Abstract
Hyaluronic acid, a nonsulfated, linear glycosaminoglycan, is ubiquitously distributed in the extracellular matrix and is known to facilitate tumor progression by enhancing invasion, growth, and angiogenesis. Native HA has been attached to substrates to create patterned surfaces resistant to cell adhesion, and has been utilized in a variety of cell adhesion studies using either non covalently bound layers patterned by soft lithography or related methods. We use a new approach to study cell interactions with HA-presenting regions, by covalently linking HA adjacent to PEG-ylated regions, which resist cell adhesion. Colon and breast cancer cells seeded on the patterned HA surfaces adhere preferentially on HA-presenting regions and proliferate there. Furthermore, we demonstrate that cell adhesion is inhibited with the blocking of HA receptor, CD44, and that cellular adhesive processes, through protrusions spreading onto the HA surface, enhance spreading and movement outside the HA-presenting regions. Overall, this approach allows high-resolution analysis of cancer cell attachment, growth, and migration on exogenous native HA.
1. Introduction
Hyaluronic acid (HA), a nonsulfated, linear glycosaminoglycan is ubiquitously distributed in the extracellular matrix (ECM), and is a well-established co-regulator for gene expression, proliferation, motility, adhesion, signaling, metastasis, and morphogenesis [1]. Specifically, HA has been shown to facilitate tumor progression by enhancing invasion, growth, and angiogenesis [2-4]. In vivo and in vitro studies focusing on the role of HA in cancer examine the effect of cellular HA synthesis, either basal or enforced, on carcinoma cell adhesion, growth, proliferation, and invasion [5-8]. For example, inhibition of HA synthesis in metastatic colon carcinoma cells decreased their adhesion to laminin, suggesting that their adhesion depends on pericellular HA [9]. However, as cell migration in vivo occurs through the ECM, it is important to study cellular interactions with exogenous HA. HA hydrogels have been utilized to allow anchorage independent growth of clusters and colonies of cells [10, 11] or as the milieu to which adhesive motifs are incorporated and enable cell attachment and growth [12]. To our best knowledge, no study so far examined adherence, growth, and migration of cancer cells on an HA-presenting substrate. In the current study, we sought to develop patterned functionalized surfaces that will enable a controllable study and high resolution visualization of cancer cell interactions with HA.
Cellular interactions with sulfated HA, which has larger electrostatic interactions than HA in its native state, have been extensively studied in patterned surfaces made by photolithographical methods [13-20]. Native HA, which is the interest of the current work, has been affixed to substrates [21] to create patterned surfaces resistant to cell adhesion and has been utilized in a variety of cell adhesion studies using either non covalently bound layers patterned by soft lithography or related methods, or covalently linked layers established using photolithographic techniques [22]. These studies reveal that HA is highly resistant to protein adhesion including BSA, fibronectin (Fn) and IgG, and to cell adhesion for a variety of cells including fibroblasts, [21, 23-25] hepatocytes, embryonic stem cells, [23-25] chondrocytes, [22] and cardiomyocytes [26]. This adhesion-resistant property has been harnessed [14, 23, 26] to create patterned cell cultures in which HA-covered, cell resistant regions are created adjacent to cell adhesive regions. Cells seeded on the surfaces adhere in patterns to the HA-free regions. In extensions of that work, patterned cell co-cultures have been created [23, 24]. Patterned cultures of primary cells were created using HA-patterned surfaces as described above. Thereafter, the HA-presenting regions were covered with cell-adherent cationic polyelectrolytes [23]. A secondary cell type was seeded on those regions. In contrast to these prior studies, in which cells are directed to avoid adhesion and growth on HA-presenting regions, we seek to direct cells, specifically cancer cells, to adhere preferentially on HA-presenting regions.
2. Materials and Methods
2.1 Silicon master microfabrication and PDMS stamp
Standard photolithography techniques were used to fabricate silicon masters patterned with 80 μm × 80 μm squares. Before use, silicon wafers were rinsed with ethanol and air-dried. An SU-2025 epoxy negative photoresist was applied by spin coating (Laurell Technologies Corp., North Wales, PA) on the silicon wafer at 600 rpm for 10 seconds to spread the photoresist and then at 3000 rpm for 40 seconds to a final 25 μm film. The silicon wafer was transferred to a hotplate for a “soft-bake” at 95 °C for three minutes to remove excess solvent. A mask with the desired pattern of 80 μm squares covered the photoresist-coated silicon wafer and was exposed to UV irradiation (350 to 450 nm) for 20 seconds. The wafer was transferred again to the hotplate for a 5-7 minute “hard-bake” at 110 °C before rinsing with SU-8 developer (Microchem Corp., Newton, MA) to remove the exposed regions of photoresist. To create a complementary elastomeric stamp, PDMS prepolymer (Sylgard 184) was mixed with a curing agent (Dow Corning, Midland, MI) in a 10:1 weight ratio, cast onto the silicon master, and cured overnight at room temperature. Cured stamps were separated from the silicon master and sonicated in ethanol for 15 minutes.
2.2 HA patterning on glass substrate
A fresh 3 percent v/v solution of 3-aminopropyl trimethoxysilane (APTMS) (Fluka Chemical Corp., Milwaukee, WI) in 95 percent v/v ethanol was prepared and reacted for five minutes to form a silanol. The APTMS solution was applied by spin coating onto the PDMS stamp at 3500 rpm for 35 seconds. The PDMS stamp was then used to transfer the APTMS to the plasma-cleaned glass substrate. Following stamping, the glass substrates were rinsed with ethanol, air-dried, and heated in an oven for one hour at 115 °C. After heating, the substrates were once again rinsed with ethanol and air-dried. To reduce the nonspecific binding of proteins and to provide a nonadhesive region surrounding the patterned areas, the APTMS patterned substrates were covered for 45 minutes at 75°C with a PEG-silane solution (prepared fresh daily) of 20 percent v/v 2 [methoxy(polyethyleneoxy)propyl]trimethoxysilane (Gelest) in toluene. Substrates were then briefly rinsed with toluene and DI water. Thereafter, the glass substrates were sterilized for one hour under UV light in a tissue culture hood. Using carbodiimide chemistry, an aqueous sterile HA solution (800 kDa, fluorescein FL-labeled; Sigma, St. Louis, MO; 50 μg/ml) was prepared with 10 mmol of EDC (Pierce Biotechnology, Inc., Rockford, IL) and 5 mmol of NHS (Pierce Biotechnology) and incubated on the glass substrates for 16 hours under sterile conditions. Excess FL-HA solution was rinsed from the glass substrate with phosphate-buffered saline (PBS), followed by incubation with 1 percent bovine serum albumin (BSA) in PBS for one hour at room temperature to reduce the nonspecific binding of proteins.
2.3 HA/FN surfaces for NIH-3T3 cell attachment
To create a surface presenting both Fn and HA, two glass coverslips were used and pieced together. Following the protocol described above, APTMS solution was transferred to glass coverslips using a planar (unpatterned) PDMS stamp. After stamping, APTMS-coated coverslips were rinsed with ethanol, air-dried, and heated for one hour at 115 °C. Thereafter, the coverslips were rinsed again with ethanol and sterilized for one hour under UV in a tissue culture hood before incubating for 16 hours with aqueous HA solution. A fresh 0.5 percent v/v solution of octadecyltrichlorosilane (OTS, Sigma) in hexane (Sigma) was prepared and transferred to a second glass coverslip by the method just described. That coverslip was rinsed with hexane and DI water and air-dried prior to sterilization under UV. Fn (Sigma) incubated on the sterilized coverslip for one hour at a concentration of 30 μg/ml. Both HA and Fn-coated coverslips were set adjacent to each other. Excess Fn and HA was rinsed from the coverslips using PBS, followed by incubation with 1 percent BSA in PBS prior to cell seeding.
2.4 Cell culture maintenance
The human colon carcinoma cell line LS174T (ATCC, Manassas, VA) was maintained in MEM medium with 10 percent fetal bovine serum (FBS) supplemented with 5 percent each of nonessential amino acids, sodium pyruvate, and an antibiotic/antimycotic solution (all from Invitrogen, Carlsbad, CA). The human breast carcinoma cell line MDA-MB-231 (ATCC) was maintained in DMEM medium (Invitrogen) supplemented with 10 percent heat-inactivated FBS. Mouse fibroblast cell line NIH-3T3 was maintained in DMEM medium with 10 percent FBS. All culture flasks were tightly sealed and stored in standard incubator conditions of 95 percent air and 5 percent CO2 at 37 °C.
2.5 Cell culture on HA functional surfaces
All cell lines were grown to confluency, washed with PBS, and digested using 0.25 percent tryspin/0.1 percent ethylenediaminetetraacetic acid (EDTA) (Invitrogen). Cell densities between 0.15 and 1×106 cells/ml were seeded onto each glass substrate patterned with FL-HA or Fn. Medium was replaced every 24 hours. Cells were imaged at the indicated time points using inverted microscopy (Olympus 1×50). Experiments were stopped between 12 and 48 hours for analysis.
2.6 Blocking experiment
To block the HA receptor (CD44), we used an established protocol [27]. Briefly, colon and breast cancer cells were passaged and seeded on HA functional surfaces at similar cell densities with or without the addition of anti-CD44 (Sigma). After 24 hours, cell attachment was documented with light microscopy, medium was removed, and slides were processed for immunofluorescent staining as described below.
2.7 Quantification of HA adsorption
Glass substrates patterned with FL-HA were mounted with fluorescent mounting medium (Dako, Glostrup, Denmark) and imaged using distinct fluorescent exposure periods (Olympus B×60). Image J software (NIH) was used to analyze the distribution of FL-HA surface densities by analyzing the grayscale intensities of the patterned regions. Using the ‘analyze surface plot’ function in Image J, surface and plot profiles were created and compared.
2.8 Immunostaining of cells in culture and on micropatterns
Cells were cultured on glass coverslips to confluency or on functional surfaces for 24 to 48 hours. The cells were then fixed using formalin-free fixative (Accustain, Sigma) or 3.7 percent formaldehyde (Fisher Chemical, Fairlawn, NJ) for 20 minutes and washed with PBS. For immunostaining, cells were permeabilized with a solution of 0.1 percent Triton-X (Sigma) for ten minutes, washed with PBS, and incubated for one hour with either anti-human CD44 (1:100; Sigma), anti-human CD168 (1:50; Novocastra, Newcastle, UK) or phalloidin (1:40, Invitrogen). Cells were rinsed twice with PBS, and samples with primary anti-human CD44 or CD168 were incubated with anti-mouse IgG Cy3 conjugate (1:50; Sigma) for one hour. To visualize Fn surfaces, substrates were incubated with anti-Fn (1:200, Sigma) and then anti-mouse IgG Cy3 conjugate. All samples were rinsed with PBS, incubated with DAPI (1:1000; Roche Diagnostics, Basel, Switzerland) for ten minutes, rinsed once more with PBS, and mounted with fluorescent mounting medium (Dako). Cells stained only with secondary antibodies were used as controls; specifically, anti-Fn (1:200; Sigma) was used to visualize Fn surfaces. The immunolabeled cells were examined using fluorescence microscopy (Olympus B×60).
2.9 Scanning Electron Microscopy (SEM)
HA-patterned surfaces were visualized using SEM to assess the cell interactions and their extensions into HA regions. Substrates were fixed with formalin-free fixative for 20 minutes and washed with PBS. Samples were postfixed with 1 percent osmium tetroxide for 20 minutes (Sigma, Allentown, PA), followed by dehydration in ethanol and a final submersion in hexamethyldisilazane (HMDS, Sigma) for ten minutes. Samples were coated with gold using a sputter coater (Anatech Hummer 6.2 Sputter Coater) and visualized using a FEI Quanta 200 ESEM.
2.10 X-ray Photoelectron Spectroscopy (XPS)
XPS was used to analyze the chemical composition of HA-coated surfaces. After incubation with HA solution, substrates were rinsed with DI water and allowed to air-dry prior to analysis using a PHI 4500 system with a magnesium PHI 04-500 Dual Anode X-ray Source operated at 15 kV and 300 W. Samples were analyzed at less than 3×10-9 Torr with a PHI 10-360 Precision Energy Analyzer operated at 178.95 eV and a 45° take-off angle. Scans between 1200 and 0 eV were performed and are displayed in Figure 1D. Atomic concentrations were determined by photoelectron peak integration.
Figure 1. Development of HA functional surfaces.
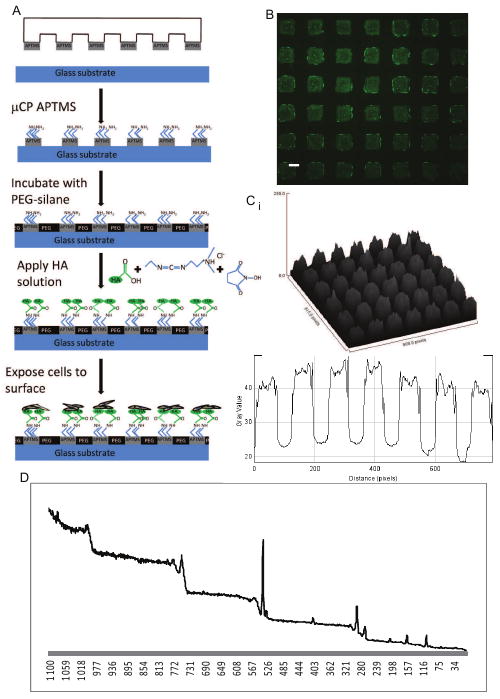
A. Schematic describing the development of HA functional surfaces B. Representative fluorescence image of fluorescein (green)-labeled HA introduced onto an array of 80 μm APTMS squares on glass otherwise covered by PEG-silane. The FL-HA adsorbed onto the APTMS patches. C. Pseudocolor fluorescence images were analyzed using image J: (i) 3D pixel surface profile and (ii) plot profile along patterned row, both demonstrating highly uniform distribution of HA in 80 μm × 80 μm squares. Scale bar is 50 μm. D. XPS spectra of HA-coated surface.
3. Results and Discussion
To direct the growth of cancer cells to HA-presenting regions, we combine soft lithography and carbodiimide chemistry to link HA covalently to glass substrates in patterned monolayers presenting HA surrounded by PEG-silane. PEG in its various forms is widely used to prevent cell adhesion [28-32]. Combining carbodiimide linking chemistry and microcontact printing (μCP), we generated well defined, discrete patterned regions of HA chemically bound to the glass substrate (Fig. 1A). Utilizing an immobilization technique, [33] PDMS stamps with square features (80 μm × 80 μm) were used to transfer APTMS (3-aminopropyl-trimethoxysilane) onto the glass substrate; the substrate was then exposed to PEG-silane which covalently attached to the non-APTMS covered regions. In contrast to previous uses, this stamping methodology allowed precise control over the location of APTMS deposition on to the glass. To deposit HA on the APTMS covered regions, an aqueous, fluorescently tagged HA solution (FL-HA; 50 μg/ml) was prepared with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) hydrochloride, a zero-length crosslinking agent that reacts with the carboxyl group of HA to form an amine-reactive intermediate. Since this intermediate is susceptible to hydrolysis, N-hydroxysuccinimide (NHS) was added to increase the efficiency of the carbodiimide reactions. A stable amide bond formed between the HA intermediate and the primary amine of the patterned APTMS on the glass substrate, generating functional surfaces presenting HA (Fig. 1B). To confirm that the HA was indeed bound to the APTMS-patterned regions, pseudocolor fluorescence intensity was quantified using Image J. A three-dimensional (3D) pixel distribution map was generated, which confirmed the surface profile of square features distributed across the glass surface (Fig. 1Ci). Pixel distance versus graph intensity further demonstrated the uniformity of the square patterns and of HA distribution within the individual squares (Fig. 1Cii). X-ray photoelectron spectroscopy (XPS) analysis showed the presence of an N(1s) photoelectron peak in spectra at approximately 400 eV (Fig. 1D), typical of organic nitrogen atoms. The ratio of carbon to nitrogen content (C/N) of coated substrates using carbodiimide chemistry was determined to be 13, closely matching that expected for HA (C/N=14), providing evidence that HA immobilization occurs on glass substrates (Fig. 1D). The difference in C/N from the expected value may be attributed to unreacted amine groups of APTMS on the substrate surface. In addition, the carbodiimide immobilization technique enabled us to generate HA patterns which were chemically bonded to the surface (rather than physically adsorbed), allowing them to remain intact after rinsing and 5 days in culture (Fig. S1).
The interaction of HA with its cell surface receptor, CD44, induces signaling events that promote anchorage-independent tumor cell growth, survival, and migration, thereby increasing metastatic spread [34, 35]. As our overall aim was to develop an in vitro culture system to study HA interactions with cancerous cells, we investigated whether the HA receptor CD44 is expressed by colon and breast cancer cells. We used the human colon cancer cell line LS174T and the human breast cancer cell line MDA-MB-231. During in vitro culture, colon cancer cells grew as colonies (Fig. 2Ai) and were characterized by membrane expression of CD44 (Fig. 2Aii). Breast cancer cells grew as a monolayer (Fig. 2Bi) and were also found to express both CD44 (Fig. 2Bii).
Figure 2. CD44 in colon and breast cancer cells.
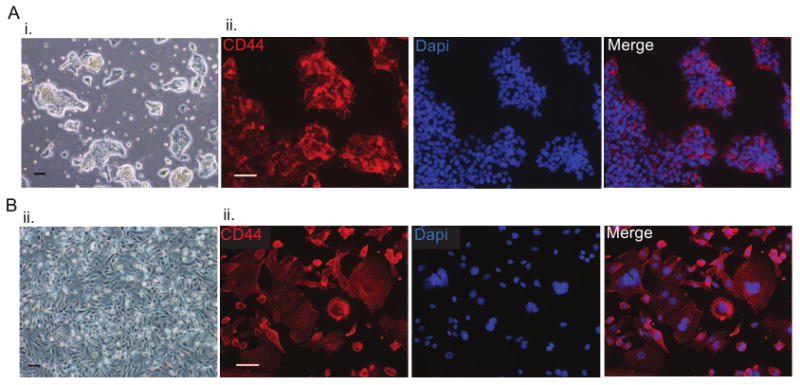
A. (i) Representative light microscopy images of colon cancer cells grow as colonies in culture; (ii) immunofluorescence staining revealed membrane expression of CD44 (CD44 in red; nuclei in blue) B.(i) Representative light microscopy images of breast cancer cells grown as a monolayer in culture; (ii) immunofluorescence staining revealing membrane expression of CD44 (CD44 in red; nuclei in blue). Scale bars are 100 μm in Ai and Bi, and 50 μm in Aii and Bii.
It has been suggested that HA is critical for colon carcinoma invasion [8, 9] and is associated with breast cancer progression [36, 37]. We next explored the utilization of the newly developed HA surfaces for cellular in vitro studies. Single-cell suspensions with cell densities of 4 and 1.5×105 of colon and breast cancer cells, respectively, were seeded on sterile FL-HA patterned surfaces. Higher cell concentrations of colon cancer cells were seeded because of their smaller cell size and growth as colonies. Within 24 hours, colonies of colon cancer cells were observed within the HA-presenting squares. After 48 hours, the colon cancer colonies grew in size, spreading beyond the perimeter of the HA patterns and extending to connect neighboring colonies (Fig. 3Ai-ii). Immunofluorescence analysis confirmed the localization of the cells within and surrounding the FL-HA patterns (Fig. 3Aiii-iv). Breast cancer cells attached to the HA patterns within 24 hours, while proliferation and minimal spreading outside the patterns was observed within 48 hours (Fig. 3Bi-ii). Breast cancer cells remained mostly restricted to HA regions, as compared to the colon cancer cells, which grow in colonies and exceeded the 80 × 80 μm HA patterns. Immunofluorescence analysis confirmed the localization of the breast cancer cells within the FL-HA patterns (Fig. 3Biii-iv). Both cancer cell types responded similarly when exposed to HA functional surfaces in subsequent trials. In contrast, no to poor cell attachment onto the patterns was observed in various surface functionalizations, all lacking HA (Table 1), while cell attachment was observed on non-patterned HA surfaces (data not shown).
Figure 3. HA surfaces for the culture of cancer cells.
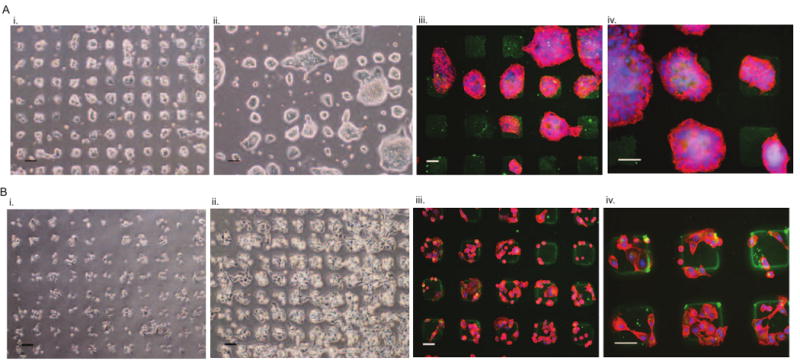
A. Colon cancer cells seeded on HA patterned surfaces: (i) formed colonies within the 80 μm × 80 μm squares after 24 hours and (ii) grew in size, with some spreading outside the squares after 48 hours of culture. Immunofluorescence analysis verified the CD44+ colon cancer cells (red; nuclei:blue) within FL-HA squares (green) at (iii) low and (iv) high magnifications. B. Breast cancer cells seeded on HA-patterned surfaces: (i) attached within the 80 μm × 80 μm squares after 24 hours, and (ii) grew outside the squares after 48 hours of culture. Immunofluorescence analysis verified the CD44+ breast cancer cells (red; nuclei in blue) within FL-HA squares (green) at (iii) low and (iv) high magnifications. Scale bars are 100 μm in Ai–ii and Bi–ii, and 50 μm in Aiii–iv and Biii–iv.
Table 1.
Colon Cancer attachment to various surface settings
| Surface Chemistry | Colon cancer cell attachment |
|---|---|
| Plain glass slide | Cells attached and remained attached after washing with PBS. |
| PEG surface | Cells initially attached, but weakly, and detached after washing with PBS at the 20 hour time point. |
| APTMS surface | Some cell attachment with no spreading (round morphology). |
| APTMS and EDC/NHS surface | Cells maintained rounded morphology as single cell, not attached to surface. |
| APTMS(patterned)/PEG | Cells attached to surface but avoided patterned areas. |
| APTMS, EDC/NHS(patterned)/PEG | Cells attached to surface, avoided patterned EDC/NHS. When rinsed with PBS, cells detached. |
To characterize the interaction of colon and breast cancer cells with the HA surface, we examined whether the adhesion to the HA surfaces was specific. Adhesive processes are known to participate in cancer cell motility and invasion [38-40]. We could observe extensions from the edge of cancer cells spreading onto the HA surface during the culture period (Fig S2). To confirm that these extensions are adhesive protrusions, high imaging analysis using SEM of adhered colon and breast cells was performed. This analysis clearly demonstrated the presence of adhesive processes via protrusions spreading onto the HA surface from breast cancer cells and the edge of colon cancer colonies, which enhance spreading and movement outside the HA patterns (Fig. 4A). As both colon and breast cancer cells were characterized by membrane expression of CD44, we examined whether blocking CD44 affected adhesion onto HA surfaces. Colon and breast cancer cells seeded onto functional HA surfaces in the presence of anti-CD44 were unable to attach onto the HA surfaces within 24 hours (Fig. 4B), confirming that adhesion occurs through surface receptor CD44. Overall, the utilization of functional HA surfaces allow us to perform high-resolution visualization to analyze cancer cell attachment and migration at a single cell level.
Figure 4. Cancer cell interaction with HA.
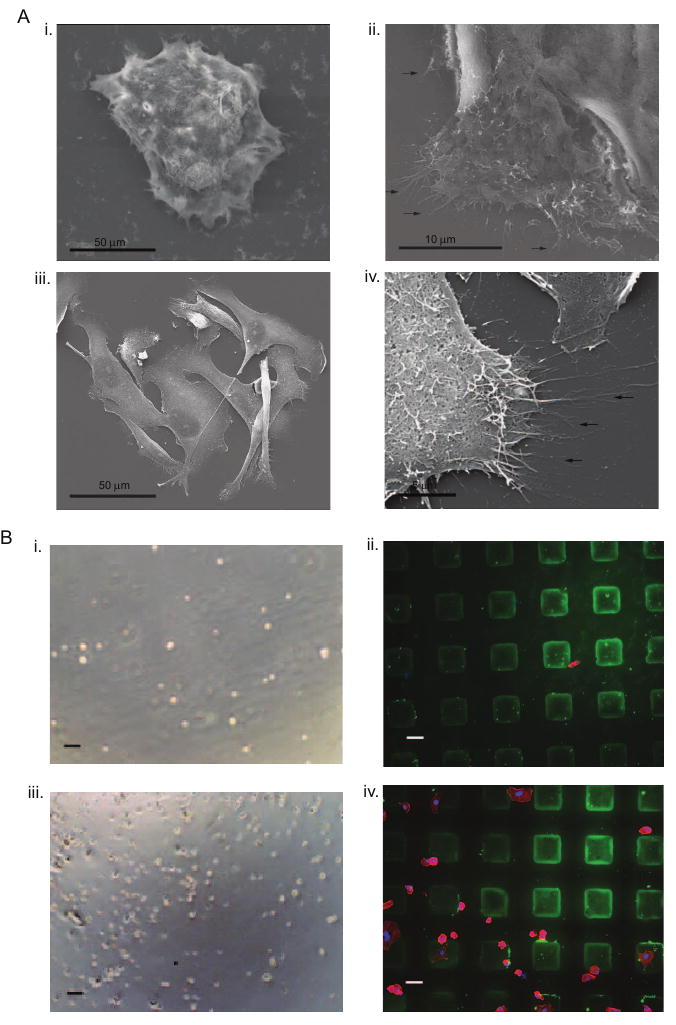
A. SEM analysis revealed adhesive protrusions of colon cancer cells cultured on HA surfaces shown at (i) low and (ii) high magnifications and of breast cancer cells cultured on HA surfaces shown at (iii) low and (iv) high magnifications (arrows indicate cell protrusions) B. Blocking of CD44 prevented (i) colon cancer cell adhesion after 24 hours of culture while (ii) HA surfaces are present (green; actin-stained colon cancer cells in red; nuclei in blue) and (iii) breast cancer cell adhesion after 24 hours of culture while (iv) HA surfaces are present (green; actin-stained breast cancer cells in red; nuclei in blue). Scale bars are 100 μm in Bi and Biii, and 50 μm in Bii and Biv.
Thus far, we have demonstrated preferred cell adhesion to HA-presenting regions rather than to neighboring regions presenting PEG. Prior work in the literature by Khademhosseini and colleagues had examined NIH-3T3 mouse fibroblast cells on surfaces patterned to present either Fn or HA; in that work, the fibroblast cells adhered preferentially to Fn neighboring regions, and were repelled from the HA presenting regions [23]. We therefore aimed to examine whether adjacent molecules affect cell adhesion onto HA-presenting regions. To achieve this, we used the same cell line, NIH-3T3 mouse fibroblast, to answer this question. When seeded on our surfaces, presenting HA and PEG, NIH-3T3 mouse fibroblast cells adhered onto HA regions (Fig 5A). When seeded on surfaces presenting HA and Fn, fibroblasts attached onto Fn surfaces (Fig. 5B-C) as previosuly demonstrated [23]. These results demonstrate that cell adhesion preference to HA-presenting region depends on the presentation of adjacent molecules. The attachment of NIH-3T3 cells reported in the present study was achievable due to the presentation of PEG rather than Fn.
Figure 5. Fibroblast cell interaction with HA-patterned surface.
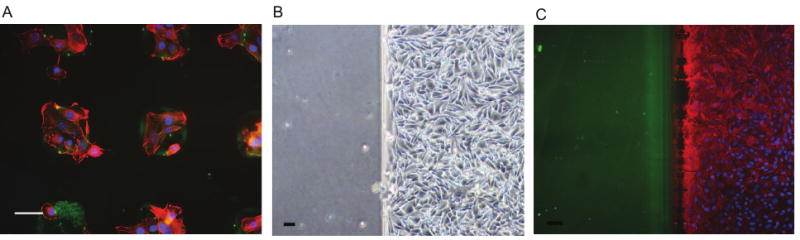
A. NIH-3T3 mouse fibroblasts seeded on newly developed HA/PEG surfaces preferentially adhered to HA regions within 6 hours B. NIH-3T3 mouse fibroblasts seeded on HA/FN surfaces preferentially adhered to Fn regions within 6 hours. C. Immunofluorescence analysis verifies attachment of fibroblast cells restricted to Fn region (red; nuclei in blue; HA in green). Scale bars are 50 μm.
4. Conclusions
Incorporating carbodiimide chemistry with μCP enables the immobilization of HA to defined regions, creating substrates suitable for in vitro applications. Both colon cancer and breast cancer cells implicitly interact with the HA surfaces, with cell adhesion occurring through CD44 surface receptors. Non cancerous fibroblast attachment onto the HA surfaces is achievable due to presentation of PEG as a competing site rather than Fn. This new approach allows high-resolution analysis of cancer cell attachment, growth, and migration on exogenous native HA.
Supplementary Material
HA patterns were subjected to cell culture conditions, incubated in colon cancer cell media at 37°C over 5 days. HA patterns remained robust during 5 days in culture. Fluorescent images were taken at the same exposure time, and the fluorescent intensity was subsequently analyzed using Image J.
A. Light microscope images of cancer cells seeded on HA surfaces display protrusions (denoted by arrows) from colon cancer cells (i) and breast cancer cells (ii). B. Immunofluorescence analysis verified protrusions(denoted by arrows) of CD44+ colon cancer cells (red, nuclei in blue) at high magnification(i) and CD44+ breast cancer cells(red, nuclei in blue) at high magnification interacting with the HA surface (green). Scale bars are 100 μm in Ai and Aii, and 50 μm in Bi and Bii.
Acknowledgments
The authors acknowledge the use of the surface analysis laboratory at Johns Hopkins, funded as part of the Materials Research Science and Engineering Center through the National Science Foundation. LED is an IGERT trainee and a National Science Foundation Graduate Fellow. This research was partially supported by NIH grant U54CA143868.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toole BP. Hyaluronan from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 2.Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of Hyaluronan by Expression of the Hyaluronan Synthase Has2 Enhances Anchorage-independent Growth and Tumorigenicity. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 3.Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. P NATL ACAD SCI USA. 1996;93:7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toole BP, Wight TN, Tammi MI. Hyaluronan-Cell Interactions in Cancer and Vascular Disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 5.Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between Hyaluronan Production and Metastatic Potential of Mouse Mammary Carcinoma Cells. Cancer Res. 1999;59:2499–2504. [PubMed] [Google Scholar]
- 6.Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan Synthase 3 Overexpression Promotes the Growth of TSU Prostate Cancer Cells. Cancer Res. 2001;61:5207–5214. [PubMed] [Google Scholar]
- 7.Simpson MA, Reiland J, Burger SR, Furcht LT, Spicer AP, Oegema TR, McCarthy JB. Hyaluronan synthase elevation in metastatic prostate carcinoma cells correlates with hyaluronan surface retention, a prerequisite for rapid adhesion to bone marrow cells. J Biol Chem. 2001;276:17949–17957. doi: 10.1074/jbc.M010064200. [DOI] [PubMed] [Google Scholar]
- 8.Kim HR, Wheeler MA, Wilson CM, Iida J, Eng D, Simpson MA, McCarthy JB, Bullard KM. Hyaluronan Facilitates Invasion of Colon Carcinoma Cells in Vitro via Interaction with CD44. Cancer Res. 2004;64:4569–4576. doi: 10.1158/0008-5472.CAN-04-0202. [DOI] [PubMed] [Google Scholar]
- 9.Laurich C, Wheeler MA, Iida J, Neudauer CL, McCarthy JB, Bullard KM. Hyaluronan mediates adhesion of metastatic colon carcinoma cells. J Surg Res. 2004;122:70–74. doi: 10.1016/j.jss.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 10.David L, Dulong V, Le Cerf D, Cazin L, Lamacz M, Vannier JP. Hyaluronan hydrogel: An appropriate three-dimensional model for evaluation of anticancer drug sensitivity. Acta Biomaterialia. 2008;4:256–263. doi: 10.1016/j.actbio.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segura T, Anderson BC, Chung PH, Webber RE, Shull KR, Shea LD. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26:359–371. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y. Micropattern immobilization of polysaccharide. J Inorg Biochem. 2000;79:77–81. doi: 10.1016/s0162-0134(99)00159-2. [DOI] [PubMed] [Google Scholar]
- 14.Barbucci R, Magnani A, Lamponi S, Pasqui D, Bryan S. The use of hyaluronan and its sulphated derivative patterned with micrometric scale on glass substrate in melanocyte cell behaviour. Biomaterials. 2003;24:915–926. doi: 10.1016/s0142-9612(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 15.Barbucci R, Lamponi S, Magnani A, Pasqui D. Micropatterned surfaces for the control of endothelial cell behaviour. Biomol Eng. 2002;19:161–170. doi: 10.1016/s1389-0344(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 16.Barbucci R, Magnani A, Chiumiento A, Pasqui D, Cangioli I, Lamponi S. Fibroblast Cell Behavior on Bound and Adsorbed Fibronectin onto Hyaluronan and Sulfated Hyaluronan Substrates. Biomacromolecules. 2005;6:638–645. doi: 10.1021/bm049642v. [DOI] [PubMed] [Google Scholar]
- 17.Di Canio C, Lamponi S, Barbucci R. Spiral and square microstructured surfaces: The effect of the decreasing size of photo-immobilized hyaluronan domains on cell growth. J Biomed Mater Res A. 2009;92A:276–284. doi: 10.1002/jbm.a.32317. [DOI] [PubMed] [Google Scholar]
- 18.Lamponi S, Di Canio C, Barbucci R. Heterotypic cell-cell interaction on micropatterned surfaces. Int J Artif Organs. 2009;32:507–516. doi: 10.1177/039139880903200805. [DOI] [PubMed] [Google Scholar]
- 19.Rossi A, Pasqui D, Barbucci R, Gerli R, Weber E. The topography of microstructured surfaces differently affects fibrillin deposition by blood and lymphatic endothelial cells in culture. Tissue Eng Part A. 2009;15:525–33. doi: 10.1089/ten.tea.2007.0421. [DOI] [PubMed] [Google Scholar]
- 20.Lamponi S, Canio CD, Forbicioni M, Barbucci R. Heterotypic interaction of fibroblasts and endothelial cells on restricted area. J Biomed Mater Res A. 2009;9999:NA. doi: 10.1002/jbm.a.32364. [DOI] [PubMed] [Google Scholar]
- 21.Morra M, Cassineli C. Non-fouling properties of polysaccharide-coated surfaces. J Biomat Sci-Poly E. 1999;10:1107–1124. doi: 10.1163/156856299x00711. [DOI] [PubMed] [Google Scholar]
- 22.Barbucci R, Torricelli P, Fini M, Pasqui D, Favia P, Sardella E, d'Agostino R, Giardino R. Proliferative and re-defferentiative effects of photo-immobilized micro-patterned hyaluronan surfaces on chondrocyte cells. Biomaterials. 2005;26:7596–7605. doi: 10.1016/j.biomaterials.2005.05.090. [DOI] [PubMed] [Google Scholar]
- 23.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Layer-by-layer depostion of hyaluronic acid and poly-l-lysine for patterned co-cultures. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, Langer R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Suh KY, Khademhosseini A, Yang JM, Eng G, Langer R. Soft lithographic patterning of hyaluronic acid on hydrophilic substrates using molding and printing. Adv Mater. 2004;16:584–588. [Google Scholar]
- 26.Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed Microdevices. 2007;9:149–157. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- 27.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. P Natl Acad Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groll J, Fiedler J, Engelhard E, Ameringer T, Tugulu S, Klok HA, Brenner RE, Moeller M. A novel star PEG-derived surface coating for specific cell adhesion. J Biomed Mater Res A. 2005;74:607–17. doi: 10.1002/jbm.a.30335. [DOI] [PubMed] [Google Scholar]
- 29.Harbers GM, Emoto K, Greef C, Metzger SW, Woodward HN, Mascali JJ, Grainger DW, Lochhead MJ. A functionalized poly(ethylene glycol)-based bioassay surface chemistry that facilitates bio-immobilization and inhibits nonspecific protein, bacterial, and mammalian cell adhesion. Chem Mater. 2007;19:4405–4414. doi: 10.1021/cm070509u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Shah SS, Yan J, Howland MC, Parikh AN, Pan T, Revzin A. Integrating sensing hydrogel microstructures into micropatterned hepatocellular cocultures. Langmuir. 2009;25:3880–6. doi: 10.1021/la803635r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord MS, Pasqui D, Barbucci R, Milthorpe BK. Protein adsorption on derivatives of hyaluronic acid and subsequent cell response. Journal of Biomedical Materials Research Part A. 2009;91:635–646. doi: 10.1002/jbm.a.32219. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim S, Joddar B, Craps M, Ramamurthi A. A surface-tethered model to assess size-specific effects of hyaluronan(HA) on endothelial cells. Biomaterials. 2007;28:825–835. doi: 10.1016/j.biomaterials.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Gotte M, Yip GW. Heparanase, Hyaluronan, and CD44 in Cancers: A Breast Carcinoma Perspective. Cancer Res. 2006;66:10233–10237. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton SR, Fard SF, Paiwand FF, Tolg C, Veiseh M, Wang C, McCarthy JB, Bissell MJ, Koropatnick J, Turley EA. The Hyaluronan Receptors CD44 and Rhamm (CD168) Form Complexes with ERK1,2 That Sustain High Basal Motility in Breast Cancer Cells. J Biol Chem. 2007;282:16667–16680. doi: 10.1074/jbc.M702078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tammi MI, Day AJ, Turley EA. Hyaluronan and Homeostasis: A Balancing Act. J Biol Chem. 2002;277:4581–4584. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- 37.Edward M, Gillan C, Micha D, Tammi RH. Tumour regulation of fibroblast hyaluronan expression: a mechanism to facilitate tumour growth and invasion. Carcinogenesis. 2005;26:1215–1223. doi: 10.1093/carcin/bgi064. [DOI] [PubMed] [Google Scholar]
- 38.Sadie A, Anne NM, William GSS, Lance AL. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMS in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 40.Hapke S, Kessler H, Luber B, Benge A, Hutzler P, Hofler H, Schmitt M, Reuning U. Ovarian cancer cell proliferation and motility is induced by engagement of integrin avB3/Vitronectin interaction. Biol Chem. 2003;384:1073–1083. doi: 10.1515/BC.2003.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HA patterns were subjected to cell culture conditions, incubated in colon cancer cell media at 37°C over 5 days. HA patterns remained robust during 5 days in culture. Fluorescent images were taken at the same exposure time, and the fluorescent intensity was subsequently analyzed using Image J.
A. Light microscope images of cancer cells seeded on HA surfaces display protrusions (denoted by arrows) from colon cancer cells (i) and breast cancer cells (ii). B. Immunofluorescence analysis verified protrusions(denoted by arrows) of CD44+ colon cancer cells (red, nuclei in blue) at high magnification(i) and CD44+ breast cancer cells(red, nuclei in blue) at high magnification interacting with the HA surface (green). Scale bars are 100 μm in Ai and Aii, and 50 μm in Bi and Bii.


