods Enzymol. 1987;138:661–709. doi: 10.1016/0076-6879(87)38060-7. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Legler G., Tlusty A., McDowell W., Schwarz R. The effect of deoxymannojirimycin on the processing of the influenza viral glycoproteins. Arch Biochem Biophys. 1984 Dec;235(2):579–588. doi: 10.1016/0003-9861(84)90232-7. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lifson J., Coutré S., Huang E., Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV) infectivity and virus-induced cell fusion. J Exp Med. 1986 Dec 1;164(6):2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell W., Romero P. A., Datema R., Schwarz R. T. Glucose trimming and mannose trimming affect different phases of the maturation of Sindbis virus in infected BHK cells. Virology. 1987 Nov;161(1):37–44. doi: 10.1016/0042-6822(87)90168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Mitchell W. M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T., Ward L. J., Semerjian A. Intracellular processing of the Newcastle disease virus fusion glycoprotein. J Virol. 1985 Mar;53(3):851–857. doi: 10.1128/jvi.53.3.851-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Gallo R. C., Sarngadharan M. G. Processing of the structural proteins of human immunodeficiency virus type 1 in the presence of monensin and cerulenin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9283–9286. doi: 10.1073/pnas.85.23.9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Kalyanaraman V. S., Hoke G. M., Sarngadharan M. G. Processing and secretion of envelope glycoproteins of human immunodeficiency virus type 1 in the presence of trimming glucosidase inhibitor deoxynojirimycin. Intervirology. 1989;30(1):27–35. doi: 10.1159/000150073. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Evidence that mannosyl residues are involved in human immunodeficiency virus type 1 (HIV-1) pathogenesis. AIDS Res Hum Retroviruses. 1987 Fall;3(3):265–282. doi: 10.1089/aid.1987.3.265. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Tung J. S., Hopkins N., Robbins P. W. Relationship of GIX antigen expression to the glycosylation of murine leukemia virus glycoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6420–6424. doi: 10.1073/pnas.77.11.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Schlesinger S., Malfer C., Schlesinger M. J. The formation of vesicular stomatitis virus (San Juan strain) becomes temperature-sensitive when glucose residues are retained on the oligosaccharides of the glycoprotein. J Biol Chem. 1984 Jun 25;259(12):7597–7601. [PubMed] [Google Scholar]
- Tsai W. P., Oroszlan S. Novel glycosylation pathways of retroviral envelope proteins identified with avian reticuloendotheliosis virus. J Virol. 1988 Sep;62(9):3167–3174. doi: 10.1128/jvi.62.9.3167-3174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A., Takeuchi K., Hishiyama M. Intracellular processing of mumps virus glycoproteins. Virology. 1988 Jul;165(1):268–273. doi: 10.1016/0042-6822(88)90681-2. [DOI] [PubMed] [Google Scholar]