In “Manganese accumulates primarily in nuclei of cultured brain cells”, Kalia et al (Kalia et al., 2008) report that upon fractionation of neuronal cells, labeled Mn was found primarily in nuclei, with virtually none in the mitochondrial fraction and therefore, that mitochondria may play an insignificant role in subcellular Mn distribution. They also say that “there has been no direct evidence --- on subcellular distribution of Mn,” and that the recent report by Morello et al (Morello et al., 2008) concluded that nuclear components may represent the “preferential targets for Mn accumulation and toxicity” (Kalia et al., 2008).
In fact, a number of other studies (Ayotte and Plaa, 1985; Lai et al., 1999; Liccione and Maines, 1988; Maynard and Cotzias, 1955; Miller et al., 1975) have determined the subcellular distribution of Mn after tissue fractionation following treatment with Mn in vivo, and all demonstrated treatment-related increases in intramitochondrial Mn. In addition, other workers have found that Mn decreases energy metabolism in vivo and in vitro, including decreases in the activities of mitochondrial enzymes, in membrane potential, and ATP production (Brouillet et al., 1993; Du et al., 1997; Galvani et al., 1995; Gavin et al., 1992; Malecki, 2001; Malthankar et al., 2004; Roth et al., 2000; Roth et al., 2002; Wolters et al., 1989; Zwingmann et al., 2003). Using electron energy-loss spectroscopy with electron microscopy, Morello et al (Morello et al., 2008) reported that although nuclei contained more Mn than mitochondria, treatment-related increases were greater in the mitochondria. They concluded that “the relevant distribution of Mn is not limited to the mitochondria.” Consideration of why the results of Kalia et al differ from those of other distribution studies requires an understanding of mitochondrial ion transport and its response to fractionation.
Mitochondrial sequestration of Ca2+ or Mn2+ does not represent simple binding but weak binding within a steady state controlled by both influx and efflux of these ions (Gunter and Pfeiffer, 1990). Mn2+ is sequestered by the mitochondrial Ca2+ uniporter, primarily energized by the internally negative membrane potential (ΔΨ), and effluxed by the Na+-independent mechanism, primarily energized by the pH gradient (Gavin et al., 1990; Gunter and Pfeiffer, 1990; Gunter and Sheu, 2008). Both are maintained by energy-dependent proton pumping across an intact inner membrane. If ΔΨ falls, uptake velocity decreases precipitously – by over 83% as ΔΨ falls from 180 to 160 mV, for example (Gunter and Sheu, 2008). If ΔΨ falls near zero, the weakly bound ions rush out by reverse uniport (Gunter et al., 1975; Gunter et al., 1978)
The “isolated mitochondria” produced by fractionation, whether by mechanical action or by detergents, represent resealed fragments of the original cellular mitochondrial network (Gunter and Sheu, 2008; Chan, 2006). In these resealed mitochondria, ΔΨ is dissipated during fractionation, then rebuilt by proton pumping energized by endogenous substrate -- e.g., pyruvate, a product of glycolysis that in the intact cell is transported continually into mitochondria for use in the TCA cycle. However, the fractionation procedure greatly dilutes glycolytic enzymes and substrates, and the amount of endogenous substrate within isolated, resealed mitochondria is greatly reduced and no longer replenished. It has been well known since the 1960’s that appreciable Ca2+ or Mn2+ uptake by these resealed mitochondria requires addition of mitochondrial substrate; however, none was added in the fractionation studies cited above. Why, then, did all except Kalia et al find Mn in the mitochondria? Maynard and Cotzias (1955) stressed that they treated animals with less Mn than that present in the food. Other ex vivo Mn distribution studies (Lai et al., 1999; Liccione and Maines, 1988) examined Mn concentrations in brain fractions; since brain Mn uptake is limited both by the blood-brain barrier and by rigorous homeostatic control of absorption and excretion, the amounts reaching mitochondria were probably not large. In contrast, Kalia et al exposed their cells for 24 hours to 100 μM Mn2+. Based on the uptake that we measured in PC12 cells at 100 μM for 24 hours (11.7 nmoles/mg cell protein) (Gunter et al., 2005), we estimate that the mitochondria of Kalia et al were exposed to [Mn2+]s over 100 times higher than those of Maynard and Cotzias and much higher than in the other fractionation studies. Following fractionation, the resealed mitochondria would begin to resequester and cycle the surrounding Mn2+, using energy from endogenous substrate. However, in the presence of large amounts of Mn2+, as in the experiments of Kalia et al, Mn cycling would quickly dissipate the endogenous substrate, ΔΨ would fall, and the Mn2+ would be released again from the mitochondria to bind to available sites such as nuclei. (For example, Mn2+ binds to DNA with an affinity of about 33 μM (Kennedy and Bryant, 1986)). In the presence of small amounts of Mn2+, as with the other fractionation studies, ion cycling would dissipate the available energy much more slowly, and the mitochondria would likely retain measurable amounts of Mn.
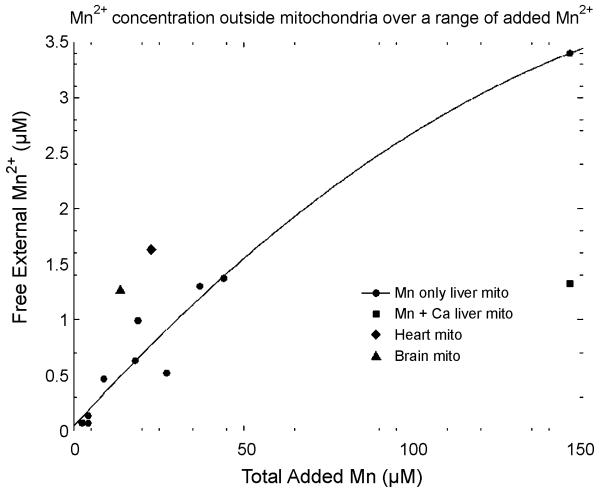
While conducting earlier experiments (Gunter et al., 2004), we determined the medium [Mn2+] to which isolated mitochondria would pump varying amounts of added Mn2+ (Fig. 1). Notice that if 2μM Mn2+ is added, the mitochondria will pump the external [Mn2+] to around 80 nM, while if 142 μM Mn2+ is added, the mitochondria will only pump the external [Mn2+] to around 3.4 μM. This is because the more extensive Mn2+ cycling in the latter case lowers ΔΨ, shifting the steady state toward less uptake. These concentrations represent the levels at which energized mitochondria compete with other cellular binding sites, such as those in the nucleus. These observations suggest that mitochondrial substrates should be added in fractionation studies to minimize redistribution of Mn2+.
Figure 1.
Concentration of free Mn2+ in the medium in the presence of several preparations of energized mitochondria (4 mg/ml) as a function of the total concentration of Mn2+ added. 160 μM Ca2+ was also added to the data indicated by the filled square to activate the uniporter.
Acknowledgments
Supported by NIH RO1 ES!0041 and Dept. of Defense (MHRP) grant W81XWH-05-1-0239.
References
- Ayotte P, Plaa L. Hepatic subcellular distribution of manganese in manganese and manganese-bilirubin induced cholestasis. Biochem. Pharm. 1985;34:3857–3865. doi: 10.1016/0006-2952(85)90435-6. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Du P, Buerstatte CR, Chan AWK, Minski MJ, Bennett L, Lai JCK. Accumulation of manganese in liver can result in decreases in energy metabolism in mitochondria. Proceedings of 1997 Conference of Hazardous Wastes and Materials.1997. pp. 1–14. [Google Scholar]
- Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur. J. Pharm. 1995;293:377–383. doi: 10.1016/0926-6917(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990;266:329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- Gunter KK, Aschner MA, Miller LM, Eliseev R, Salter J, Anderson K, Hammond S, Gunter TE. Determining the oxidation states of manganese in PC12 and NGF-induced PC12 cells. Free Radic Biol Med. 2005;39:164–181. doi: 10.1016/j.freeradbiomed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Puskin JS, Russell PR. Quantitative magnetic resonance studies of manganese uptake by mitochondria. Biophys J. 1975;15:319–333. doi: 10.1016/S0006-3495(75)85822-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Puskin JS, Russell PR. Efflux of Ca2+ and Mn2+ from rat liver mitochndria. Biochem. 1978;17:339–345. doi: 10.1021/bi00595a023. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Miller LM, Gavin CE, Eliseev R, Salter J, Buntinas L, Alexandrov A, Hammond S, Gunter KK. Determination of the oxidation states of manganese in brain, liver, and heart mitochondria. J Neurochem. 2004;88:266–280. doi: 10.1046/j.1471-4159.2003.02122.x. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Sheu S-S. Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. BBA Bioenergetics. 2008 doi: 10.1016/j.bbabio.2008.12.011. (in press)doi:10.1016/j.bbabio.2008.12.011: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia K, Jiang W, Zheng W. Manganese accumulates primarily in nuclei of cultures brain cells. Neurotoxicology. 2008;29:466–470. doi: 10.1016/j.neuro.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SD, Bryant RG. Manganese-deoxyribonucleic acid binding modes: Nuclear magnetic resonance dispersion results. Biophy. J. 1986;50:669–676. doi: 10.1016/S0006-3495(86)83507-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JCK, Minski MJ, Chan AWK, Leung TKC, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. Arnold, 1999 #1331. [PubMed] [Google Scholar]
- Liccione JJ, Maines MD. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther. 1988;247:156–161. [PubMed] [Google Scholar]
- Malecki EA. Manganese toxicity is associated with mitochondrial disfunction and DNA fragmentation in rat primary striatal neurons. Brain Res. Bull. 2001;55:225–228. doi: 10.1016/s0361-9230(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Malthankar GV, White BK, Bhushan A, Daniels CK, Rodnick KJ, Lai JC. Differential lowering by manganese treatment of activities of glycolitic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem Res. 2004;29:709–717. doi: 10.1023/b:nere.0000018841.98399.ce. [DOI] [PubMed] [Google Scholar]
- Maynard LS, Cotzias GL. The partition of manganese among organs and intracellular organelles of the rat. J Biol Chem. 1955;214:489–495. [PubMed] [Google Scholar]
- Miller ST, Cotzias GC, Evert HA. Control of tissue manganese: Initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol. 1975;229:1080–1084. doi: 10.1152/ajplegacy.1975.229.4.1080. [DOI] [PubMed] [Google Scholar]
- Morello M, Canini A, Mattioli P, Sorge RP, Almonti A, Bocca B, Forte G, Martorana A, Bernardi G, Sancesario G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats: An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotox. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Walowitz J, Brown RW. Manganese-induced rat pheochromocytoma (PC12) cell death is independent of caspase activation. J Neurosci Res. 2000;61:162–171. doi: 10.1002/1097-4547(20000715)61:2<162::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Roth JA, Horbinski C, Higgins D, Lein P, garrick MD. Mechanisms of manganese-induced rat pheochromocytoma (PC 12) cell death and cell differentiation. Neurotoxicology. 2002;23:147–157. doi: 10.1016/s0161-813x(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Wolters EC, Huang C-C, Clark C, Peppard RF, Okada J, Chu N,-S, Adam MJ, Ruth TJ, Li D, Calne DB. Positron emission tomography in manganese intoxication. Ann Neurol. 1989;26:647–651. doi: 10.1002/ana.410260510. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Leibfritz D, Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: Relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab. 2003;23:756–771. doi: 10.1097/01.WCB.0000056062.25434.4D. [DOI] [PubMed] [Google Scholar]