Abstract
Cognitive aging studies have suggested that older adults show declines in both sustained and transient cognitive control processes. However, previous neuroimaging studies have primarily focused on age-related change in the magnitude, but not temporal dynamics, of brain activity. The present study compared brain activity dynamics in healthy old and young adults during task switching. A mixed blocked/event-related functional magnetic resonance imaging design enabled separation of transient and sustained neural activity associated with cognitive control. Relative to young adults, older adults exhibited not only decreased sustained activity in the anterior prefrontal cortex (aPFC) during task-switching blocks but also increased transient activity on task-switch trials. Another pattern of age-related shift in dynamics was present in the lateral PFC (lPFC) and posterior parietal cortex (PPC), with younger adults showing a cue-related response during task-switch trials in lPFC and PPC, whereas older adults exhibited switch-related activation during the cue period in PPC only. In all 3 regions, these qualitatively distinct patterns of brain activity predicted qualitatively distinct patterns of behavioral performance across the 2 age groups. Together, these results suggest that older adults may shift from a proactive to reactive cognitive control strategy as a means of retaining relatively preserved behavioral performance in the face of age-related neurocognitive changes.
Keywords: cognitive control, mixed blocked/event-related fMRI, parietal cortex, prefrontal cortex, task set
Introduction
Impairments in executive control form a core component of age-related cognitive decline (Craik and Salthouse 2007). Cognitive neuroscience studies have demonstrated not only age-related shifts in the magnitude of neural responses, including reduced activation in many of the brain regions that support executive control, such as lateral prefrontal cortex (lPFC) (e.g., Logan et al. 2002), but also activity increases in widespread regions that are typically interpreted in terms of a compensation pattern (e.g., Cabeza et al. 2002; Persson et al. 2004) or a breakdown of inhibitory mechanisms (e.g., Dickerson et al. 2004; Gazzaley et al. 2005). In the current report, we focus on how an analysis of the temporal dynamics of brain activity, rather than simple activation magnitudes, might provide new insights into the nature of age-related changes in executive control.
Experimental studies of multitasking or task switching have become one of the most popular means of studying executive control. In task-switching paradigms, individuals are required to perform 2 or more different tasks in a rapidly intermixed manner (Jersild 1927; Allport et al. 1994; Rogers and Monsell 1995), with an advance cue typically indicating the task to perform on the upcoming trial (Meiran 1996). These paradigms place a high demand on both sustained and transient cognitive control, with the former involved in actively maintaining the relevant task sets or rules in a highly accessible state and the latter involved in preparatory and updating processes occurring when the next task and/or a task switch is indicated. Behavioral studies have demonstrated reliable age-related declines in performance. Interestingly, the declines have often been observed in terms of mixing costs that index sustained changes during task-switching block (De Jong 2001; Reimers and Maylor 2005; Verhaeghen and Basak 2005). However, other studies have focused on age-related changes in switching costs that may reflect transient processes occurring during task-switch trials (Salthouse et al. 1998; Kramer et al. 1999; Mayr 2001; Kray et al. 2002). The behavioral literature thus suggests cognitive control changes in both sustained and transient components during aging and further that these are potentially dissociable (Mayr 2001; Braver and West 2007).
Neuroimaging methods (e.g., functional magnetic resonance imaging [fMRI] and event-related potential [ERP]) provide a more direct window into the temporal dynamics of executive control processes during task switching and how they might change with age. ERP studies have consistently observed distinct frontal and parietal components not only associated with task-switch trials (Friedman et al. 2008; West and Travers 2008) but also with mixed-task blocks (i.e., both switch and repeat trials) compared with single-task blocks. Moreover, in older adults, cue-related potentials show increased latency, whereas target-related potentials were found to be of higher amplitude, potentially indicating a compensatory process (Kray et al. 2005). Event-related fMRI studies have reliably also observed transient cue-related activation during task switching within posterior parietal cortex (PPC) and lPFC (Dove et al. 2000; Kimberg et al. 2000; MacDonald et al. 2000; Rushworth et al. 2002; Sakai and Passingham 2003; Crone et al. 2006). Several fMRI studies have also examined task switching in older adults and confirmed that age-related changes in activity magnitude are most prominent in the lPFC and PPC regions (DiGirolamo et al. 2001; Gold et al. 2008). However, the studies have utilized blocked designs, which do not permit an examination of age-related changes in the temporal dynamics of brain activation.
The mixed blocked/event-related fMRI design may be the ideal one for dissociating distinct task-switching control processes because it enables decomposition of activity dynamics during task blocks into both transient (i.e., trial related) and sustained (i.e., present during intertrial intervals [ITIs]) components (Visscher et al. 2003). Using this approach, we found sustained activity in anterior PFC (aPFC) during mixed-task blocks that was dissociable from the transient responses in PPC and lPFC (Braver et al. 2003). The approach has also been employed with older adults in a study of long-term memory retrieval (Dennis et al. 2007), with older adults showing a reduction in sustained activity within the PFC. This finding suggests that older adults may fail to initiate sustained control processes that preserve flexible and rapid task performance under cognitively demanding conditions. However, the reduction in sustained activity in that study was not linked to additional changes in transient activity. It is thus possible that, in older adults, the disruption of the sustained processes may result in behavioral impairment or be compensated by transient processes by changing task strategy (Braver and West 2007).
In the current study, we utilized the mixed blocked/event-related fMRI design to compare the brain activity dynamics of older and younger adults during task switching. Specifically, we tested the hypothesis that aging would lead to a shift in the temporal dynamics of brain activity, such that under switching conditions older adults would show not only reduced sustained activity but also increased transient activity in brain regions supporting cognitive control (e.g., aPFC, lPFC, and PPC), potentially as a compensatory mechanism. Moreover, we predicted that older adults would also exhibit impairments in responding to cue stimuli on task-switch trials, which would be reflected in additional changes in transient activation dynamics within these regions.
Materials and Methods
Participants
Fourteen right-handed older adults (OLD) and 13 right-handed young adults (YOUNG) participated in the study. Participants were screened for any sign of medical disorders, including treated or untreated hypertension diabetes and thyroid problems, neurological disorders past head injuries involving loss of consciousness for 5 or more minutes or a documented concussions, psychiatric disorders, medication histories that could inference cognitive performance, or any other contraindication for MRI. Older participants were further administered the Blessed Orientation-Memory-Concentration Test (Katzman et al. 1983), and individuals with 5 or more errors did not participate in the present study. All participants gave informed consent per guidelines set by the Washington University Medical Center Human Studies Committee and were paid $25 for each hour of participation. The OLD and YOUNG groups underwent identical experimental procedures as part of the study. The primary results of the YOUNG have been reported previously (Braver et al. 2003). The OLD group consisted of 4 males and 10 females with a mean age of 73 years (age range 65–87 years), and the YOUNG group consisted of 8 males and 5 females with a mean age of 21 years (age range 19–26 years). The educational history of the OLD group was 14.5 ± 2.0 (mean ± standard deviation [SD]), which is comparable with that of the YOUNG group, consisting of undergraduate university students.
It is worth noting that although the gender distribution appeared somewhat different in the 2 groups (more female participants in the OLD group), this bias did not appear to confound our results. Indeed, we reanalyzed both the behavioral and imaging data, statistically controlling for gender in analysis of covariance, and confirmed that none of the results were changed. It is also important that there is virtually no gender difference in brain activity during executive control (Haut and Barch 2006).
Behavioral Tasks
The experimental paradigm that participants performed was identical to our previous report (Braver et al. 2003). Briefly, the paradigm involved 2 semantic classification tasks performed under either single-block or mixed-block conditions. One classification task required a decision as to whether a visually presented word described an object that is either larger (LARGE; e.g., “truck”) or smaller (SMALL; e.g., “carrot”) than a standard computer monitor. The other task required a decision as to whether the object was manmade (e.g., truck) or natural (e.g., carrot). For both tasks, a task cue appeared prior to the target word and signaled the classification judgment to be made (LRG-SML or MAN-NAT). In the mixed-block condition, the classification task to be performed varied randomly from trial to trial. In the single-task condition, only a single task was performed during the entire block. Thus, in the single-task condition, the task cue information could be ignored. All words varied on both the MAN-NAT and LRG-SML dimensions with each possible combination presented with equal frequency (manmade/large, manmade/small, natural/large, and natural/small). Furthermore, in mixed blocks, there were approximately equal numbers of task-switch and task-repeat trials. Moreover, the word list assigned to each task condition (single vs. mixed block) was counterbalanced across participants.
The words for both conditions were presented centrally on a visual display in 36-point Helvetica font. Words were taken from standardized lists of concrete nouns. All words were 3–7 letters in length and consisted of 1 or 2 syllables. Responses to stimuli were made by pressing different buttons on a handheld response box with either the index or the middle finger of the right hand. The stimulus–response mappings were counterbalanced across participants. However, it is important to note the response overlap across tasks, in that the same button marked a particular feature in each of the 2 semantic dimensions (e.g., the right button could indicate that an item was manmade or large and the left button indicate that an item was natural or small). Such response overlap (and associated ambiguity) is considered to be an important feature of task-switching paradigms that contribute to the demands for cognitive control (Meiran et al. 2000; see also Supplementary Materials). Within each trial, the timing and sequence of events was as follows. First, the task cue was presented for 750 ms, followed by a 1750-ms delay. Next, the semantic target word was presented for 2000 ms, during which responses were recorded. Participants were instructed to make a classification decision as quickly and accurately as possible following target onset and indicate this decision with a button press. Next, a variable ITI occurred between 500 and 5500 ms in steps of 2500 ms (mean = 1.75 s, SD = 1.87 s). The variability in ITI allowed for estimation of the event-related hemodynamic response on each trial, as described below (Friston et al. 1995). Each scanning run consisted of 40 trials. Two scanning runs were performed for each condition, yielding 80 single-task trials and 80 mixed-task trials.
Prior to the scanning session, participants were given instructions regarding all tasks to be performed. Participants were then given practice trials in which to perform each task. During practice trials, the experimenter answered any further questions, validated that instructions were understood, and ensured that the tasks were performed appropriately and with a reasonably high level of accuracy.
Functional Imaging
Images were acquired on a Siemens 1.5-T Vision System (Erlangen, Germany) with a standard circularly polarized head coil. A pillow and tape were used to minimize head movement. Headphones dampened scanner noise and enabled communication with participants. Both structural and functional images were acquired at each scan. High-resolution (1.25 × 1 × 1) structural images were acquired using a sagittal magnetization prepared rapid gradient echo 3D T1-weighted sequence (time repetition [TR] = 9.7 mm, time echo [TE] = 4, flip = 12°, time to inversion = 300 ms) (Mugler and Brookeman 1990). Functional images were acquired using an asymmetric spin-echo echo-planar sequence (TR = 2500, TE = 50 ms, flip = 90°). Each image consisted of 18 contiguous, 7-mm-thick axial slices acquired parallel to the anterior–posterior commissure plane (3.75 × 3.75 mm in-plane), allowing complete brain coverage at a high signal-to-noise ratio (Conturo et al. 1996). Participants were scanned in 4 separate scanning runs, with 2 scans each of the single-task and mixed-task conditions. For the single-task condition, 1 run was performed of each classification task (MAN-NAT and LRG-SML). Each run consisted of alternating cycles of task (TSK) and fixation (FIX) blocks with the following structure: FIX, TSK, FIX, TSK, FIX. The inclusion of fixation blocks was an important feature of the scanning design to enable us to conduct mixed blocked and event-related fMRI analyses (see below). Task blocks were 140 s (20 trials) in duration. Fixation blocks (denoted by a centrally presented crosshair) were 37.5 s in duration. Finally, the first 4 images in each scanning run were used to allow the scanner to reach steady state and hence were discarded. Each run lasted approximately 6.5 min, and a 2-min delay occurred between runs, during which time participants rested.
Visual stimuli were presented using PsyScope (Cohen et al. 1993) running on an Apple PowerMac G4. Stimuli were projected to participants with an AmPro LCD projector (model 150) onto a screen positioned at the head end of the bore. Participants viewed the screen through a mirror attached to the head coil. A fiber-optic, light-sensitive key press interfaced with the PsyScope Button Box was used to record participants’ behavioral performance.
Data Analysis
Behavioral performance data were analyzed for age and task-switching effects by conducting analysis of variance (ANOVAs) or t-tests on accuracy and reaction time (RT). Functional imaging data were preprocessed prior to statistical analysis according to the following procedures. All functional images were first temporally aligned across the brain volume, corrected for movement using a rigid-body rotation and translation correction (Friston et al. 1996; Snyder 1996), and then registered to the participant's anatomical images (in order to correct for movement between the anatomical and function scans). The data were then intensity normalized (to an arbitrary value of 1000), resampled into 3-mm isotropic voxels, and spatially smoothed with a 9-mm full-width half-maximum (FWHM) Gaussian kernel. Participants’ structural images were transformed into standardized atlas space (Talairach and Tournoux 1988) using a 12D affine transformation (Woods et al. 1992, 1998). Importantly, the structural images were registered to locally developed template that involved averaging across an equal number of older and younger adult brains to insure that registration was not age biased (Buckner et al. 2004). The functional images were then registered to the reference brain using the alignment parameters derived for the structural scans.
A general linear model (GLM) approach (Friston et al. 1995) was used to estimate parameter values for both event-related (transient) activity and for sustained activity associated with the entire task block. Importantly, the sustained and transient effects are simultaneously and independently coded within the same GLM, which allowed us to dissociate these effects. Sustained effects were coded by a boxcar function using an assumption of a fixed-shape response of long duration (i.e., boxcar convolved with a gamma function; Boynton et al. 1996). Event-related effects were coded by using series of time points along the hemodynamic response epoch. The duration of this epoch was taken to be 20 s (8 scanning frames). These time points were individually estimated in the GLM.
The logic of the GLM estimation approach is that event-related effects will decay back to baseline during the ITI, whereas sustained effects should remain relatively constant and of increased amplitude relative to control (fixation) blocks. This approach to GLM coding of sustained and transient responses has been validated via both simulation and empirically based methodological studies (Visscher et al. 2003). With this GLM coding for the sustained and transient events, the baseline state in the GLM reflects activity during the fixation blocks that were interleaved with the task blocks.
The event-related and sustained estimates for the time course data were then submitted to a group analysis using voxel-wise random-effects model ANOVAs. For analyses directly comparing transient and sustained activity, the magnitude of the event-related response was computed by computing a cross-correlation (i.e., the inner product) with a standard hemodynamic response function (i.e., a gamma function; Boynton et al. 1996), providing comparable measurement with the sustained effect. Note that the time courses in Figure 1c are based on the parameter estimate for each time point, whereas the signal magnitudes in Figure 1b are based on the cross-correlation (see also Supplementary Materials for complementary analysis).
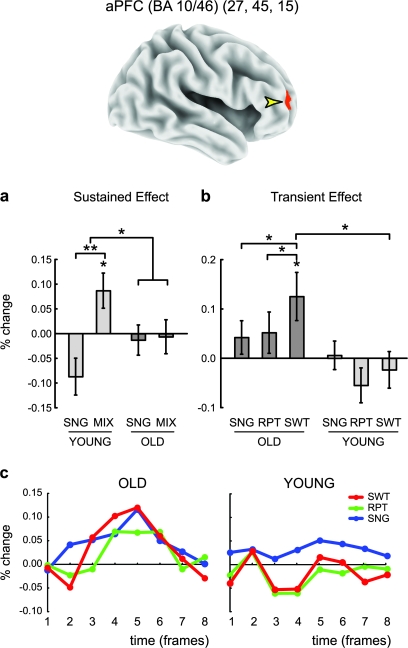
Figure 1.
Sustained and transient activity in the aPFC. The ROI is indicated by a yellow arrow in the 3D surface of the brain. (a) Sustained activity in each age group (OLD and YOUNG) in the single- (SNG) and mixed (MIX)-task blocks (expressed in terms of mean percent signal change relative to fixation). (b) Transient activity in the same region. The format is similar to (a) but showing activity in the mixed block divided into switch (SWT) and repeat (RPT) trial types. The magnitudes were computed by a cross-correlation with a standard hemodynamic response function (see also Materials and methods). The activity in the mixed task is divided into repeat and switch trials. SNG, single; RPT, repeat; SWT, switch. (c) Time course of MRI signal for the transient activity over the course of the trial for the OLD and YOUNG groups in the same region. The x-axis indicates time in fMRI frames (i.e., 2.5-s TRs). Significant differences and interactions are indicated by asterisks (*P < 0.05, **P < 0.01); error bars are standard error of the mean across participants.
The primary analysis approach was region-of-interest (ROI) based. Specifically, analyses focused on 3 ROIs in the aPFC, lPFC, and PPC. These brain regions have repeatedly been activated during task switching in the previous literature (Dove et al. 2000; Kimberg et al. 2000; MacDonald et al. 2000; Sohn et al. 2000; DiGirolamo et al. 2001; Brass and von Cramon 2002, 2004; Bunge et al. 2002; Brass et al. 2003; Braver et al. 2003; Sakai and Passingham 2003, 2006; Reynolds et al. 2004; Yeung et al. 2006). Specifically, these regions were functionally defined based on the coordinates reported in meta-analyses for cognitive control (Table 4 in Wager and Smith 2003; Table II in Owen et al. 2005). Spherical regions with 10 mm radius were then created from each of the seed point coordinates, with the regions merged into a composite for each volume of interest. The aPFC region included the posterior part of Brodmann area (BA) 10 and the most anterior portions of BA 46, the lPFC region included the posterior inferior portion of PFC incorporating BA 44 and BA 45, and the PPC region included the portion of parietal cortex near the boundary of the superior cortex and PPC (BA 7 and BA 40). This ROI-based approach is identical to one we have used in previous studies (Emery et al. 2008; Fales et al. 2008; Locke and Braver 2008); the exact anatomical masks are available from the authors upon request. Within each ROI, voxel clusters were identified and selected for further analyses based on statistical contrasts of interest. These voxel clusters were assessed for significance using the AlphaSim Monte Carlo procedure (http://afni.nimh.nih.gov/afni/), using the anatomically/functionally defined ROI as a small-volume mask. All contiguous voxel clusters reported below passed the threshold for significance (alpha < 0.05) when correcting for multiple comparisons within the small volume.
aPFC Analyses
A set of analyses focused on age differences in aPFC activity. These analyses primarily focused on the age-related differences in activity dynamics in terms of “sustained” and “transient” processes based on the previous findings (Braver et al. 2003; Owen et al. 2005; Dosenbach et al. 2007). The first analysis identified aPFC regions showing significant sustained activity that differed across the 2 age groups using the following 3 contrasts: 1) significantly increased sustained activity in the mixed block relative to the single block in either the OLD or the YOUNG group (P < 0.05); 2) a significant group (OLD and YOUNG) by block (single and mixed) interaction for sustained activity (P < 0.05); and 3) significant group (OLD and YOUNG) by dynamics (sustained and transient) interaction (P < 0.05; c.f., Braver et al. 2003). For this last contrast, the magnitude of the event-related response computed by computing a cross-correlation with a standard hemodynamic response function (Boynton et al. 1996) in order to permit a direct comparison between the sustained and transient patterns. It is important to note that the employed contrast approach is unbiased in terms of age, such that it will identify regions showing sustained activity during the mixed block that satisfy either a YOUNG > OLD or OLD > YOUNG pattern. Moreover, the contrast does not reflect any a priori hypothesis regarding the pattern of transient activity in the aPFC ROI.
A second analysis identified aPFC regions showing significant between-subjects correlations between activation and behavioral performance, examining each age group separately. The following 2 contrasts were used in each analysis: 1) voxel-wise sustained activity in the mixed block relative to the single block (P < 0.05) and 2) a significant voxel-wise correlation coefficient (P < 0.05) between increased sustained activity in the mixed block (i.e., mixed − single) and the behavioral mixing cost effect (repeat trial – single trial reaction time). A separate analysis identified aPFC regions in an analogous way but focused on transient rather than sustained activity related to task switching. Thus, the contrasts identified regions showing 1) voxel-wise event-related activity in the task-switch trials relative to the repeat trials (P < 0.05) and 2) a significant voxel-wise correlation coefficient (P < 0.05) between increased transient switch-related activity (i.e., switch trials − repeat trials) and the behavioral switch cost effect (switch trial - repeat trial reaction time).
By conducting separate voxel-wise analyses for effects of experimental condition and brain–behavior correlations, it is possible to circumvent the inherent trade-off in variance partitioning associated with each analysis. Specifically, the first analysis identifies voxel clusters in which the variance due to experimental conditions is much greater than between-subjects variability. Conversely, in the second analysis, high between-subjects variability is preferred (but which reduces the significance of experimental condition effects).
lPFC and PPC Analyses
Another set of analyses focused on age differences in lPFC and PPC activity. These analyses primarily focused on transient activity dynamics within these regions based on the previous findings (Dove et al. 2000; Kimberg et al. 2000; MacDonald et al. 2000; Sohn et al. 2000; DiGirolamo et al. 2001; Brass and von Cramon 2002, 2004; Bunge et al. 2002; Brass et al. 2003; Braver et al. 2003; Sakai and Passingham 2003, 2006; Wager and Smith 2003; Reynolds et al. 2004; Yeung et al. 2006; Dosenbach et al. 2007). The first analysis identified regions within lPFC and PPC showing significant transient activity related to task switching by identifying voxels with significantly increased event-related response on task-switch trials relative to single-task trials (averaging across YOUNG and OLD groups, P < 0.01), ensuring that the voxel clusters included in the ROIs were unbiased with respect to age.
The use of the single-task trial, instead of task-repeat trial, as control trial would provide appropriate analogue for the sustained effect (mixed vs. single). Moreover, in order to examine cue-related effects, the task-repeat trials might not be an appropriate baseline (although they may be appropriate for examining target-related effects) because both types of trials might involve cue-triggered activation of preparatory control (Gilbert and Shallice 2002; Ruge et al. 2005; Sakai 2008). However, this case is unlikely in single-task trials, for which the cue is always uninformative, again appropriate in control trial with the task-switch trial.
Two subsequent analyses were conducted on the regions identified in this first analysis. One of these decomposed the transient response into both cue-related and target-related components (Fox et al. 2007). Cue-related activity was defined as differences in parameter estimates between the average of frames 2 and 3 and the average of frames 1,7, and 8. Target-related activity was defined as the difference in parameter estimate between the average of frames 4, 5, and 6 and the average of frames 1, 7, and 8. It is possible that some of this activation level was influenced by blood oxygen level–dependent effects bleeding over from the cue period. However, with the time constant of the hemodynamic response (3–6 s), it is still most likely that time points 4–6 were dominated by target rather than cue effects.
Another analysis examined the relationship between trial-by-trial fluctuations in reaction time and the magnitude of the event-related response. Event-related modulations due to reaction time fluctuations were estimated separately for each of the 3 trial types (single task, task repeat, and task switch). Reaction time speed was coded via a binning procedure, with trials sorted into 10 bins (0–9) ranging fastest to slowest in 10% increments. The bin code for each trial was then used as a parametric regressor within the GLM. Based on the regression parameter, the event-related response at each identified ROI was calculated for the fastest and slowest bins. Thus, the estimated effect of RT on event-related activity involved the whole data set (i.e., not just a small subset of extreme fastest and slowest bins) but did allow us to plot the predicted values for the extreme fastest and slowest bins. These estimates were then subjected to statistical analyses to examine whether response speed influenced task-switching effects on brain activation dynamics. This analysis was only applied to examine transient effects and not sustained effects because by definition, the sustained effect persists throughout task block (i.e., across multiple trials).
Results
Behavioral Results
Task accuracy was high in both of the groups, over 85% correct for all conditions (Table 1). The accuracy of the OLD group was significantly lower than the YOUNG group (OLD vs. YOUNG; overall: t25 = −2.58, P < 0.05; single: t25 = −2.32, P < 0.05; repeat: t25 = −2.37, P < 0.05; switch: t25 = −1.72, P < 0.10). In both of the groups, the mixing cost on accuracy (mix vs. single) was not significant (OLD: t13 = −1.32, P = 0.21; YOUNG: t12 = −0.43, P = 0.65), but the switch cost in accuracy (switch vs. repeat) was near significance in both groups (OLD: t13 = −1.90, P = 0.08; YOUNG: t12 = −2.00, P = 0.07). There was no group difference in switch cost (i.e., switch vs. repeat) (t25 = −0.04) or mixing cost (i.e., repeat vs. single) (t25 = 0.69).
Table 1.
Behavioral performance (mean (standard error of the mean))
| Group | Single | Repeat | Switch | Overall |
| Accuracy | ||||
| Old | 0.925 (0.011) | 0.906 (0.014) | 0.873 (0.021) | 0.901 (0.013) |
| Young | 0.953 (0.005) | 0.947 (0.010) | 0.913 (0.010) | 0.938 (0.005) |
| Reaction time (ms) | ||||
| Old | 1171.5 (59.0) | 1251.6 (59.0) | 1362.5 (65.9) | 1261.9 (62.7) |
| Young | 969.8 (25.1) | 1058.2 (38.6) | 1124.3 (42.7) | 1050.8 (33.7) |
Consistent with the accuracy effects, reaction times in the OLD group were significantly longer than the YOUNG group (OLD vs. YOUNG; overall: t25 = 3.13, P < 0.01; single: t25 = 3.15, P < 0.01; repeat: t25 = 2.74, P < 0.05; switch: t25 = 3.03, P < 0.01). Although there were significant mixing and switch costs in both of the groups (OLD—switch cost: t13 = 4.56, P < 0.001; mixing cost: t13 = 2.72, P < 0.05; YOUNG—switch cost: t12 = 3.61, P < 0.01; mixing cost: t12 = 3.74, P < 0.01), the group differences were again not significant (switch cost: t25 = −1.4; mixing cost: t25 = 0.59).
These behavioral observations suggest that the OLD group experienced robust cognitive declines in task performance. However, the lower performance of participants in the OLD group was likely due to a general cognitive decline, which is typical of older adults, and was relatively independent of a specific decline in task-switching performance. The overall performance of the OLD group was relatively high and may reflect relatively preserved cognitive functioning in the recruited participants. This would be consistent with our other previous studies of cognition in older adults using the same participant pool that exhibited comparable behavioral performance to young adults during performance of a context processing task (the AX continuous performance task), frequently used to probe executive functions (Paxton et al. 2008). Moreover, the relative comparability of behavioral performance across the 2 groups indicates that the imaging results can be interpreted without concerns regarding confounds due to behavior change (Reuter-Lorenz and Lustig 2005; Braver and West 2007).
Imaging Results
Sustained versus Transient aPFC Activity
Within the right hemisphere aPFC ROI, a voxel cluster was identified (26 voxels; peak coordinate: [27, 45, 15]; BA 46/10; see Fig. 1a). Inspection of the sustained activation pattern in this region confirmed the presence of a significant group (OLD vs. YOUNG) × task block (mix vs. single) interaction (F1,25 = 6.69, P < 0.05). The source of this interaction was that in younger adults, there was significant sustained activity in the MIX condition (t12 = 2.44, P < 0.05), and this was also significantly greater than SINGLE (mix vs. single: t12 = 4.08, P < 0.002), whereas in older adults, sustained activity was absent (mix: t13 = −0.42, P = 0.68; mix vs. single: t13 = 0.13, P = 0.90). The anatomical location of this aPFC region is within 20 mm from other aPFC foci identified in previous task-switching studies (Sakai and Passingham 2003, 2006; Crone et al. 2006), thus validating its use in the current study.
The above result suggests that in the OLD group, there was a reduction of sustained activity associated with cognitive control during task switching. We confirmed this directly through a whole-brain exploratory analysis. For this analysis, we used the identical criteria for identification of sustained effects that was employed in our previous study of the YOUNG group (Braver et al. 2003; see also Supplementary Materials). The whole-brain analysis confirmed that there was an absence of sustained activity in the OLD group throughout the whole PFC and PPC (Fig. S1a and Table S1 in Supplementary Materials). In contrast, when analyzed separately, the YOUNG group exhibited significant sustained activity in the aPFC region, as reported previously (see Table 2 and Fig. 2 in Braver et al. 2003). These results validate the present ROI approach in terms of aPFC sustained/transient activity (i.e., absence of PFC/PPC sustained activity in the OLD group and presence of aPFC sustained activity in the YOUNG group).
A further analysis examined the pattern of transient, event-related responses in this aPFC region. As reported previously, in the YOUNG group, there was no event-related activation in aPFC for any of the trial types (max t < 1.3, min P > 0.20; Braver et al. 2003). However, as shown in Figure 1b,c, the OLD group showed an increased transient response in this same region compared with the YOUNG group (age main effect: F1,25 = 4.37, P < 0.05). Furthermore, in the OLD group, event-related activation on switch trials was significantly increased relative to nonswitch trials (switch: t13 = 2.56, P < 0.05; switch vs. repeat: t13 = 2.58, P < 0.05; switch vs. single: t13 = 2.20, P < 0.05). A 2-way repeated-measures ANOVA on the transient activity pattern, including trial type (single and switch) and group (OLD and YOUNG) as factors, revealed a significant interaction effect (F1,25 = 4.79, P < 0.05), with neither of the main effects significant (age: F1,25 = 3.75, P = 0.06; trial: F1,25 = 1.09, P = 0.30). Figure 1b,c shows the magnitude and time course of the MRI signal for event-related effects (single, task-repeat, and task-switch trials), demonstrating transient activity in the OLD group and the absence of the transient activity in the YOUNG group. A supplementary analysis confirmed this interaction pattern using a different approach to estimate event-related activation magnitude (see Supplementary Materials).
Together, these observations indicate a double dissociation in the aPFC in terms of the group and the dynamics of activity (i.e., sustained vs. transient) during the mixed block. To statistically verify this dissociation, we computed the task-switching effect for both the sustained (mix block − single block) and transient (switch trial − single trial) activity measures and subjected these to a 2-way repeated-measures ANOVA with group (OLD an YOUNG) and dynamics (sustained and transient) as factors. A significant crossover interaction effect was observed (F1,25 = 7.23, P < 0.05; main effects not significant: max F < 2.53; min P > 0.11), consistent with a double dissociation pattern.
Finally, we examined whether this age-related double dissociation observed in aPFC was idiosyncratic to this region or rather representative of a brain-wide shift. We calculated, using a liberal threshold (P < 0.05, uncorrected), the number of voxels across the whole brain showing sustained activity (mixed block - single block), in which activation levels were significantly different across the 2 age groups (OLD vs. YOUNG). The same calculation was performed for transient activity (switch trial − single trial). The results of this analysis indicated that of the 6053 voxels showing age differences in sustained activity, 58% were of the YOUNG > OLD pattern. Moreover, and consonant with the results described above, the OLD > YOUNG reversed pattern was not observed for sustained activity (i.e., mix minus single) within any region of PFC. Conversely, of the 7746 voxels showing age differences in transient activity, 89% were of the OLD > YOUNG pattern. Thus, the age-related shift in activation dynamics observed in aPFC seems fairly representative of a brain-wide pattern.
Brain–Behavior Relationship in aPFC
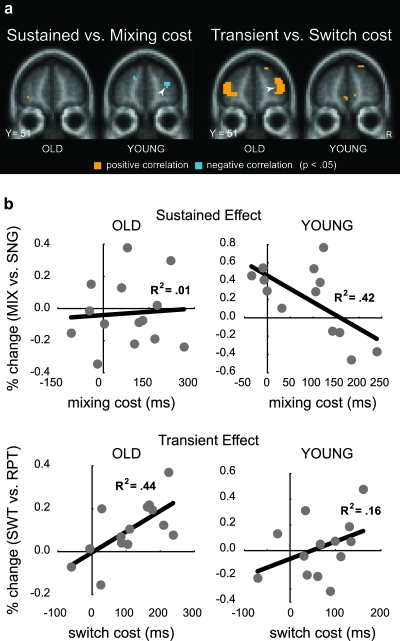
We next examined whether the age differences in sustained and transient activity within aPFC were related to behavioral performance effects. Specifically, a voxel-wise correlation analysis was performed within the aPFC ROI in order to examine whether between-subjects differences in the magnitude of sustained and transient activity were correlated with mixing and switching reaction time costs. As shown in Figure 2a,b (left, top), the YOUNG group exhibited a significant negative correlation between sustained activity and mixing cost (peak correlation: [30, 48, 18]; r = −0.64, t12 = −2.81, P < 0.05; cluster size: 20 voxels). Thus, greater sustained activity in the YOUNG group was associated with a smaller cost on performance. However, there were no such correlations within aPFC for the OLD group (for the region identified in the YOUNG group correlation, r = 0.07, P = 0.81). In contrast, when transient activity was examined in relationship to switching cost (switch trials - repeat trials), a significant correlation was observed for the OLD group (peak correlation: [33, 48, 24]; r = 0.66, t13 = 3.1, P < 0.01; cluster size: 28 voxels). Interestingly, the direction of this correlation was positive, indicating that more transient activity was associated with a larger switch cost. There were no significant correlations in transient activity observed for the YOUNG group (for the region identified in the OLD, r = 0.39, P = 0.18).
Figure 2.
Brain–behavior relationship in the aPFC. (a) Correlation maps (coronal sections at labeled Talairach coordinate) showing significant relationship in aPFC between sustained/transient activity and mixing/switch cost (and masked by corresponding activation contrast). Correlation coefficient was calculated between the activation (sustained: MIX vs. SNG; transient: SWT vs. RPT) and corresponding cost on reaction time (i.e., mixing or switch). (b) Scatterplot of correlations for sustained effect (upper row) in aPFC region indicated by arrow in left panel of (a) and for transient effect (lower row) in aPFC region indicated by arrow in right panel of (a). Leftmost plots are for older participants (OLD) and rightmost plots are for younger participants (YOUNG); each dot indicates 1 participant. Data are plotted with percent signal change (MIX vs. SNG or SWT vs. RPT) on y-axis and behavioral cost (mixing or switch) on x-axis.
Although the aPFC regions showing these significant correlations were not fully overlapping with each other or the region showing the double dissociation in activation dynamics (the correlation failed to reach significance in this region), the peak coordinates were located within 12 mm distance from each other (i.e., [27, 45, 15], [30, 48, 18], and [33, 48, 24]). As spatial smoothing was used in the present study (9-mm FWHM), it is not possible to rule out the hypothesis that the smoothing created disparate peaks in what might have been a single right hemisphere aPFC functional region.
Transient Activity in lPFC and PPC
Within the left hemisphere lPFC and PPC ROIs, numerous voxel clusters were identified. These included a cluster in the posterior part of the lPFC (BA 44/45; 68 voxels; peak coordinate: [−48, 12, 15]) and the PPC (BA 40/39; 93 voxels; peak coordinate: [−30, −54, 42]). The anatomical locations of the posterior lPFC and PPC are close to the regions identified in the previous studies showing transient task-switching effects (Dove et al. 2000; Kimberg et al. 2000; Sohn et al. 2000; Brass and von Cramon 2002, 2004; Brass et al. 2003; Braver et al. 2003; Bunge et al. 2003; Sakai and Passingham 2003, 2006; Asari et al. 2005; Crone et al. 2006; Yeung et al. 2006).
A complementary whole-brain exploratory analysis was also performed separately for the OLD group using the identical criteria in our previous study to identify regions in the YOUNG group (Braver et al. 2003; see also Supplementary Materials). The OLD group exhibited significant transient activity in multiple cortical regions during task-switch trials, including PPC and posterior lPFC regions near the previous ROIs in our previous report (Fig. S1 and Table S1 in Supplementary Materials; see also Table 2 and Fig. 2 in Braver et al. 2003). This finding validates the present ROI approach in terms of lPFC/PPC transient activity that was observed independently of age group. Thus, subsequent analyses focused on the PPC and posterior lPFC regions.
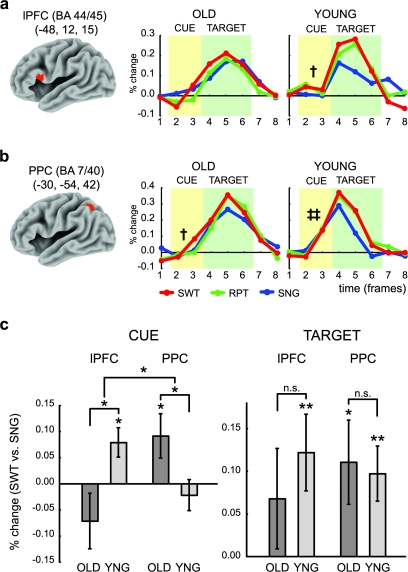
Figure 3a shows the time course of MRI signal in the posterior lPFC and PPC regions, demonstrating that both the YOUNG and the OLD groups exhibited robust transient responses that increased on task-switch trials. However, this transient activation comprised event-related responses to both the cue/delay periods and target/response periods occurring during the trial. We thus examined these 2 periods separately in follow-up analyses. In terms of cue-related activation, we found that in the posterior lPFC region, the YOUNG group showed an increased response on switch trials relative to single trials (t12 = 2.80, P < 0.05), whereas the OLD group did not show such an effect (t13 = −1.35, P = 0.20). The age group difference in cue-related activity was also statistically reliable (t25 = 2.67, P < 0.05). Conversely, when we examined the PPC, the opposite pattern was observed. The OLD group showed a significant switch-related cue response (t13 = 2.20, P < 0.05), but the YOUNG group did not (t12 = −0.74, P = 0.47), with the age difference again being statistically reliable (t25 = 2.31, P < 0.05). These findings indicate a double dissociation between the posterior lPFC and PPC in terms of aging effects on the switch-related cue response. A 2-way repeated-measures ANOVA confirmed that the age × region interaction was statistically reliable (F1,25 = 6.35, P < 0.05; see Fig. 3c).
Figure 3.
Cue- and target-related components of the transient activity in the lPFC and PPC near intraparietal sulcus. (a) Leftmost panel shows the lPFC ROI on 3D brain surface. Time course of MRI signal in OLD (middle) and YOUNG (right) groups, with similar format to Figure 1c. Cue- and target-related components are highlighted by yellow and green background, respectively. (b) Leftmost panel shows the PPC ROI on 3D brain surface. The format is similar to that in the panel (a). Significant cue-related activity in the switch trials (red line) is indicated by daggers. (†P < 0.05), and significant cue-related activity across trial types are indicated by double daggers (‡‡P < 0.01). (c) Decomposition of transient activity into cue (left panel) and target (right panel) components in each region and for each age group. The y-axis indicates percent signal change (SWT vs. SNG). Significant activity, difference, and interactions are indicated by asterisks (*P < 0.05, **P < 0.01).
It is important to note that the age differences in the PPC were observed not because the YOUNG group failed to show a cue response in this region but rather because the region showed a strong cue response on all trials in the YOUNG group (single: t12 = 3.26, P < 0.01; repeat: t12 = 3.39, P < 0.01; switch: t12 = 3.34, P < 0.01; Fig. 3b right) but only on switch trials in OLD group. Yet, the reverse pattern was not true for the OLD group in the lPFC. In fact, the lPFC did not show a cue response on any of the trial types for this group (max |t| < 1.3, P > 0.20). These observations regarding cue-related activity collectively indicate that the double dissociation revealed in Figure 3c (left) cannot be explained by a simple age-related switch or reversal in the functional role played by lPFC and PPC. Rather, it suggests that whereas in the YOUNG group, PPC serves a general cue-related function with lPFC becoming additionally recruited on switch trials, in the OLD group, only the switch trial cue response in PPC is preserved.
Analyses of the target-related response indicated a significant switch-related increase in activity in the YOUNG for both the PPC (switch vs. single: t12 = 2.99, P < 05) and lPFC (t12 = 2.50, P < 0.05). In the OLD, the effect was also significant for the PPC (t13 = 2.26, P < 0.05), but not for the lPFC (t13 = 1.42, P = 0.17). However, in neither region was the effect of age significant (max |t| < 1). Together, these results indicate that cognitive aging has effects on the cue but not target-related responses in these regions.
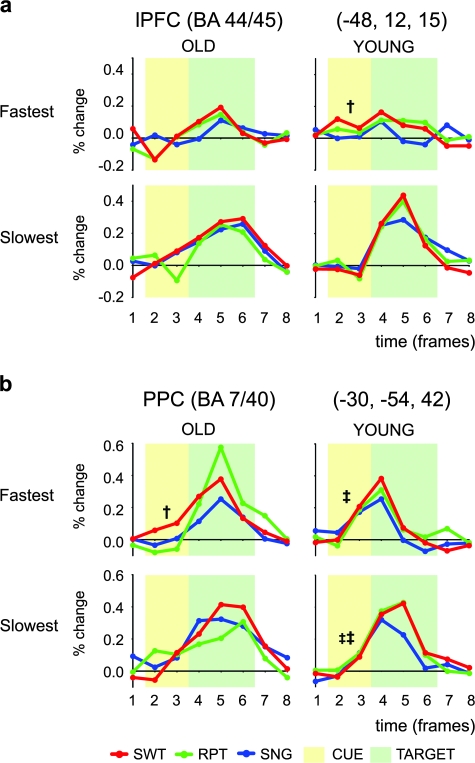
Brain–Behavior Relationships in lPFC and PPC
We examined the relationship between the magnitude of the event-related response in the lPFC and PPC regions and trial-by-trial fluctuations in response speed. In the lPFC, the effect of response speed impacted the magnitude of the cue-related response in the YOUNG group, with the fastest switch trials showing increased cue-related activity relative to both slow switch trials (t12 = 2.80, P < 0.05) and fast single trials (t12 = 3.19, P < 0.01). However, no such differentiation was observed in the OLD group (Fig. 4a). This pattern was confirmed by a statistically significant 3-way interaction between group (OLD and YOUNG), trial (switch and single), and RT (fastest and slowest) (F1,25 = 4.07, P = 0.05). In the PPC, the opposite pattern appeared, with the effect of response speed impacting the magnitude of the cue-related response in the OLD group but not the YOUNG group. For the OLD group, increased cue-related activation was only observed on the fastest switch trials (switch vs. single, fast: t13 = 2.24, P < 0.05; switch vs. single, slow: t13 = 0.89, P = 0.36; Fig. 4b right). On the other hand, in the YOUNG group, cue-related activity was significant in both of the fastest and the slowest trials (slow: F1,12 = 11.79, P < 0.01; fast: F1,12 = 8.07, P < 0.05), but main effect of RT was not significant (F1,12 = 0.06, P = 0.81; Fig. 4b left). Thus, the relationship between cue-related activity and RT was consistent with the average activation patterns described in the previous section, in that cue-related activity in lPFC seems to play a stronger functional role in task performance for the YOUNG group, whereas cue-related activity in PPC seemed more strongly linked to performance for the OLD group.
Figure 4.
Transient activity as a function of trial RT in the OLD and YOUNG group. (a) Time courses of MRI signal in lPFC estimated for fastest (upper panels) and slowest (lower panels) RT trials. (b) Time courses of MRI signal in PPC. The format is similar to that in Figure 3a. Significant cue-related activity in the switch trials is indicated by daggers (†P < 0.05) and across trial types is indicated by double daggers (‡P < 0.05, ‡‡P < 0.01).
In terms of target-related activity, the effect of RT was significant for the lPFC (slowest > fastest: F1,25 = 35.30, P < 0.0001), such that target responses were larger on the slowest trials. However, this effect did not interact with age or trial type (Fs < 1.5, P values > 0.26). The effect of RT was not only significant for the PPC (slowest > fastest: F1,25 = 6.71, P < 0.05) but also significantly interacted with age (YOUNG > OLD: F1,25 = 5.27, P < 0.05). Thus, in both groups, trials for which performance is poor (i.e., RTs are slow) are associated with a substantial increase in target-related activation of lPFC and PPC. However, the larger effect in PPC for the YOUNG group suggests that they may have had an easier time transiently recruiting additional mechanisms to assist in response selection on such trials. This effect might partially potentially explain the slower RTs and lower accuracy of the OLD group on this task, which occurred independently of trial type (as did the RT effect on target-related activation in these regions).
Discussion
The results of this study demonstrate 2 age-related double dissociations during task switching: 1) older adults showed a reduction in sustained activation of aPFC but an increase in switch-related transient activity in this region and 2) older adults had a reduced switch-related response to task cues in posterior lPFC but an increased response in PPC. Together, these findings suggest that cognitive aging produces a qualitative shift in the temporal dynamics of brain activity and may indicate that older adults utilize different control strategies during task performance. In particular, as described further below, the results are consistent with the hypothesis that younger adults primarily rely on proactive cognitive control during task switching, whereas older adults rely more heavily on a reactive control strategy (Braver and West 2007; Paxton et al. 2008; Braver et al. 2009).
Age-Related Changes in aPFC Function
Previous neuroimaging studies have suggested that the aPFC is implicated in higher level cognitive representations such as goal structure (Koechlin et al. 1999, 2003; Burgess et al. 2000; Braver and Bongiolatti 2002), relational integration (Christoff et al. 2001; Bunge et al. 2005; De Pisapia et al. 2006), and task rules (Bunge et al. 2003; Sakai and Passingham 2003, 2006). Consistent with these previous observations, the sustained aPFC activity observed here in young adults may reflect active maintenance of task sets or rules. In particular, aPFC may contribute to task–rule representation by implementing a top-level node in a decision tree that reflects the hierarchical structure of the experimental context (see also Fig. S2 in Supplementary Materials), such that the different tasks are represented as alternative (and mutually exclusive) pathways or branches (Koechlin et al. 2003; Bunge et al. 2005; Botvinick 2007; Badre 2008; Sakai 2008). Thus, sustained aPFC activation may reflect a proactive control strategy that facilitates rapid interpretation of task cues in a top-down manner. This interpretation is supported by the finding that in younger adults, aPFC sustained activity was negatively correlated with the mixing cost, indicating that higher activation levels were associated with better management of the multitask environment.
A surprising finding was that older adults showed increased transient activation in the same region of aPFC. Moreover, in older adults, aPFC activity was positively rather than negatively correlated with behavior and was associated with the switch cost rather than mixing cost. The positive direction of correlation indicates that increased aPFC activity was linked to poorer switching performance, probably reflecting a compensatory processes recruited in some older adult participants. It is thus likely that the transient, target-driven activity dynamics reflects a reactive control strategy, as is suggested by behavioral studies (Mayr 2001). This hypothesis is noteworthy in terms of the seeming inconsistency of the brain–behavior correlations observed here with prior findings in which increased activity among older adults was associated with more effective compensation (e.g., Cabeza 2002). One speculation is that previous event-related and blocked fMRI studies may not have been able to detect such dissociable dynamics because the transient and sustained activity shifted in opposite directions. Nevertheless, the present study suggests that aPFC is also important for both proactive and reactive cognitive control strategies in certain tasks but with the proactive strategy being more strongly linked to optimal performance in this region.
One possible functional interpretation is that older adults transiently recruit aPFC to resolve ambiguity, competition, or interference between task sets that may be greatest on task-switch trials and especially under conditions in which a hierarchical rule representation was not previously active (Botvinick 2007; Badre 2008; Sakai 2008). Or, alternatively, aPFC may implement a task–rule integration function, which is recruited on a post-target basis (Christoff et al. 2001; Bunge et al. 2005). Thus, in older adults, aPFC may have helped to facilitate responding but at a cost of increased response slowing. Regardless of which particular explanation of aPFC function is correct, the sustained-to-transient shift in this region's dynamics indicates that access to high-level cognitive representations is changed in a temporal rather than anatomical manner with increasing age.
One interesting question may be how transient and sustained activity cooperatively contribute to behavioral performance. Specifically, we found that sustained aPFC positively contributed to performance in the YOUNG group, whereas transient aPFC negatively contributed to performance in the OLD group. Because the older adults did not show sustained activity, it would be natural to assume that there was no relationship between this activity and their performance. A similar inference could apply for aPFC transient activity in young adults. On the other hand, a clear extension of our interpretation would be to assume that sustained aPFC contributes positively, whereas transient aPFC activity contributes negatively to performance in both groups. Such within-groups double dissociations in brain–behavior relationships might be possible to observe in larger sample studies that have sufficient statistical power to detect such complex between-subjects effects (i.e., correlations).
Age-Related Changes in lPFC and PPC Function
The current results indicated robust transient activity in lPFC and PPC associated with task switching. This finding replicates many prior reports (Dove et al. 2000; Kimberg et al. 2000; Sohn et al. 2000; DiGirolamo et al. 2001; Wallis et al. 2001; Brass and von Cramon 2002, 2004; Brass et al. 2003; Bunge et al. 2003; Sakai and Passingham 2003, 2006; Wallis and Miller 2003; Crone et al. 2006) and suggests a fairly consistent role for these regions in the updating of task sets or task rules. Importantly, however, even though both older and younger adults robustly engaged these regions, a more detailed examination of the temporal dynamics also revealed important age differences. Within lPFC, cue-related activity was increased on switch trials and contributed to fast performance in younger but not older adults. Conversely, within PPC, younger adults showed consistent cue-related activity that contributed to fast performance on all trials, whereas in older adults, cue-related responses were present only on switch trials. This suggests that younger adults more actively engaged preparatory control mechanisms to facilitate responding, whereas older adults primarily engaged in preparatory activation to manage the increased control demands present on task-switch trials.
Imaging studies of brain structure in older adults have revealed regional cortical volume reductions in some of the same lPFC and PPC regions that are the focus of the current results (e.g., Raz et al. 2005; Smith et al. 2007). Moreover, in recent work, age-related changes in lPFC and PPC activity during task switching have been linked to a reduction in white matter integrity in pathways that might link these structures (Gold et al. 2008; Madden et al. 2009). The older adults participating in the current study may have also suffered from exhibited age-related volume reductions or white matter changes in PFC, PPC, and other regions. However, this cannot fully explain the observed pattern of results because these were more complex than a simple change in activation magnitude. In particular, the lPFC and PPC reductions were selectively observed during the cue period and not the target period.
Recent event-related brain potential studies have also revealed age-related differences during task switching, with prolonged potential and increased latency in older adults during cue period (Kray et al. 2005; Friedman et al. 2008; West and Travers 2008). Moreover, the older adults revealed increased magnitude during target period, which might reflect compensatory potential (Kray et al. 2005). These results are consistent with the reactive strategy hypothesis in the older adults.
A possible functional interpretation of lPFC and PPC is that they both mediate a form of task-based attention that can be constrained in a top-down manner by advance cues as well as in a bottom-up manner by target stimuli. Previous research and theories suggest a natural division of labor between the 2 regions (Fig. S2 in Supplementary Materials; see also Snyder et al. 2000; Bunge et al. 2001), in which lPFC mediates attention more toward abstract stimulus dimensions, whereas the PPC might mediate attention toward response dimensions or stimulus–response mapping rules.
The age-related shift in dynamics across the 2 regions also suggests a change in the cognitive control strategies used by younger and older adults. For younger adults, cue-related activation of lPFC and PPC might indicate a proactive control strategy of preparatory attentional focusing to facilitate rapid target classification decisions in terms of both the selection of task-relevant features and responses. The consistent preparatory activation of lPFC and PPC might also arise as a result of top-down sustained signals arising from aPFC, providing input from a higher order task representation that is being tonically maintained. Such effective use of cue information allowed the young adults to achieve a proactive strategy. Of course, further support for such a hypothesis would require an in-depth analysis of functional connectivity dynamics between the regions, which is beyond the scope of the current study. In contrast for older adults, activation of lPFC and PPC might primarily occur in a reactive manner, as a means of resolving competition or ambiguity between activated stimulus dimensions or mapping rules. The shift toward reactive control might also have occurred as a direct consequence of impaired cue utilization in older adults; such an interpretation would be consistent with findings from behavioral studies (Cepeda et al. 2001; Mayr and Liebscher 2001).
Age-Related Changes in Brain Activity versus Behavior
One caveat of the present results is that aging effects on brain activity dynamics were observed in the absence of significant age-related differences in behavioral switch and mixing costs. The preserved behavioral performance found in the older adults may be not only due to the high-functioning older adults that participated in the present study, but also a function of the lower task-switching demands imposed by the experimental paradigm. Specifically, in the current study, switching occurred between 2 different semantic classification tasks rather than involving a cross-modal task switch (e.g., between lexical/phonological vs. semantic dimensions; Sakai and Passingham 2006). Indeed, in such cross-modal switching paradigms, the magnitude of switch and mixing costs are typically larger (in both younger and older adults) than that observed in the current study (c.f., Rogers and Monsell 1995; De Jong 2001; Mayr 2001; Reimers and Maylor 2005). The positive side of the present situation was that it enabled interpretation of the imaging results without the presence of confounds derived from age-related behavioral impairment. However, the negative side is that it becomes more difficult to determine the relationship between activation changes and such behavioral impairments.
Future studies might resolve this issue by utilizing both a low-demand task-switching paradigm during scanning along with a higher demand paradigm administered (to the same participants) performed outside the scanner. In this manner, age-related changes in neural activity during in-scanner task performance can be more tightly linked to the degree of behavioral impairment observed in the out-of-scanner, higher demand task. In general, such experimental designs might provide an especially useful means of establishing changes in brain–behavior relationships among older adults during executive control tasks and as such better reveal the neural mechanisms underlying this age-related cognitive decline.
Supplementary Material
Supplementary material can be found at http://www.cercor.oxfordjournals.org/
Funding
NIH R01 MH66078 to T.S.B.; Research Fellowship from the Uehara Memorial Foundation to K.J.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and performance XV. Cambridge (MA): MIT Press; 1994. pp. 421–452. [Google Scholar]
- Asari T, Konishi S, Jimura K, Miyashita Y. Multiple components of lateral posterior parietal activation associated with cognitive set shifting. Neuroimage. 2005;26:694–702. doi: 10.1016/j.neuroimage.2004.12.063. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Multilevel structure in behaviour and in the brain: a model of Fuster's hierarchy. Philos Trans R Soc Lond B Biol Sci. 2007;362:1615–1626. doi: 10.1098/rstb.2007.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Ruge H, Meiran N, Rubin O, Koch I, Zysset S, Prinz W, von Cramon DY. When the same response has different meanings: recoding the response meaning in the lateral prefrontal cortex. Neuroimage. 2003;20:1026–1031. doi: 10.1016/S1053-8119(03)00357-4. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, West RL. Working memory, executive processes, and aging. In: Craik FI, Salthouse TA, editors. Handbook of aging and cognition. New York: Psychology Press; 2007. pp. 311–372. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–4119. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Conturo TE, McKinstry RC, Akbudak E, Snyder AZ, Yang T, Raichle ME. Sensitivity optimization and experimental design in functional magnetic resonance imaging. Soc Neurosci Abstr. 1996;26:7. [Google Scholar]
- Craik MI, Salthouse TA. The handbook of aging and cognition. New York: Psychology Press; 2007. [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- De Jong R. Adult age differences in goal activation and goal maintenance. Eur J Cogn Psychol. 2001;13:71–89. [Google Scholar]
- De Pisapia N, Slomski JA, Braver TS. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cereb Cortex. 2006;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiol Aging. 2007;28:1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Emery L, Heaven TJ, Paxton JL, Braver TS. Age-related changes in neural activity during performance matched working memory manipulation. Neuroimage. 2008;42:1577–1586. doi: 10.1016/j.neuroimage.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Johnson R, Jr, Ritter W, Bersick M. Age-related changes in executive function: an event-related potential (ERP) investigation of task-switching. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:95–128. doi: 10.1080/13825580701533769. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magn Res Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Shallice T. Task switching: a PDP model. Cogn Psychol. 2002;44:297–337. doi: 10.1006/cogp.2001.0770. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.005. Publish online May 20; doi:10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut KM, Barch DM. Sex influences on material-sensitive functional lateralization in working and episodic memory: men and women are not all that different. Neuroimage. 2006;32:411–422. doi: 10.1016/j.neuroimage.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Jersild AT. Mental set and shift. Arch Psychol. 1927;89:5–82. [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M. Modulation of task-related neural activity in task-switching: an fMRI study. Brain Res Cogn Brain Res. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: explorations of executive control processes in the task switching paradigm. Acta Psychol. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A. Age differences in attentional control: an event-related potential approach. Psychophysiology. 2005;42:407–416. doi: 10.1111/j.1469-8986.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Kray J, Li KZ, Lindenberger U. Age-related changes in task-switching components: the role of task uncertainty. Brain Cogn. 2002;49:363–381. doi: 10.1006/brcg.2001.1505. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: the role of inhibition, stimulus ambiguity, and response-set overlap. Psychol Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Mayr U, Liebscher T. Is there an age deficit in the selection of mental sets? Eur J Cogn Psychol. 2001;13:47–69. [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn. 1996;22:1423–1442. [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cogn Psychol. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Mugler JPI, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP-RAGE) Magn Res Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: effects of age on general and specific switch costs. Dev Psychol. 2005;41:661–671. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, Donaldson DI, Wagner AD, Braver TS. Item- and task-level processes in the left inferior prefrontal cortex: positive and negative correlates of encoding. Neuroimage. 2004;21:1472–1483. doi: 10.1016/j.neuroimage.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY. Advance preparation and stimulus-induced interference in cued task switching: further insights from BOLD fMRI. Neuropsychologia. 2005;43:340–355. doi: 10.1016/j.neuropsychologia.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. J Cogn Neurosci. 2002;14:1139–1150. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task switching to speed, age, and fluid intelligence. Psychol Aging. 1998;13:445–461. doi: 10.1037/0882-7974.13.3.445. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Snyder AZ. Difference image versus ratio image error function forms in PET-PET realignment. In: Bailer D, Jones T, editors. Quantification of brain function using PET. San Diego (CA): Academic Press; 1996. [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Sohn M, Ursu S, Anderson JR, Stenger VA, Carter C. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Verhaeghen P, Basak C. Ageing and switching of the focus of attention in working memory: results from a modified N-back task. Q J Exp Psychol A. 2005;58:134–154. doi: 10.1080/02724980443000241. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed block/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- West R, Travers S. Differential effects of aging on processes underlying task switching. Brain Cogn. 2008;68:67–80. doi: 10.1016/j.bandc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD. Between-task competition and cognitive control in task switching. J Neurosci. 2006;26:1429–1438. doi: 10.1523/JNEUROSCI.3109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.